SAT Subject Test Physics (2012)
PART III. PHYSICS TOPIC REVIEW
Chapter 18. ATOMIC PHYSICS
Much of your review of physics has involved understanding phenomena above the atomic level. However, many interactions can be understood only by considering matter at the subatomic level, which involves the particles, forces, and energy within individual atoms. In this chapter, you will review the particle nature of light, atomic models, and nuclear interactions.
The Photoelectric Effect
So far you have treated light as a wave that could be approximated as a ray. In some situations, however, light must be described by a particle nature as well. The particles are individual bundles of energy called photons. In the 19th century, Heinrich Hertz discovered that if he shined ultraviolet light on a sample of zinc, the zinc acquired a positive charge. Shortly after, Max Planck defined the photon and related it to the frequency of light according to the following equation.
![]()
The letter h represents a value known as Planck’s constant, which equals 6.63 × 10–34 J s. Because frequency equals speed divided by wavelength, the equation can be rewritten as follows.
![]()
Now it becomes obvious that a photon’s energy is directly related to its frequency but inversely related to its wavelength.
Putting together Hertz’s observation and Planck’s idea, Einstein was able to propose an explanation. He suggested that the zinc that Hertz observed became positively charged because it emitted electrons. The electrons were emitted because the metal absorbed photons from the light that struck it. The emitted electrons were, therefore, described as photoelectrons, and the phenomenon became known as the photoelectric effect.
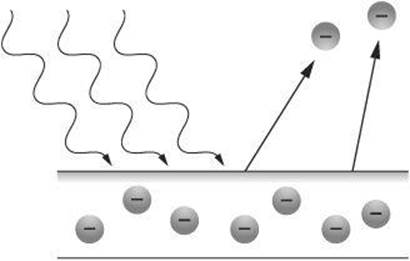
Shining visible light on the same sample of zinc does not have the same effect as shining ultraviolet light. The reason is that each metal has a minimum, or threshold frequency (fo), required for electrons to be emitted. The threshold frequency is the lowest frequency, and therefore the longest wavelength, that enables photoelectrons to be ejected from the surface. The photoelectrons ejected at this frequency have no leftover kinetic energy. At any higher frequency, they have leftover kinetic energy that enables them to travel away from the surface. Einstein rewrote Planck’s equation as follows.
![]()
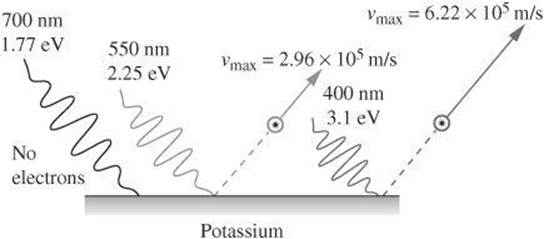
In this equation, E is the energy supplied by the photon striking the surface of the material, KEmax is the maximum kinetic energy of the emitted photo-electron, and Φ is the energy needed to remove the photoelectron from the surface (also known as the work function). The following diagram shows that red light striking the surface of potassium does not cause photoelectrons to be emitted. Both green and blue light emit photoelectrons. However, blue light provides enough leftover energy to causes the photoelectron to move at a greater speed than green light.
Atomic Models
An understanding of the photoelectric effect involves a useful model of the atomic structure. The earliest description of the nature of matter was provided more than 2000 years ago by the ancient Greeks. In fact, the modern term atom comes from the Greek word atomos, which means indivisible. The Greek model described matter as consisting of small particles considered to be not only indivisible, but also indestructible.
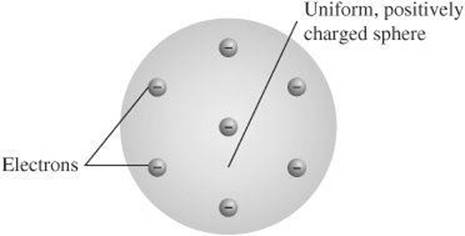
At the end of the 19th century, J. J. Thompson proved that the atom was divisible by discovering charged particles that would later become known as electrons. As a result, he suggested a model of the atom as a sphere of positive charge with negatively charged electrons embedded in it. It became known as the plum pudding model because it resembled a popular dessert at the time.
