MCAT Biology and Biochemistry: New for MCAT 2015 (2014)
Chapter 4. Biochemistry
The notion of life refers to both the activities and the physical structures of living organisms. Both the storage/utilization of energy and the synthesis of structures depend on a large number of chemical reactions that occur within each cell. Fortunately, these reactions do not proceed on their own spontaneously, without regulation. If they did, each cell’s energy would rapidly dissipate and total disorder would result. Most reactions are slowed by a large barrier known as the activation energy (Ea ), discussed below. The Ea is a bottleneck in a reaction, like a nearly closed gate. The role of the enzyme is to open this chemical gate. In this sense, the enzyme is like a switch. When the enzyme is on, the gate is open (low Ea ), and the reaction accelerates. When the enzyme is off, the gate closes and the reaction slows. Before we discuss how enzymes work, we must digress a bit to review the basics of thermodynamics. Then we can review some of the major metabolic pathways in the cell.
4.1 THERMODYNAMICS
Thermodynamics is the study of the energetics of chemical reactions. There are two relevant forms of energy in chemistry: heat energy (movement of molecules) and potential energy (energy stored in chemical bonds). [What is the most important potential energy storage molecule in all cells?1] The first law of thermodynamics, also known as the law of conservation of energy, states that the energy of the universe is constant. It implies that when the energy of a system decreases, the energy of the rest of the universe (the surroundings) must increase, and vice versa. The second law of thermodynamics states that the disorder, or entropy, of the universe tends to increase. Another way to state the second law is as follows: Spontaneous reactions tend to increase the disorder of the universe. The symbol for entropy is S, and “a change in entropy” is denoted ∆S, where ∆S = Safter – Sbefore. [If the ∆S of a system is negative, has the disorder of that system increased or decreased?2]
A practical way to discuss thermodynamics is the mathematical notion of free energy (Gibbs free energy), defined by Josiah Gibbs as follows:3
Eq. 1 ∆G = ∆H – T∆S
T denotes temperature, and H denotes enthalpy, which is defined by another equation:
Eq. 2 ∆H = ∆E – P∆V
Here E represents the bond energy of products or reactants in a system, P is pressure, and V is volume. [Given that cellular reactions take place in the liquid phase, how is H related to E in a cell?4] ∆G increases with increasing ∆H(bond energy) and decreases with increasing entropy.
• Given the second law of thermodynamics and the mathematical definition of ∆G, which reaction will be favorable: one with a decrease in free energy (∆G < 0) or one with an increase in free energy (∆G > 0)?5
The change in the Gibbs free energy of a reaction determines whether the reaction is favorable (spontaneous, ∆G negative) or unfavorable (nonspontaneous, ∆G positive). In terms of the generic reaction
A + B → C + D
the Gibbs free energy change determines whether the reactants (denoted A and B) will stay as they are or be converted to products (C and D).
Spontaneous reactions, ones that occur without a net addition of energy, have ∆G < 0. They occur with energy to spare. Reactions with a negative ∆G are exergonic (energy exits the system); reactions with a positive ∆G are endergonic. Endergonic reactions only occur if energy is added. In the lab, energy is added in the form of heat; in the body, endergonic reactions are driven by reaction coupling to exergonic reactions (more on this later). Reactions with a negative ∆H are called exothermic and liberate heat. Most metabolic reactions are exothermic (which is how homeothermic organisms such as mammals maintain a constant body temperature). Reactions with a positive ∆Hrequire an input of heat and are referred to as endothermic. (Thermodynamics will be discussed in more detail in MCAT General Chemistry Review and MCAT Physics and Math Review.)
The signs of thermodynamic quantities are assigned from the point of view of the system, not the surroundings or the universe. Thus, a negative ∆G means that the system goes to a lower free energy state, and a system will always move in the direction of the lowest free energy. As an analogy, visualize a spinning top as the system. What happens to the top? Does it spin faster and faster? No. It moves towards the lowest energy state. Let’s expand the analogy, using an equation:
motionless top → spinning top
Here the “reactant” is the motionless top, and the “product” is the spinning top. Which is lower: the free energy of product or reactant? The reactant. Is the reaction “spontaneous” as written? No; in fact, the reverse reaction is spontaneous. Hence,
Gspinning > Gmotionless
and thus,
Greaction as written (motionless to spinning; left to right) > 0
So the reaction is nonspontaneous. In other words, it requires energy input, namely, energy from your muscles as you spin the top. [If the products in a reaction have more entropy than the reactants, and the enthalpy (H) of the reactants and the products are the same, can the reaction occur spontaneously?6]
The value of ∆G depends on the concentrations of reactants and products, which can be variable in the body. Therefore, to compare reactions, biochemists calculate a standard free energy change, denoted ∆G°, with all reactants and products present at 1 M concentration. Furthermore, the biochemist’s standardized ∆G determined at pH 7 is denoted ∆G°′.
∆G°′ is related to the equilibrium constant for a reaction by the following equation:
Eq. 3 ΔG°′ = −RT In K′eq
where R is the gas constant (which would be given on the MCAT, along with the entire equation), and K′eq is the ratio of products to reactants at equilibrium:
K′eq = 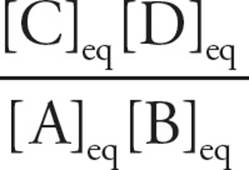
K′eq is the ratio of products to reactants when enough time has passed for equilibrium to be reached [When K′eq =1, what is ∆G°′?7]
But what if we wanted to calculate ∆G for a reaction in the body? In this case, we need one more equation:
Eq. 4 ΔG = ΔG°′ + RT In Q, where Q = 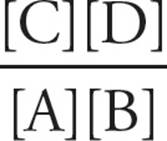
Here, Q is calculated using the actual concentrations of A, B, C, and D (for example, the concentrations in the cell). Equation 4 is simply a conversion from ∆G°′ (the laboratory standard ∆G with initial concentrations at 1 M) to the real-life here-and-now ∆G. Note that if we put 1 M concentrations of A, B, C, and D into a beaker (at pH 7), we have recreated the laboratory standard initial set-up: Q = 1, so ln Q = 0, which means ∆G = ∆G°′.
• You are studying a particular reaction. You find the reaction in a book and read ∆G°′ from a table. Can you calculate ∆G for this reaction in a living human being without any more information?8
Remember that Q and Keq are not the same. Q is the ratio of products to reactants in any given set-up; Keq is the ratio at equilibrium. Equilibrium is defined as the point where the rate of reaction in one direction equals the rate of reaction in the other. At equilibrium, there is constant product and reactant turnover as reactants form products and vice versa, but overall concentrations stay the same. Theoretically (given enough time), all reactant/product systems will eventually reach this point.
