5 Steps to a 5 500 AP Physics Questions to Know by Test Day (2012)
Chapter 10. Thermodynamics
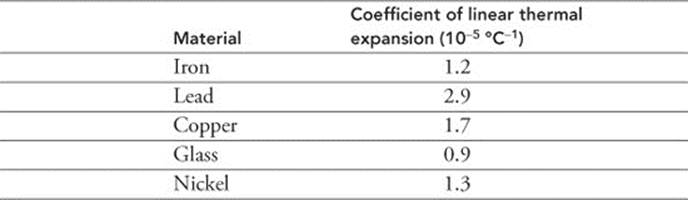
271. A 1.0-m-long section of iron railroad gets warmed by the Sun from 25°C to 40°C. The coefficient of thermal linear expansion for iron is 1.2 × 10-5°C−1. How much did the length of the iron rail change?
(A) −0.18 cm
(B) −0.18 mm
(C) 0 cm
(D) 0.18 mm
(E) 0.18 cm
272. You have 20 moles of a gas in a 1-m3 sealed container at 125°C. How many atmospheres of pressure are inside the container?
(A) 0.1 atm
(B) 0.6 atm
(C) 1.0 atm
(D) 1.2 atm
(E) 1.8 atm
273. If you have 2 moles of gas at room temperature (25°C), then what is the internal energy of the gas?
(A) 720 J
(B) 3,600 J
(C) 7,200 J
(D) 36,000 J
(E) 72,000 J
274. The mass of an oxygen atom is 5 × 10−26 kg. What is the average speed of an oxygen molecule at room temperature (25°C)?
(A) 50 m/s
(B) 100 m/s
(C) 250 m/s
(D) 500 m/s
(E) 1,000 m/s
275. In an adiabatic process, 0.5 moles of gas at 1,000 K expand to reach a final temperature of 500 K. How much work was done?
(A) −5,000 J
(B) −3,000 J
(C) 0 J
(D) 3,000 J
(E) 5,000 J
276. An engine converts 5,000 J of thermal energy into 2,500 J of work. What is the efficiency of the engine?
(A) 10 percent
(B) 20 percent
(C) 50 percent
(D) 100 percent
(E) 200 percent
277. An ideal heat engine has an efficiency rate of 20 percent. If the heat reservoir has a temperature of 200°C, then what is the temperature of the heat sink?
(A) 0°C
(B) 50°C
(C) 110°C
(D) 200°C
(E) 300°C
278. If 500 J of work was done on a volume of gas in a container with a moveable piston at a constant atmospheric pressure, what was the change in gas volume?
(A) −0.05 m3
(B) −0.005 m3
(C) 0 m3
(D) 0.005 m3
(E) 0.05 m3
279. The pressure, volume, and temperature of a gas were changed from Point A (low temperature) to Point B (high temperature) as shown in this graph. How much work was done by the gas?
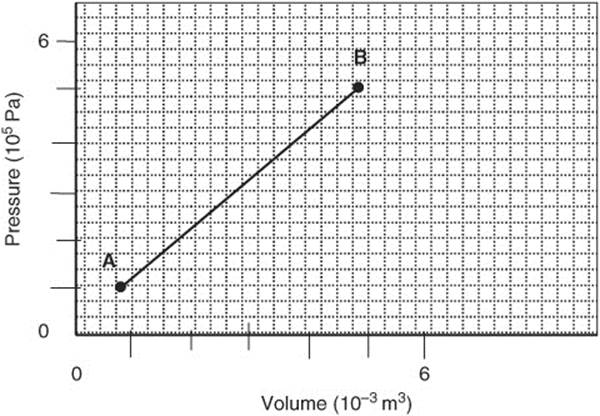
(A) −1,200 J
(B) −120 J
(C) 0 J
(D) 120 J
(E) 1,200 J
280. In which of the following processes is no work done on or by the gas?
(A) Adiabatic
(B) Isobaric
(C) Isothermal
(D) Isochoric
(E) None, all of the processes do work
281. A copper rod has a diameter of 2 cm and a length of 0.5 m. It is placed against a heat source. The temperature of the rod increases from 25°C to 50°C. The thermal conductivity of copper is 390 J/s.m.°C. What is the rate of heat transfer into the copper rod?
(A) 6 J/s
(B) 15 J/s
(C) 2,000 J/s
(D) 2,500 J/s
(E) 3,000 J/s
282. A 20-kg crate slides horizontally on a floor at 0.5 m/s and comes to rest in 25 s. What is the rate of thermal energy transferred between the crate and the floor by friction?
(A) 0.1 W
(B) 0.5 W
(C) 1.0 W
(D) 2.0 W
(E) 10 W
283. One kJ of thermal energy is transferred to a gas in a cylinder with a movable piston. At the same time, 200 J of work is done on the system. What is the change in internal energy of the system?
(A) 5 J
(B) 800 J
(C) 1,000 J
(D) 1,200 J
(E) 5,000 J
284. If 500 J of thermal energy is added to a gas in a cylinder and the temperature remains the same, then which of the following statements is true?
(A) The internal energy increases by 500 J.
(B) The internal energy decreases by 500 J.
(C) The gas does 500 J of work.
(D) 500 J of work is done on the gas.
(E) The pressure inside increases.