Several years later, Ernest Rutherford conducted his now-famous gold foil experiment. By aiming a stream of positive charges at a thin gold foil and observing the path of the particles upon striking the gold foil, Rutherford was able to develop a new atomic model.
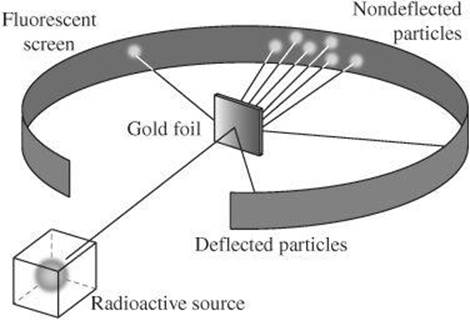
According to Rutherford’s model, atoms consist of mostly empty space. Almost all of the atom’s mass is concentrated in the center, or nucleus. The nucleus, which repelled the stream of particles, must have a positive charge. Positively charged protons, therefore, are located in the nucleus, whereas negative-charged electrons are located in the region around the nucleus.
The next development in the atomic model came from Niels Bohr. Using the hydrogen atom, Bohr revised the atomic model to show that electrons orbit the nucleus in definite, circular orbits. In the case of hydrogen, there is a single electron orbiting the nucleus, which has a single proton. An electron has a ground state, or orbit, with the least amount of energy with respect to the nucleus. If an atom gains energy, an electron can be raised to a higher energy level. An electron rises to a higher energy level only when it absorbs definite, quantized amounts of energy. When an electron returns to its ground state, it emits photons of energy equal to the amount that had been absorbed. This means that an electron does not emit light while in an energy level, but only when it drops from a higher energy to a lower one.
Energy levels are denoted as ![]() ,
, ![]() ,
, ![]() , and so on. Each level is associated with a different amount of energy.
, and so on. Each level is associated with a different amount of energy.
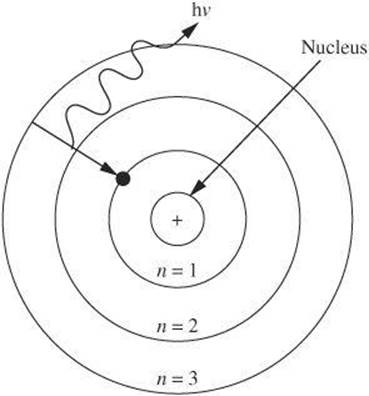
Atomic Nucleus
In addition to protons, it was later discovered that neutrons are also found within the nucleus of an atom. Because they are both found in the nucleus, protons and neutrons are often described as nucleons. Unlike protons and electrons, neutrons are electrically neutral. The number of protons Z in an atom defines the type of element it is. All atoms of a given element have the same number of protons, or atomic number. The number of neutrons N can vary among atoms of the same element. Atoms of the same element with different numbers of neutrons are known as isotopes of one another. In a neutral atom, the number of electrons equals the number of protons.
The total number of protons and neutrons is the mass number A of an atom. A symbol in the form of the one in the following diagram describes the atomic number and the mass number of an atom.
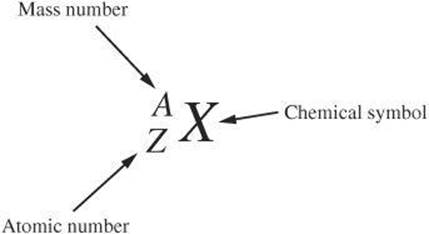
Example:
How many protons and neutrons are in a neutral atom of titanium ![]() Ti?
Ti?
The number of protons is the lower number, which is 22. The atomic number of titanium is 22. The mass number is the top number, which is 48. The number of neutrons is ![]() .
.
Nuclear Stability
The nucleus consists of tightly packed protons and neutrons. Recall that protons are positively charged and neutrons are electrically neutral. Knowing that positive charges repel one another, you might wonder why the positively charged protons do not repel one another and fly apart. They do indeed repel one another, but another force, known as the strong force, overcomes the repulsion. This force is different from other forces you have reviewed. It is extremely short range and acts only across distances of about 10–15 m. In addition, it is independent of electric charge. The force has the same magnitude between two protons or two neutrons at the same distance.