While all reactions will eventually reach an equilibrium defined by the constant above, we can disturb this balance with the addition or removal of a reactant or product. This causes a change in Q but not Keq and the reaction will proceed in the direction necessary to re-establish equilibrium. (The shift to restore equilibrium is a demonstration of Le Châtelier’s principle which will be discussed in further detail in MCAT General Chemistry Review.) Using this principle, a reaction which favors reactants at equilibrium can be driven to generate additional products (such strategies are employed frequently in cellular respiration).
• How can ∆G be negative if ∆G°′ is positive (which indicates that the reaction is unfavorable at standard conditions)?9
• Does Keq indicate the rate at which a reaction will proceed?10
• When Keq is large, which has lower free energy: products or reactants?11
• When Q is large, which has lower free energy: products or reactants?12
• Which direction, forward or backward, will be favored in a reaction if ∆G = 0? (Hint: What does Equation 4 look like when ∆G = 0?)13
• Radiolabeled chemicals are often used to trace constituents in biochemical reactions. The following reaction with ∆G = 0 is in aqueous solution:
A = ![]() B + C, Keq =
B + C, Keq = 
A small amount of radiolabeled B is added to the solution. After a period of time, where will the radiolabel most likely be found: in A, in B, or in both?14
In summary, then, there are two factors that determine whether a reaction will occur spontaneously (∆G negative) in the cell:
1) The intrinsic properties of the reactants and products (∆G°′)
2) The concentrations of reactants and products (RT ln Q)
(In the lab there is third factor: temperature. If ln Q is negative and the temperature is high enough, ∆G will be negative, regardless of the value of ∆G°′.)
Thermodynamics vs. Reaction Rates
The term spontaneous is used to describe a reaction system with ∆G < 0. This can be misleading, since the common usage of the word spontaneous has a connotation of rapid rate; this is not what spontaneous means in the context of chemical reactions. For example, many reactions have a negative ∆G, indicating that they are “spontaneous” from a thermodynamic point of view, but they do not necessarily occur at a significant rate. Spontaneous means that a reaction may proceed without additional energy input, but it says nothing about the rate of reaction.
Thermodynamics will tell you where a system starts and finishes but nothing about the path traveled to get there. The difference in free energy in a reaction is only a function of the nature of the reactants and products. Thus, ∆Gdoes not depend on the pathway a reaction takes or the rate of reaction; it is only a measurement of the difference in free energy between reactants and products.
• How does the ∆G for a reaction burning (oxidizing) sugar in a furnace compare to the ∆G when sugar is broken down (oxidized) in a human?15
4.2 Kinetics and Activation Energy (Ea)
The reason some spontaneous (i.e., themodynamically favorable) reactions proceed very slowly or not at all is that a large amount of energy is required to get them going. For example, the burning of wood is spontaneous, but you can stare at a log all day and it won’t burn. Some energy (heat) must be provided to kick-start the process.
The study of reaction rates is called chemical kinetics. All reactions proceed through a transient intermediate that is unstable and takes a great deal of energy to produce. The energy required to produce the transient intermediate is called the activation energy (Ea). This is the barrier that prevents many reactions from proceeding even though the ∆G for the reaction may be negative. The match you use to light your fireplace provides the activation energy for the reaction known as burning. It is the activation energy barrier that determines the kinetics of a reaction. [How would the rate of a spontaneous reaction be affected if the activation energy were lowered?16]
The concept of Ea is key to understanding the role of enzymes, so let’s spend some time on it. To illustrate, take this reaction:
Bobwithout a job + job → Bobwith a job
Is this a favorable reaction, i.e., will the universe be better off, with less total (nervous) energy, if Bob gets the job? Will things settle down? Let’s assume yes. However, between the two states (without/with) there is an intermediate state, namely, Bobapplying for job. So the reaction will look this way:
Bobwithout a job + job → [Bobapplying for job]‡ → Bobwith a job
The middle term is the transition state (TS), traditionally written in square brackets with a double-cross symbol: [TS]‡. It exists for a very, very short time, either moving forward to form product or breaking back down into reactants. The energy required for Bob to be job hunting is much higher than the energy of Bob with a job or Bob without a job. As a result, he may not go job hunting, even though he’d be happier in the long run if he did. In this model, we can describe the Ea as the energy necessary to get Bob to apply for a job.
A catalyst lowers the Ea of a reaction without changing the ∆G. The catalyst lowers the Ea by stabilizing the transition state, making its existence less thermodynamically unfavorable. The second important characteristic of a catalyst is that it is not consumed in the reaction; it is regenerated with each reaction cycle.
In our model, an example of a catalyst would be a career planning service (CPS). Adding a CPS won’t make Bobwithout a job any happier or sadder, nor will it make Bobwith a job happier or sadder. But it will make it much easier for Bob to move between the two states: without a job vs. with a job. The traditional way to represent a reaction system like this is using a reaction coordinate graph, as shown in Figure 1. This is just a way to look at the energy of the reaction system as compared to the three possible states of the system: 1) reactants, 2) [TS]‡, and 3) products. The x-axis plots the physical progress of the reaction system (the “reaction coordinate”), and the y-axis plots energy.