285. A 1-m rod of material is heated so that its temperature is increased by 10°C. The rod expands by 0.17 mm. What material is the rod made of?
(A) Glass
(B) Copper
(C) Iron
(D) Lead
(E) Nickel
286. Two moles of oxygen gas are in a container with a movable piston at 25°C. The pressure is 6,400 Pa. What is the volume of the gas?
(A) 0.001 m3
(B) 0.01 m3
(C) 0.1 m3
(D) 1.0 m3
(E) 10 m3
287. One mole of hydrogen gas has an internal energy of 4.3 kJ. What is the temperature of the gas?
(A) 2 K
(B) 16 K
(C) 160 K
(D) 260 K
(E) 360 K
288. A heat engine has a 20 percent efficiency rating. If you put in 2 kJ of thermal energy, how much work will the engine do?
(A) 20 J
(B) 40 J
(C) 200 J
(D) 400 J
(E) 800 J
289. If a heat source for an ideal heat engine has a temperature of 1,000°C and the temperature of the heat sink is 100°C, what is its efficiency?
(A) 7 percent
(B) 21 percent
(C) 42 percent
(D) 64 percent
(E) 70 percent
290. You change the temperature of one mole of nitrogen gas from 25°C to −175°C. What is the change in the gas’s internal energy?
(A) −2,400 J
(B) −1,200 J
(C) 0 J
(D) 1,200 J
(E) 2,400 J
291. A concrete slab of a sidewalk heats up in the Sun. The temperature changes from 20°C to 40°C. The coefficient of thermal linear expansion for concrete is 1.2 × 10−5 °C−1. What is the percentage change in the length of the slab?
(A) 0.02 percent
(B) 0.2 percent
(C) 1 percent
(D) 2 percent
(E) 20 percent
292. At a constant pressure, the volume of a gas triples from its original volume. Which of the following statements is true?
(A) The work done on the gas increases by one-third.
(B) The work done by the gas increases by one-third.
(C) The work done by the gas increases threefold.
(D) The work done on the gas increases threefold.
(E) No work is done on or by the gas.
293. A 0.001-m3 sealed cylinder contains gas at a pressure of 2,500 N/m2, with a temperature of 25°C. How many gas molecules are in the cylinder?
(A) 3 × 1020
(B) 6 × 1020
(C) 3 × 1023
(D) 6 × 1023
(E) 1.2 × 1024
294. A 1.0-m brass rod with a 1-cm radius gets heat transferred to it at a rate of 1 J/s. If the coefficient of linear thermal expansion is 110 J/s. m. °C, then what is the change in the rod’s temperature?
(A) 3°C
(B) 6°C
(C) 12°C
(D) 18°C
(E) 30°C
295. If the internal energy change during an isochoric process is 1,000 J, how much heat is transferred?
(A) −1,000 J
(B) −100 J
(C) 0 J
(D) 500 J
(E) 1,000 J
296. Assuming a temperature of 125°C for steam, how fast, on average, would a water molecule move?
(A) 75 m/s
(B) 150 m/s
(C) 300 m/s
(D) 600 m/s
(E) 750 m/s
297. You push on the piston of a gas cylinder with a constant force of 2,000 N and the piston adiabatically depresses by 1 m. What was the change in internal energy of the gas?
(A) 100 J
(B) 500 J
(C) 1,000 J
(D) 1,500 J
(E) 2,000 J
298. On which of the following devices must work be done for it to achieve its function?
(A) Steam engine
(B) Electrical motor
(C) Air conditioner
(D) Gasoline engine
(E) All of these devices must have work done on them
299. Ten moles of a gas undergo a pressure and volume change from Point A to Point B as indicated by the arrows in this graph.
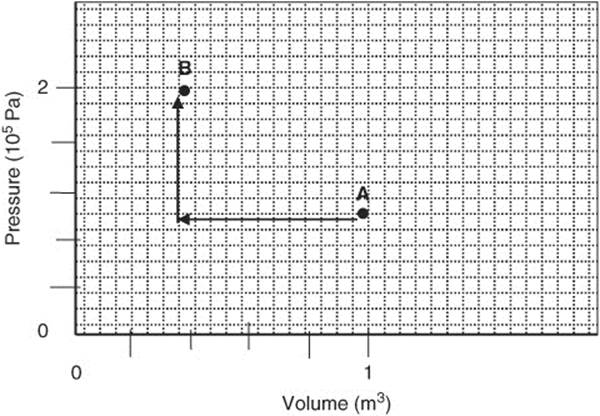
(a) Indicate on the graph how you would calculate the work involved.
(b) Calculate the work involved.
(c) Assuming that the change occurred adiabatically, calculate the change in internal energy.
(d) Calculate the temperature change that occurred.
(e) If the temperature at Point A was 50 K, what was it at Point B?
300. A silver wire has a radius of 0.5 mm and a length of 10 cm. Answer the following questions:
(a) If the wire is heated at one end and the temperature of the wire increases by 200°C, then how much will the wire expand linearly? (The coefficient of linear thermal expansion for silver is 1.9 × 10−5°C−1.)
(b) What percentage change is the expansion?
(c) If it takes 30 s for the wire to change temperature, how much heat was transferred? (The thermal conductivity for silver is 420 J/[s. m.°C].)