The relationship between the number of particles in a nucleus and the strong force determines the stability of a nucleus. In the following graph, the solid line represents nuclei for which the number of neutrons equals the number of protons. Only lighter nuclei fall on the line, whereas heavier nuclei fall above the line. What does this mean? Consider the nucleus in terms of the strong force. When the strong force balances the repulsion between protons, a nucleus is stable. The strong force is short range and acts only between particles and their nearest neighbors. The repulsive electric force, however, is long range and acts between all protons in the nucleus. For the forces to remain balanced, as the number of protons increases, the number of neutrons must also increase to add enough attractive force to maintain stability. Therefore, heavy nuclei are stable only when they have more neutrons than protons.
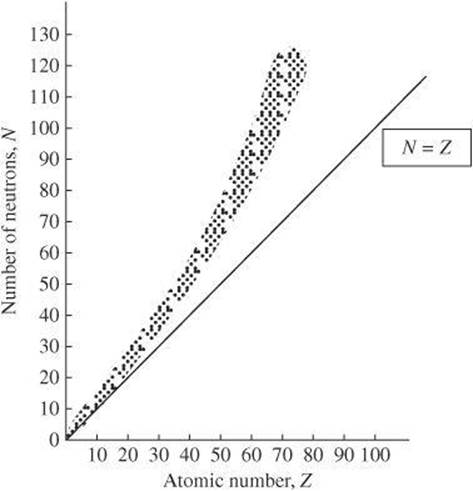
Once the number of number of protons exceeds 83, additional neutrons cannot compensate for the repulsive forces. As a result, atoms with atomic numbers greater than 83 do not have stable nuclei.
Binding Energy
The particles bound together in a nucleus are at a lower energy state than the particles would have individually. This difference in energy is known as the binding energy of the nucleus. The binding energy can be calculated using the following equation.
![]()
The quantity Δm is the difference between the mass of the unbound nucleons minus the mass of the bound nucleons. It is known as the mass defect.
Nuclear Decay
Nuclei that are not stable tend to break apart into other particles in a process known as nuclear decay. This process can occur naturally or be induced artificially. Either way, particles, photons, or both may be emitted. The nucleus that decays is known as the parent nucleus, and the nucleus that results is the daughter nucleus. The spontaneous emission of radiation is known as radioactivity. There are three basic types of radiation: alpha (α), beta (β), and gamma (γ).
Alpha Decay An alpha particle ![]() is emitted during alpha decay. A nucleus loses two protons and two neutrons, so its mass decreases and its atomic number decreases. A common example of alpha decay occurs when uranium-238 breaks down as shown here.
is emitted during alpha decay. A nucleus loses two protons and two neutrons, so its mass decreases and its atomic number decreases. A common example of alpha decay occurs when uranium-238 breaks down as shown here.
![]()
This expression is known as a nuclear equation. This particular equation shows that the parent nucleus ![]() emits an alpha particle
emits an alpha particle ![]() to become the daughter nucleus
to become the daughter nucleus ![]() . Notice that the mass numbers and atomic numbers balance across the equation.
. Notice that the mass numbers and atomic numbers balance across the equation.
Beta Decay Either an electron ![]() or a positron
or a positron ![]() is emitted during beta decay. A positron is like an electron except that it has a positive charge. Because there are no electrons in the nucleus of an atom, the particles emitted during beta decay must have a different source. Consider the following example, in which the parent nucleus is carbon-14, and the daughter nucleus is nitrogen–14.
is emitted during beta decay. A positron is like an electron except that it has a positive charge. Because there are no electrons in the nucleus of an atom, the particles emitted during beta decay must have a different source. Consider the following example, in which the parent nucleus is carbon-14, and the daughter nucleus is nitrogen–14.
![]()
A neutron in the nucleus becomes a proton and an electron. The proton remains in the nucleus, increasing the atomic number by 1, and the electron is emitted. The mass number remains the same because the proton essentially replaced the neutron.