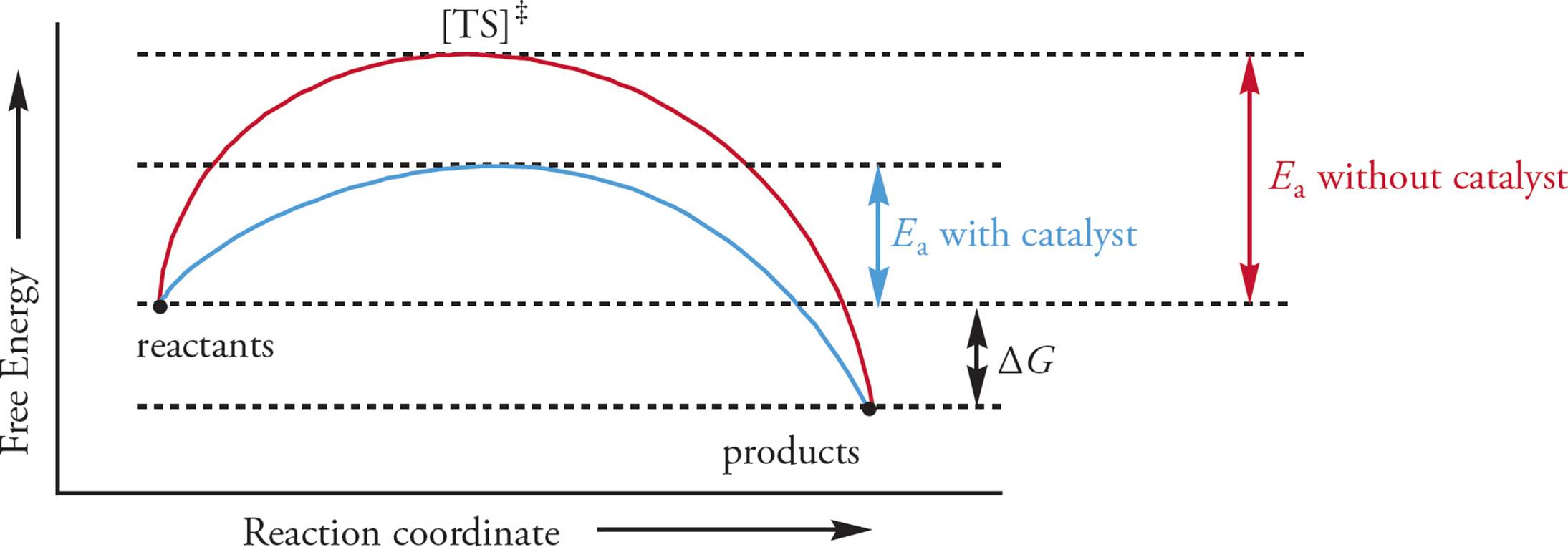
Figure 1 The Reaction Coordinate Graph
Enzymes are catalysts. They increase the rate of a reaction by lowering the reaction’s activation energy, but they do not affect ∆G between reactants and products. As catalysts, enzymes have a kinetic role, not a thermodynamic one. [Will an enzyme alter the concentration of reagents at equilibrium?17] Enzymes may alter the rate of a reaction enormously: A reaction that would take a hundred years to reach equilibrium without an enzyme may occur in just seconds with an enzyme. (Contrast the kinetic role of enzymes with collision kinetics as discussed in MCAT General Chemistry Review.)
Given that thousands of enzymes have been discovered, scientists frequently classify them based upon reaction type. Table 1 below lists several examples but note that enzymes cannot control the direction in which a reaction proceeds so it is common to see enzymes in a given class function in reverse.
|
Enzyme Class |
Reaction |
|
Hydrolase |
hydrolyzes chemical bonds (includes ATPases, proteases, and others) |
|
Isomerase |
rearranges bonds within a molecule to form an isomer |
|
Ligase |
forms a chemical bond (e.g., DNA ligase) |
|
Lyase |
breaks chemical bonds by means other than oxidation or hydrolysis (e.g., pyruvate decarboxylase) |
|
Kinase |
transfers a phosphate group to a molecule from a high energy carrier, such as ATP (e.g., phosphofructokinase [PFK]) |
|
Oxidoreductase |
runs redox reactions (includes oxidases, reductases, dehydrogenases, and others) |
|
Polymerase |
polymerization (e.g., addition of nucleotides to the leading strand of DNA by DNA polymerase III) |
|
Phosphatase |
removes a phosphate group from a molecule |
|
Phosphorylase |
transfers a phosphate group to a molecule from inorganic phosphate (e.g., glycogen phosphorylase) |
|
Protease |
hydrolyzyes peptide bonds (e.g., trypsin, chymotrypsin, pepsin, etc.) |
Table 1 Enyzme Classes
ATP as an Energy Source: Reaction Coupling
Enzymes increase the rate of reactions that have a negative ∆G. These reactions would occur on their own without an enzyme (they are spontaneous) but far more slowly than with one. However, there are many reactions in the body that occur which have a positive ∆G. The biosynthesis of macromolecules such as DNA and protein is not spontaneous (∆G > 0), but clearly these reactions do take place (or we wouldn’t be here). How can this be? Thermodynamically unfavorable reactions in the cell can be driven forward by reaction coupling. In reaction coupling, one very favorable reaction is used to drive an unfavorable one. This is possible because free energy changes are additive. [What is the favorable reaction that the cell can use to drive unfavorable reactions?18] In the lab, the ∆G°′ for the hydrolysis of one phosphate group from ATP is −7.3 kcal/mol, so it is a very favorable reaction. In the cell, ∆G is about −12 kcal/mol, so in the cell it is even more favorable. [What’s the difference between the situation in vitro (lab) and in vivo (cell)?19]
How does ATP hydrolysis drive unfavorable reactions? There are many ways. One example is by causing a conformational change in a protein; in this way ATP hydrolysis can be used to power energy-costly events like transmembrane transport. Another example is by transfer of a phosphate group from ATP to a substrate. Take the unfavorable reaction A + B → C. Let’s say that Reactant A must proceed through an intermediate, APO42– in order to participate. Let’s say ∆G = +7 kcal/mol for the overall reaction. What if the two partial reactions have ∆Gs as follows:
|
A + PO42– → APO42– |
∆G = +2 kcal/mol |
|
APO42– + B → C + PO42– |
∆G = +5 kcal/mol |
|
Total |
∆G = +7 kcal/mol |
These reactions will not proceed, because the overall ∆G will be +7 kcal/mol. What will be the overall ∆G if we couple the reaction A + B → C to the hydrolysis of one ATP? All we have to do is add up all the ∆G values, as follows:
|
ATP → ADP + PO42– |
∆G = −12 kcal/mol |
|
A + PO42– → APO42– |
∆G = +2 kcal/mol |
|
APO42– + B → C + PO42– |
∆G = +5 kcal/mol |
|
Total |
∆G = −5 kcal/mol |
Now the overall reaction, shown below, is thermodynamically favorable. We have coupled the unfavorable reaction A + B → C to the highly favorable hydrolysis of ATP:
A + B + ATP → C + ADP + PO42– ∆G = −5 kcal/mol
Note that we first stated that the enzyme has only a kinetic role (influencing rate only), not a thermodynamic one (determining favorability). Then we went on to discuss reaction coupling, which allows enzymes to promote otherwise unfavorable reactions. There is no contradiction, however. The only difference is viewing reactions in an isolated manner or in the complex series of linked reactions more commonly found in the body. The same rule applies in either case: ∆G must be negative for either a single reaction or a series of linked reactions to occur spontaneously. In summary:
• One reaction in a test tube: the enzyme is a catalyst with a kinetic role only. It influences the rate of the reaction, but not the outcome.
• Many “real life” reactions in the cell: enzyme controls outcomes by selectively promoting unfavorable reactions via reaction coupling.
4.3 ENZYME STRUCTURE AND FUNCTION
Most enzymes are proteins that must fold into specific three-dimensional structures to act as catalysts. (Some enzymes are RNA or contain RNA sequences with catalytic activity. Most catalyze their own splicing, and the rRNA in ribosomes helps in peptide-bond formation.) An enzyme may consist of a single polypeptide chain or several polypeptide subunits held together in a __20 (primary? secondary? etc.) structure. The reason for the importance of folding in enzyme function is the proper formation of the active site, the region in an enzyme’s three-dimensional structure that is directly involved in catalysis.
[What shape are enzymes more likely to have: fibrous/elongated or globular/spherical?21] The reactants in an enzyme-catalyzed reaction are called substrates. (Products have no special name; they’re just “products.”) What is the role of the active site, that is, how do enzymes work? The active site model, commonly referred to as the “lock and key hypothesis,” states that the substrate and active site are perfectly complementary. This differs from the induced fit model which asserts that the substrate and active site differ slightly in structure and that the binding of the substrate induces a conformational change in the enzyme. The induced fit model has gained greater acceptance in recent years, but regardless of the model, enzymes accelerate the rate of a given reaction by helping to stabilize the transition state. For example, if a transition state intermediate possesses a transient negative charge, what amino acid residues might be found at the active site to stabilize the transition state?22 This lowers the activation energy barrier between reactants and products. In our previous example of Bob looking for a job, the use of a career planning service would function as an enzyme by making the process of job hunting easier.
• Is it possible that amino acids located far apart from each other in the primary protein equence may play a role in the formation of the same active site?23
• If, during an enzyme-catalyzed reaction, an intermediate forms in which the substrate is covalently linked to the enzyme via a serine residue, can this occur at any serine residue or must it occur at a specific serine residue?24
• Compound A converts into Compound B in solution: The reaction has the following equilibrium constant: Keq = [B]eq/[A]eq = 1000. If pure A is dissolved in water at 298 K, will ∆G for the reaction A ![]() B be positive or negative? Is it possible to answer this question without knowing ∆G°′?25
B be positive or negative? Is it possible to answer this question without knowing ∆G°′?25
• Regarding the reaction described in the previous question, if pure B is put into solution in the presence of an enzyme that catalyzes the reaction between A and B, which one of the following will be true?26
A) All the B will be converted into A, until there is 1000 times more A than B.
B) All of the B will remain as B, since B is favored at equilibrium.
C) The enzyme will have no effect, since enzymes act on the transition state and there is no transition state present.
D) The reaction that produces A will predominate until ∆G = 0.
The active site for enzymes is generally highly specific in its substrate recognition, including stereospecificity (the ability to distinguish between stereoisomers). For example, enzymes which catalyze reactions involving amino acids are specific for D or L amino acids, and enzymes catalyzing reactions involving monosaccharides may distinguish between stereoisomers as well. [Which configurations are found in animals?27]
Many proteases (protein-cleaving enzymes) have an active site with a serine residue whose OH group can act as a nucleophile, attacking the carbonyl carbon of an amino acid residue in a polypeptide chain. Examples are trypsin, chymotrypsin, and elastase. These enzymes also usually have a recognition pocket near the active site. This is a pocket in the enzyme’s structure which attracts certain residues on substrate polypeptides. The enzyme always cuts polypeptides at the same site, just to one side of the recognition residue. For example, chymotrypsin always cuts on the carboxyl side of one of the large hydrophobic residues Tyr, Trp, Phe, and Met. Enzymes that act on hydrophobic substrates have hydrophobic amino acids in their active sites, while hydrophilic/polar amino acids will comprise the active site of enzymes with hydrophilic substrates.
Given the importance of the active site, it becomes clear that small alterations in its structure can drastically alter enzymatic activity. Therefore, both temperature and pH play a critical role in enzymatic function. As temperature increases, the thermal motion of the peptide and surrounding solution destabilize its structure. If the temperature rises sufficiently, the protein denatures and loses its orderly structure. The pH of the surrounding medium also impacts protein stability; several amino acids possess ionizable –R groups that change charge depending on pH. This can decrease the affinity of a substrate for the active site and, if the pH deviates sufficiently, the protein can denature.
• The transition state intermediate for a reaction possesses a transient negative charge. The active site for an enzyme catalyzing this reaction contains a His residue to stabilize the intermediate. If the His residue at the active site is replaced by a glutamate which is negatively charged at pH 7.0, what effect will this have on the reaction, assuming that the reactants are present in excess compared to the enzyme?
A) The repulsion caused by the negative charge in the glutamate at the altered active site will increase the activation energy and make the reaction proceed more slowly than it would in solution without enzyme.
B) The rate of catalysis will be unaffected, but the equilibrium ratio of products and reactants will change, favoring reactants.
C) The transition state intermediate will not be stabilized as effectively by the altered enzyme, lowering the rate relative to the rate with catalysis by the normal enzyme.
D) The rate of catalysis will decrease, and the equilibrium constant will change.28
Enzymatic function can also depend upon the association of additional molecules. Cofactors, which are metal ions or small molecules (not themselves a protein), are required for activity in many enzymes. In fact, the majority of the vitamins in our diet serve as precursors for cofactors (e.g., niacin [B3] is ultimately transformed into NAD+). When a cofactor is an organic molecule, it is referred to as a coenzyme; these often bind to the substrate during the catalyzed reaction. One prime example of a coenzyme, which we will focus on later in the chapter, is coenzyme A (CoA).
4.4 REGULATION OF ENZYME ACTIVITY
Metabolic pathways in the cell are not all continually on, but must be tightly regulated to maintain health. For example, if glycogen synthesis and breakdown occur in the same cell at the same time, a great deal of energy will be wasted without accomplishing anything. Therefore, the activity of key enzymes in metabolic pathways is usually regulated in one or more of the following ways:
1) Covalent modification. Proteins can have several different groups covalently attached to them, and this can regulate their activity, lifespan in the cell, and/or cellular location. The addition of a phosphoryl group from a molecule of ATP by a protein kinase to the hydroxyl of serine, threonine, or tyrosine residues is the most common example. Phosphorylation of these different sites on an enzyme can either activate or inactivate the enzyme. Protein phosphorylases also phosphorylate proteins, but use free-floating inorganic phosphate (Pi) in the cell instead of ATP. Protein phosphorylation can be reversed by protein phosphatases.
2) Proteolytic cleavage. Many enzymes (and other proteins) are synthesized in inactive forms (zymogens) that are activated by cleavage by a protease.