The symbol ![]() represents an electron. Unlike the symbols for the nuclei, the symbol for the electron provides information about charge. The -1 indicates that the charge is negative with a magnitude equal to that of the proton. The 0 indicates that the electron does not contribute to the mass number. The type of beta decay in which an electron is emitted is known as beta-minus decay.
represents an electron. Unlike the symbols for the nuclei, the symbol for the electron provides information about charge. The -1 indicates that the charge is negative with a magnitude equal to that of the proton. The 0 indicates that the electron does not contribute to the mass number. The type of beta decay in which an electron is emitted is known as beta-minus decay.
The ![]() represents a particle called an antineutrino. Experimental evidence showed that a new type of particle must be produced in order to conserve energy and momentum. The basic particle, the neutrino v, has no electric charge and almost no (if any) mass. The antiparticle of the neutrino is the antineutrino.
represents a particle called an antineutrino. Experimental evidence showed that a new type of particle must be produced in order to conserve energy and momentum. The basic particle, the neutrino v, has no electric charge and almost no (if any) mass. The antiparticle of the neutrino is the antineutrino.
The following nuclear equation shows another type of beta decay. In this decay, known as beta-plus decay, a positron and a neutrino are emitted.
![]()
In this process, a proton becomes a positron and a neutron. The positron is emitted along with a neutrino. The atomic number decreases by 1 because a proton is lost, but the mass number remains the same because a neutron is gained. The symbol ![]() represents a positron, which resembles an electron with a positive charge. The symbol v represents a neutrino. Beta-plus decay is typically not tested on SAT Physics.
represents a positron, which resembles an electron with a positive charge. The symbol v represents a neutrino. Beta-plus decay is typically not tested on SAT Physics.
Gamma Decay When one or more nucleons in an unstable nucleus transition from a higher energy state to a lower one, photons known as gamma rays are emitted. During this type of decay, known as gamma decay, energy is released but the atomic number and mass number are not changed.
Gamma decay is often associated with alpha and beta decay. During these processes, the nucleus is left in an excited state, which then returns to normal through gamma decay.
Half-Life
One way to measure the rate of radioactive decay is by identifying half-life. The half-life of a substance is the time it takes for half of the radioactive nuclei in a sample to decay. You may often see a decay curve to represent the number of radioactive nuclei remaining in a sample as a function of time. The decay curve below represents the amount of a sample remaining after several half-lives of iodine-131.
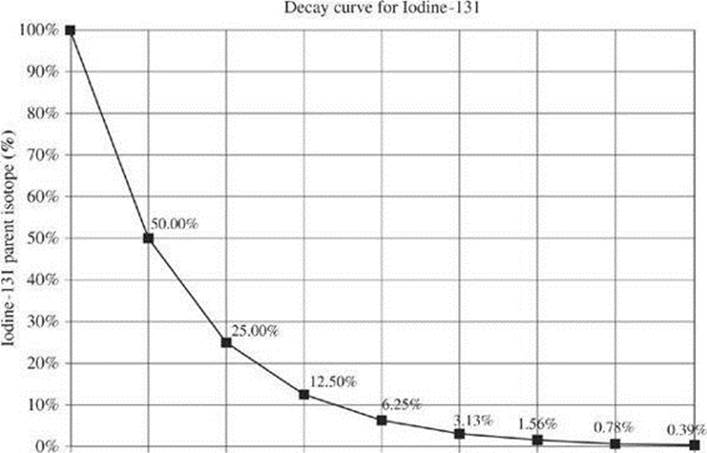
The half-life of iodine-131 is about 8 days. You can see that half of a sample remains after 8 days (1 half-life). Another half of that, or 25%, remains after 16 days (2 half-lives). Half of that, or 12.50%, remains after 24 days (3 half-lives). The amount of radioactive sample remaining continues to decrease exponentially over time.
Half-lives are useful for identifying substances because they are generally unique. In addition, measuring amounts of substances of known half-lives, such as carbon-14, can be used to determine the ages of objects, such as fossils.
Nuclear Reactions
In chemical reactions, only the electrons are involved. As a result, the identity of an element does not change. In nuclear reactions, the composition of the nucleus changes. When the atomic number changes, the identity of the element changes. Two types of nuclear reactions are fusion and fission.