3) Association with other polypeptides. Some enzymes have catalytic activity in one polypeptide subunit that is regulated by association with a separate regulatory subunit. For example, there are some proteins that demonstrate continuous rapid catalysis if their regulatory subunit is removed; this is known as constitutive activity (constitutive means continuous or unregulated). There are other proteins that require association with another peptide in order to function. Still other proteins can bind many regulatory subunits. There are numerous examples of this in the cell, and many of them have diverse and complex regulatory mechanisms that all revolve around the theme of “associations with other polypeptides can affect enzyme activity.”
4) Allosteric regulation. The modification of active-site activity through interactions of molecules with other specific sites on the enzyme (called allosteric sites). Let’s look at this in a little more detail.
Allosteric Regulation
If the cell is to make use of the enzyme as a biochemical switch, there must be a way to turn the enzyme on or off. One mechanism of regulation is the binding of small molecules to particular sites on an enzyme that are distinct from the active site; this is allosteric regulation. This name comes from the fact that the particular spot on the enzyme which can bind the small molecule is not located close to the active site; allo means “other,” and steric refers to a location in space (as in “steric hindrance”), so allosteric means “at another place.” The binding of the allosteric regulator to the allosteric site is generally noncovalent and reversible. When bound, the allosteric regulator can alter the conformation of the enzyme to increase or decrease catalysis, even though it may be bound to the enzyme at a site distant from the active site or even on a separate polypeptide.
Feedback Inhibition
Enzymes usually act as part of pathways, not alone. Rather than regulate every enzyme in a pathway, usually there are one or two key enzymes that are regulated, such as the enzyme that catalyzes the first irreversible step in a pathway. The easiest way to explain this is with an example. Three enzymes (E1, E2, and E3) catalyze the three steps required to convert Substrate A to Product D. When plenty of D is around, it would be logical to shut off E1 so that excess B, C, and D are not made. This would conserve A and would also conserve energy. Commonly, an end-product such as D will shut off an enzyme early in the pathway, such as E1. This is called negative feedback, or feedback inhibition.
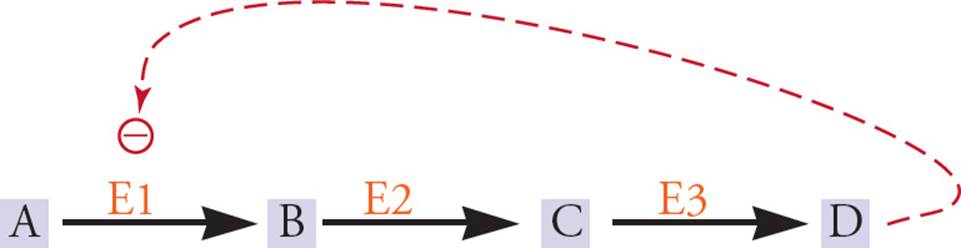
Figure 2 Feedback Inhibition
There are examples of positive feedback (“feedback stimulation”), but negative feedback is by far the most common example of feedback regulation. On the other hand, feedforward stimulation is common. This involves the stimulation of an enzyme by its substrate, or by a molecule used in the synthesis of the substrate. For example, in Figure 2, A might stimulate E3. This makes sense because when lots of A is around, we want the pathway for utilization of A to be active.
Allosteric regulation can be quite complex. It is possible for more than one small molecule to be capable of binding to an allosteric site. For example, imagine a reaction pathway from A through Z, where each step (A → B, B → C, etc.) is catalyzed by an enzyme. Let’s say that an allosteric enzyme called E15 catalyzes the reaction O → P. It would be possible for A to allosterically activate E15 (feedforward stimulation) and for Z to allosterically inhibit E15 (feedback inhibition). This may sound complex, but it’s quite logical. What it means is that when lots of A is around, E15 will be stimulated to use the molecules made from A (B, C, D, etc.) to make P, which could then be used to make Q, R, S, etc., all the way up to Z. On the other hand, if a lot of excess Z built up, it would inhibit E15, thereby conserving the supply of A, B, C, etc. and preventing more build-up of Z, Y, X, etc. Hence, in addition to acting as switches, enzymes act as valves, because they regulate the flow of substrates into products.
4.5 BASIC ENZYME KINETICS
Enzyme kinetics is the study of the rate of formation of products from substrates in the presence of an enzyme. The reaction rate (V, for velocity) is the amount of product formed per unit time, in moles per second (mol/s). It depends on the concentration of substrate, [S], and enzyme.29 If there is only a little substrate, then the rate V is directly proportional to the amount of substrate added: double the amount of substrate and the reaction rate doubles, triple the substrate and the rate triples, and so forth. But eventually there is so much substrate that the active sites of the enzymes are occupied much of the time, and adding more substrate doesn’t increase the reaction rate as much, that is, the slope of the V vs. [S] curve decreases. Finally, there is so much substrate that every active site is continuously occupied, and adding more substrate doesn’t increase the reaction rate at all. At this point the enzyme is said to be saturated. The reaction rate when the enzyme is saturated is denoted Vmax; see Figure 3. This is a property of each enzyme at a particular concentration of enzyme. You can look it up in a book for the common ones. [If a small amount of enzyme in a solution is acting at Vmax, and the substrate concentration is doubled, what is the new reaction rate?30]
Another commonly used parameter on these enzyme kinetics graphs is the Michaelis constant Km. Km is the substrate concentration at which the reaction velocity is half its maximum. To find Km on the enzyme kinetics graph, mark the Vmax on the y-axis, then divide this distance in half to find Vmax/2. Km is found by drawing a horizontal line from Vmax/2 to the curve, and then a vertical line down to the x-axis. Km is unique for each enzyme-substrate pair and gives information on the affinity of the enzyme for its substrate. If an enzyme-substrate pair has a low Km, it means that not very much substrate is required to get the reaction rate to half the maximum rate; thus the enzyme has a high affinity for this particular substrate.
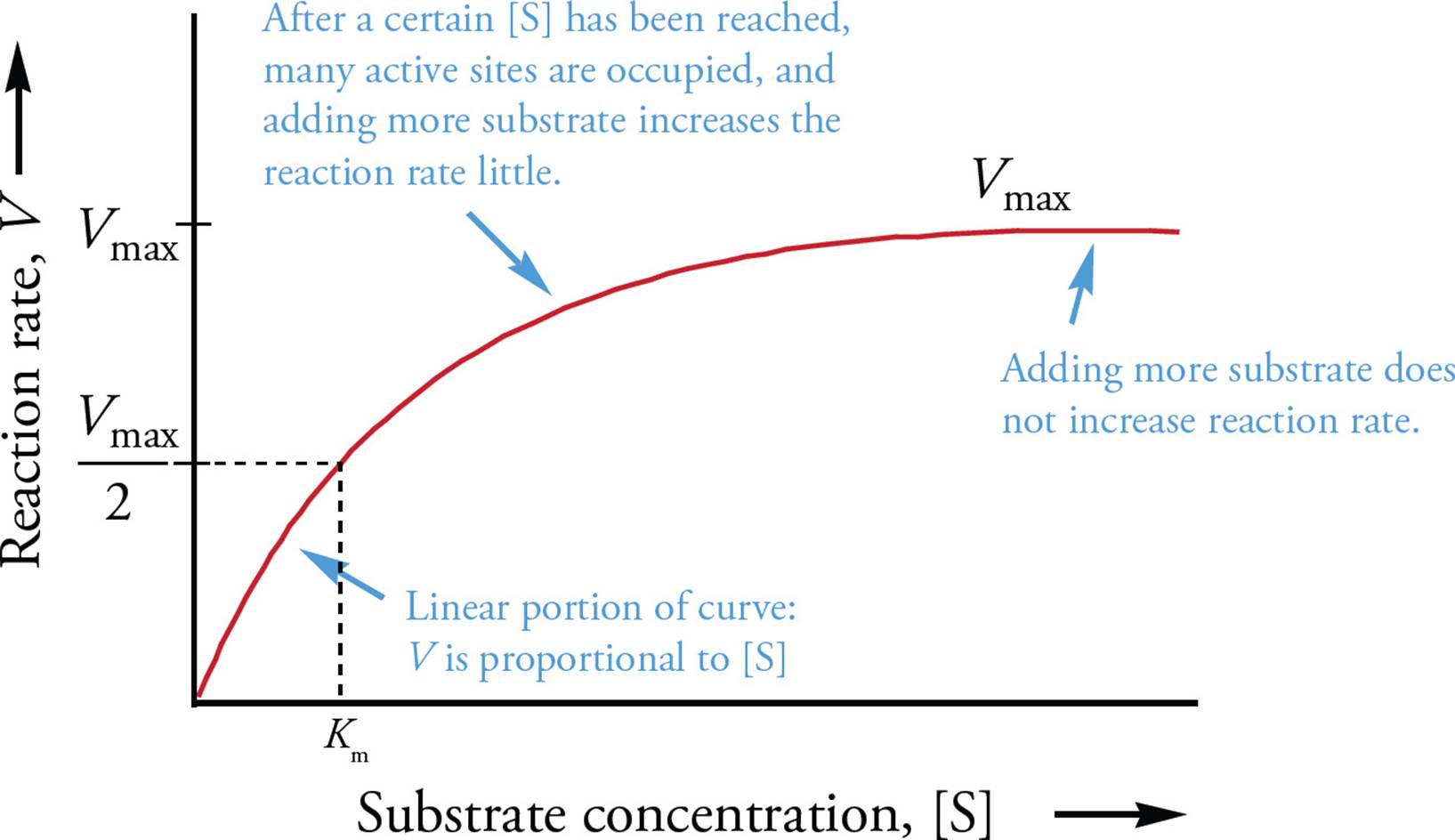
Figure 3 Saturation Kinetics
Cooperativity
Many multi-subunit enzymes do not behave in the simple kinetic manner described above. In such enzymes, the binding of substrate to one subunit allosterically increases the affinity of other subunits for substrate. The conformation of the enzyme prior to substrate binding, with low substrate affinity, is sometimes termed “tense,” and the conformation of enzyme with increased affinity is termed “relaxed.”31 Such enzymes are said to bind substrate cooperatively(Figure 4). This term just indicates that the substrates “cooperate” with each other. The binding of one substrate molecule to the enzyme complex enhances the binding of more substrate molecules to the same complex. Cooperative enzymes must have more than one active site. They are usually multisubunit complexes, composed of more than one protein chain held together in a quaternary structure. They may also be a single-subunit enzyme with two or more active sites.
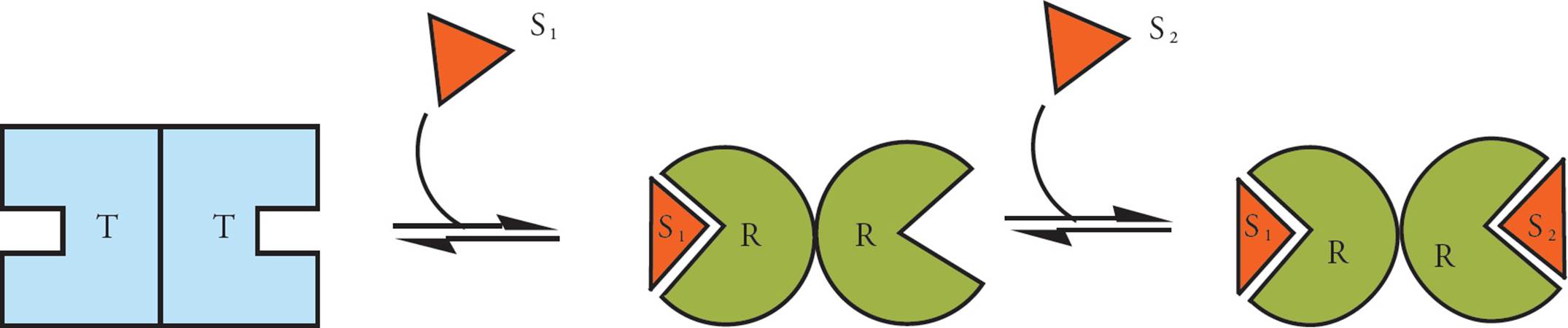
Figure 4 Enzyme Cooperativity
A sigmoidal curve results from cooperative binding. In Figure 5 below, the flat part at the bottom left (Region 1) is explained by the notion that at low [S] the enzyme complex has a low affinity for substrate (is in the tense state), and adding more substrate increases the rate little. The steep part in the middle of the curve (Region 2) represents the range of substrate concentrations where adding substrate greatly increases the reaction rate, because the enzyme complex is in the relaxed state. [The leveling off at the upper right part of the curve (Region 3) represents what?32]
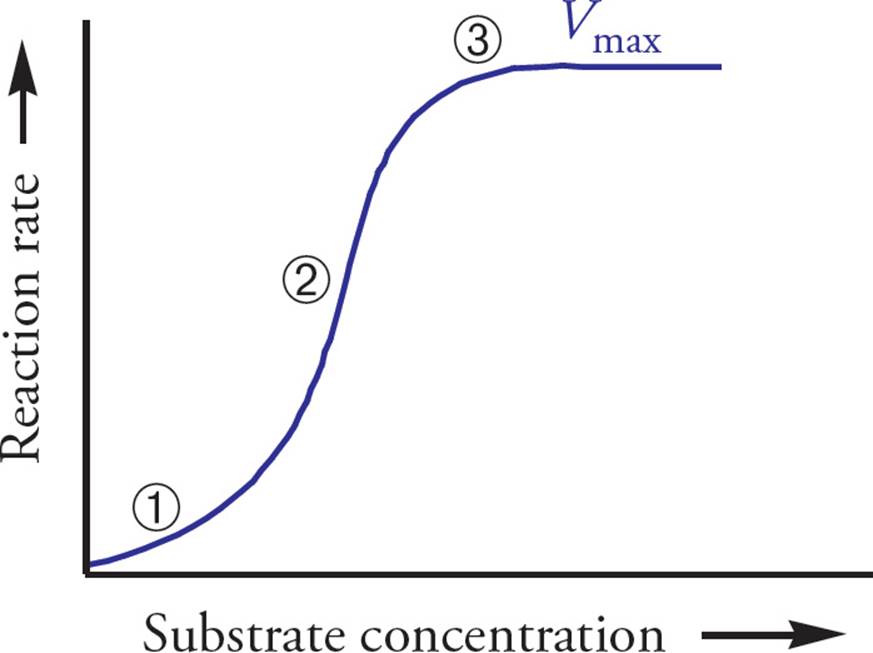
Figure 5 Sigmoidal Kinetics of Cooperativity