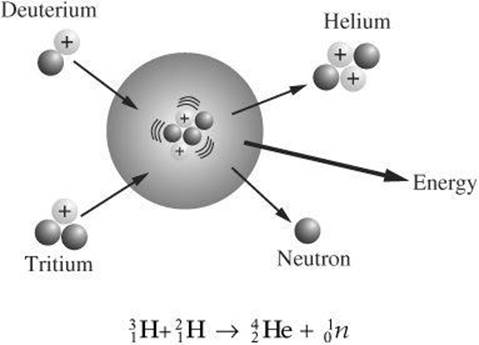
Fusion Two smaller nuclei combine to form a larger one during nuclear fusion. Energy is released during this process. The following example shows fusion between two forms of hydrogen, deuterium and tritium. Helium is formed along with the release of a neutron and energy.
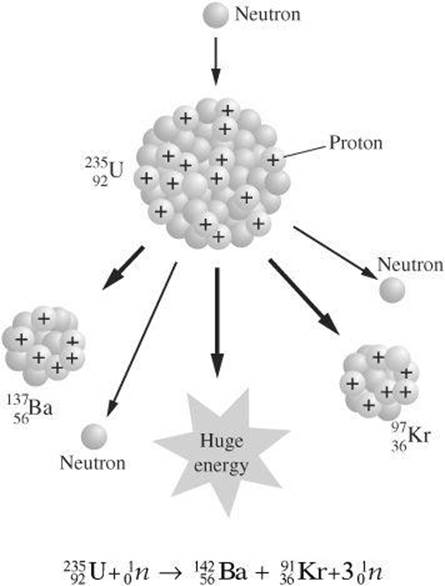
Fission One large nucleus splits to form smaller nuclei during nuclear fission. To initiate fission, a large nucleus is bombarded with a slow neutron. The large nucleus then splits into smaller nuclei as additional neutrons and energy are released. The following example shows the one fission reaction of uranium-235.
Test-Taking Hint
Practice balancing nuclear equations as you do for chemical equations. This will help you identify missing particles if incomplete nuclear equations are provided to you.
REVIEW QUESTIONS
Select the choice that best answers the question or completes the statement.
1. Light can be described as discrete bundles of energy called
(A) nuclei
(B) neutrons
(C) waves
(D) protons
(E) photons
2. Light with a wavelength of 1.20 × 1015 Hz is directed at a sample of pure silver. If the work function is 4.73 eV, what will be the speed of the photo-electrons that are emitted? [h = 4.14 × 10–15 eV · s and the mass of an electron is 9.11 × 10–31 kg]
(A) 3.81 × 10–20 m/s
(B) 2.89 × 105 m/s
(C) 2.89 × 105 m/s
(D) 2.54 × 1014 m/s
(E) 5.68 × 1015 m/s
3. In what way was the atomic model changed based on Rutherford’s gold foil experiment?
(A) The atom was recognized to contain negative charges.
(B) Neutral particles known as neutrons were discovered.
(C) Matter was found to consist of indivisible particles.
(D) Most of the mass was shown to be concentrated in the nucleus.
(E) Photons were observed when electrons dropped to lower energy levels.
4. When an electron in a hydrogen atom drops from the ![]() state to the
state to the ![]() state, a photon is emitted.
state, a photon is emitted.
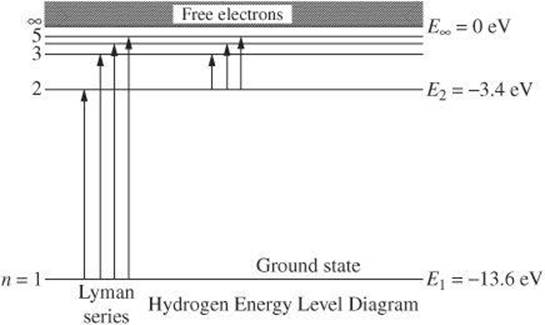
What is the wavelength of the photon? [6.63 × 10–34 J • s and 1 ![]() ]
]
(A) 41.4 nm
(B) 122 nm
(C) 163 nm
(D) 199 nm
(E) 406 nm
5. How many neutrons are in an atom of ![]() ?
?
(A) 8
(B) 10
(C) 16
(D) 18
(E) 26
6. Which of the following always occurs when a hydrogen atom undergoes nuclear fusion?
(A) It shares an electron with another atom.