Cooperativity does not apply just to catalytic enzymes. For example, hemoglobin (Hb) is a protein complex made of four polypeptide subunits, each of which contains a heme prosthetic group with a single O2-binding site. (So one Hb has four hemes and four binding sites.) Hb is a carrier (of oxygen), not a catalyst of any reaction (not an enzyme). It exhibits cooperative O2 binding. This is why the Hb-O2 dissociation curve is sigmoidal. [What is the relationship between the two notions allosteric and cooperative?33]
Inhibition of Enzyme Activity
Enzyme inhibitors can reduce enzyme activity by a few different mechanisms, including competitive inhibition and noncompetitive inhibition. Competitive inhibitors are molecules that compete with substrate for binding at the active site. [You can predict that structurally, competitive inhibitors resemble what?34] The key thing to remember about competitive inhibitors is that their inhibition can be overcome by adding more substrate; if the substrate concentration is high enough, the substrate can outcompete the inhibitor. Hence, Vmax is not affected. You can get to the same Vmax, but it takes more substrate (see Figure 6). Therefore, the Km of the reaction to which a competitive inhibitor has been added is increased compared to the Km of the uninhibited reaction. [If an enzyme has a reaction rate of 1 µmole/min at a substrate concentration of 50 µM and a rate of 10 µmole/min at a substrate concentration of 100 µM, does this indicate the presence of a competitive inhibitor?35]
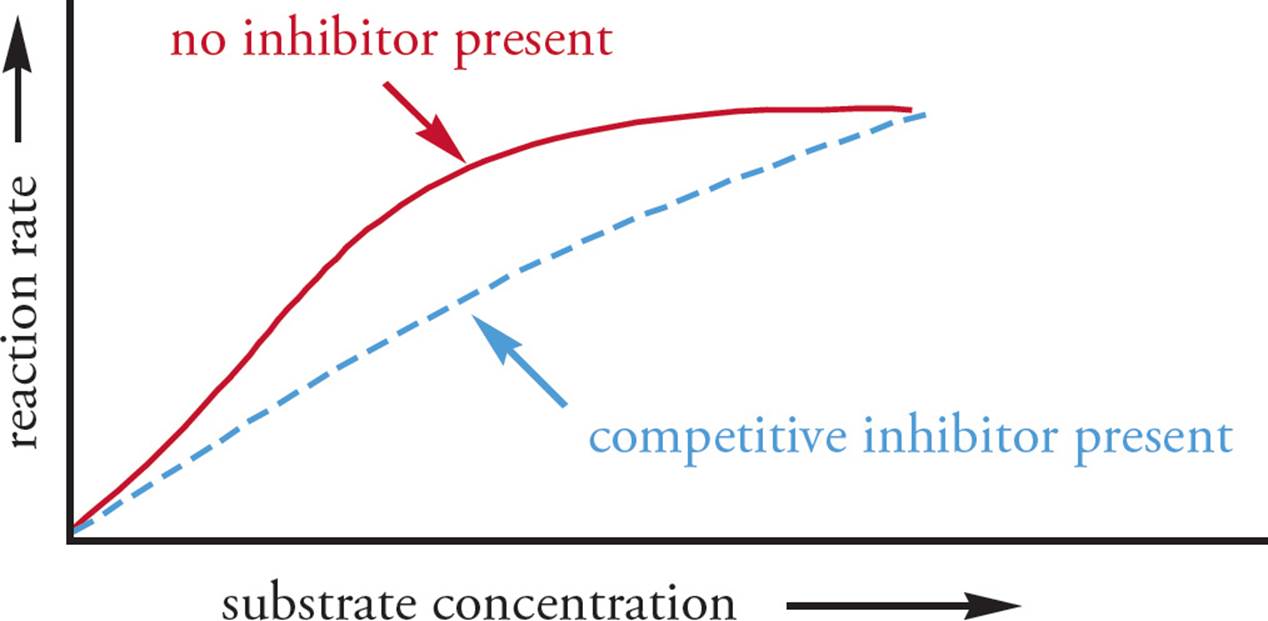
Figure 6 Competitive Inhibition
Noncompetitive inhibitors bind at an allosteric site, not at the active site. No matter how much substrate you add, the inhibitor will not be displaced from its site of action (see Figure 7). Hence, noncompetitive inhibition doesdiminish Vmax. Remember that Vmax is always calculated at the same enzyme concentration, since adding more enzyme will increase the measured Vmax. Addition of a noncompetitive inhibitor changes the Vmax and Vmax/2 of the reaction, but typically does not alter Km. This is because the substrate can still bind to the active site, but the inhibitor prevents the catalytic activity of the enzyme.