(B) It transfers its electron to another atom.
(C) It emits a positron.
(D) It absorbs a neutron.
(E) It combines with another light nucleus.
7. A substance undergoes alpha decay according to the following equation.
![]()
What is the missing substance?
![]()
![]()
![]()
![]()
![]()
8. A scientist places 200 g of a radioactive substance in a container at 3:00 P.M. At 9:00 P.M. the next night, only 25 g of the sample remain. What is the half-life of the substance?
(A) 10 hours
(B) 12 hours
(C) 15 hours
(D) 18 hours
(E) 30 hours
Questions 9 and 10 relate to the diagram below, which represents the decay curve for carbon-14.
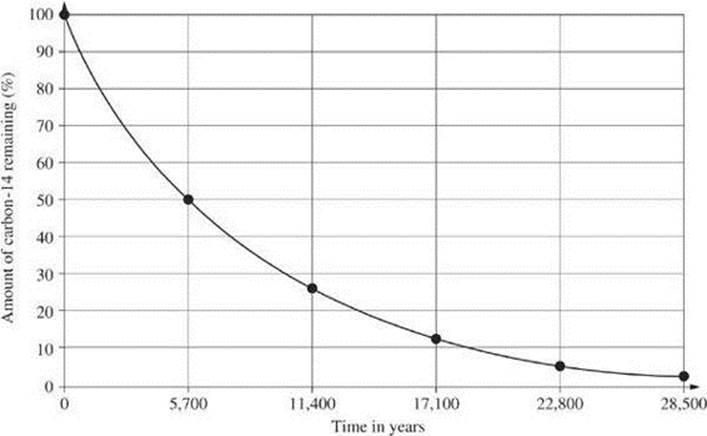
9. Based on the graph, what is the approximate half-life of carbon-14?
(A) 500 years
(B) 2,850 years
(C) 5,700 years
(D) 11,400 years
(E) 28,500 years
10. How much of a 500.0-g sample will remain in 22,800 years?
(A) 250.0 g
(B) 125.0 g
(C) 65.20 g
(D) 31.25 g
(E) 15.60 g
QUESTION ANSWERS AND EXPLANATIONS
1. E According to the particle theory of light, light consists of discrete bundles of energy called photons.
2. B Rearrange Einstein’s equation ![]() to solve for the maximum kinetic energy:
to solve for the maximum kinetic energy: ![]() . Then use the equation for kinetic energy to solve for speed:
. Then use the equation for kinetic energy to solve for speed:
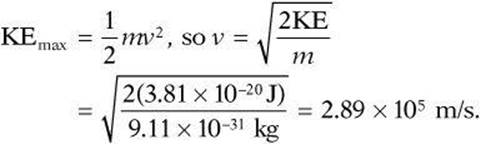
3. D Rutherford’s experiment involved aiming a stream of alpha particles at gold atoms. Most of the particles went directly through the empty space of the atoms, but some were deflected by the positively charged nucleus. The nucleus was defined as containing most of the mass of the atom, while negative charged electrons orbited around it.
4. B When the electron makes the transition, the electron loses 10.2 eV of energy. Convert this energy to joules: ![]() . Then use the energy to calculate the wavelength:
. Then use the energy to calculate the wavelength:
![]()
5. B In a nuclear symbol, the top number is the mass number, or total number of protons and neutrons. The bottom number is the number of neutrons. Therefore, the number of neutrons is the difference between the top and bottom numbers.
6. E Nuclear fusion occurs when light nuclei combine to form a heavier nucleus.
7. A The mass number should be the sum of the mass numbers of the particles produced. The atomic number should be the sum of the atomic numbers of the particles produced. Mass number = ![]() and atomic number =
and atomic number = ![]() .
.
8. A If 200 g have decayed such that only 25 g remain, three half-lives have passed. The time required for this change is 30 hours, so the half-life must be 10 hours.
9. C The half-life occurs when half, or 50%, of the original sample remains. This occurs at 5700 years on the graph. The actual half-life is 5730 years.
10. D Four half-lives pass in 22,800 years. At four half-lives, 6.25 percent of the original sample remains. ![]() .
.