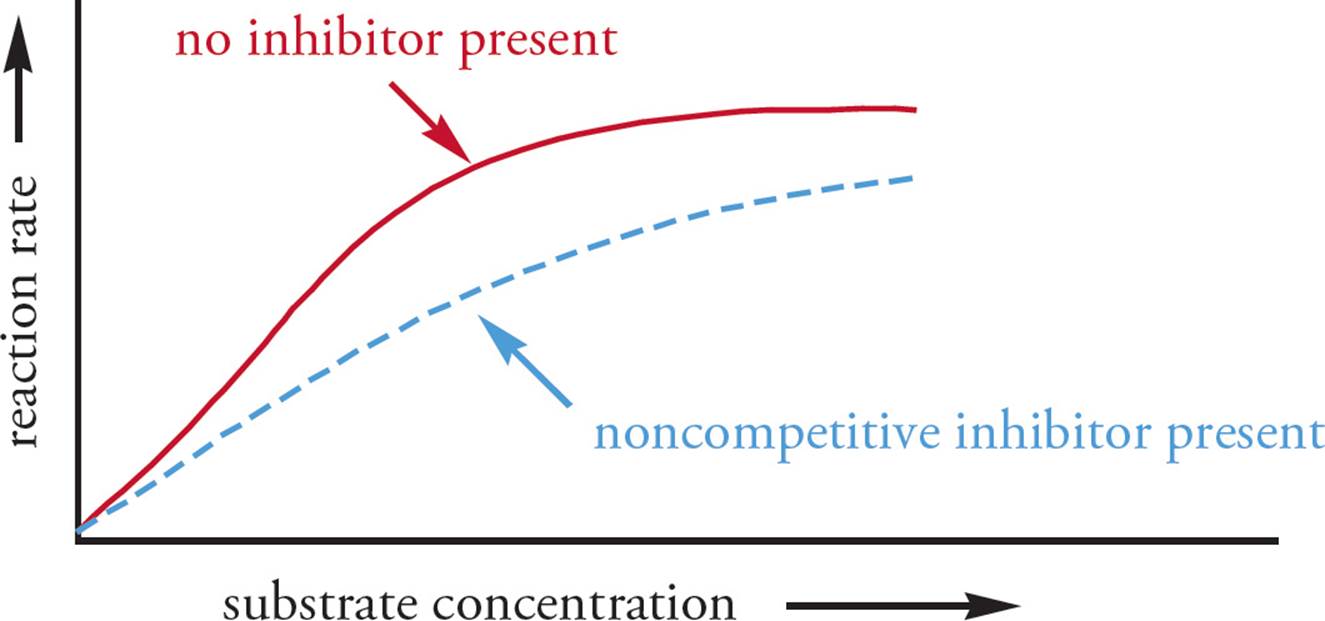
Figure 7 Noncompetitive Inhibition
• Carbon dioxide is an allosteric inhibitor of hemoglobin. It dissociates easily when Hb passes through the lungs, where the CO2 can be exhaled. Carbon monoxide, on the other hand, binds at the oxygen-binding site with an affinity 300 times greater than oxygen; it can be displaced by oxygen, but only when there is much more O2 than CO in the environment. Which of the following is/are correct?36
I. Carbon monoxide is an irreversible inhibitor.
II. CO2 is a reversible inhibitor.
III. CO2 is a noncompetitive inhibitor.
• In the Figure below, the kinetics of an enzyme are plotted. In each case, an inhibitor may be present or absent. Which one of the following statements is true?37
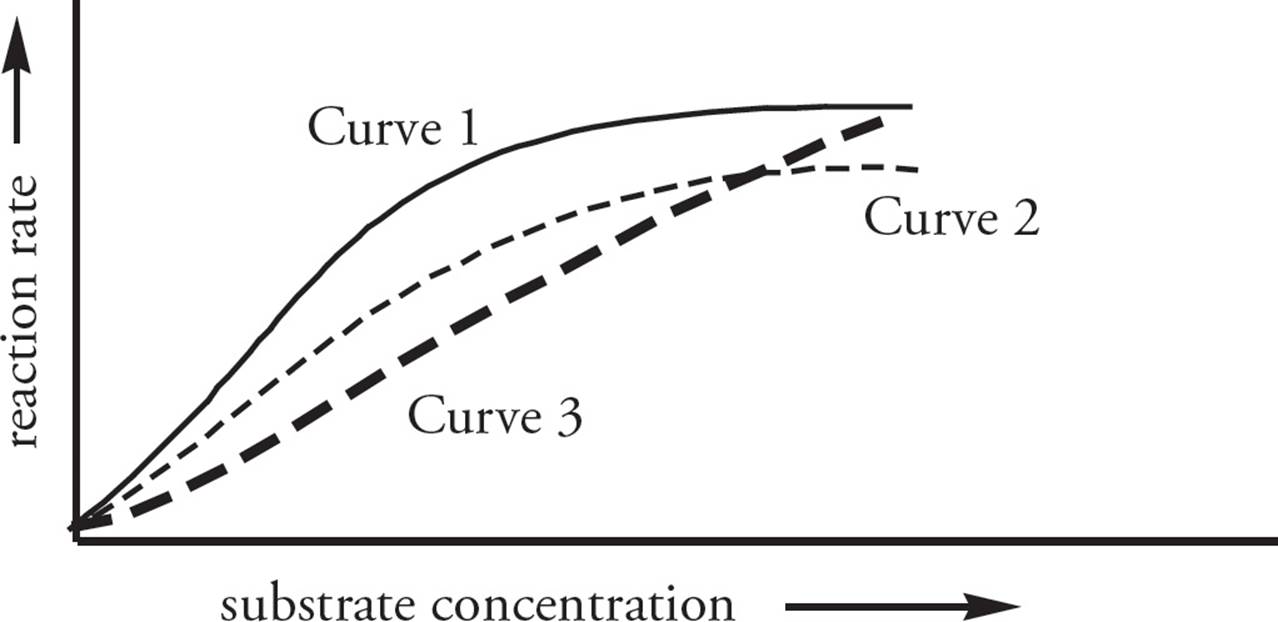
A) Curve 3 represents noncompetitive inhibition of the enzyme.
B) Curve 1 represents noncompetitive inhibition of the enzyme.
C) The Vmax values of Curve 2 and Curve 3 are the same.
D) Curve 3 represents competitive inhibition of the enzyme, and the enzyme is uninhibited in Curve 1.
If an inhibitor is only able to bind to the enzyme-substrate complex (that is, it cannot bind before the substrate has bound), it is referred to as an uncompetitive inhibitor. This effectively decreases Vmax by limiting the amount of available enzyme-substrate complex which can be converted to product. By sequestering enzyme bound to substrate, this increases the apparent affinity of the enzyme for the substrate as it cannot readily dissociate (decreasing Km).
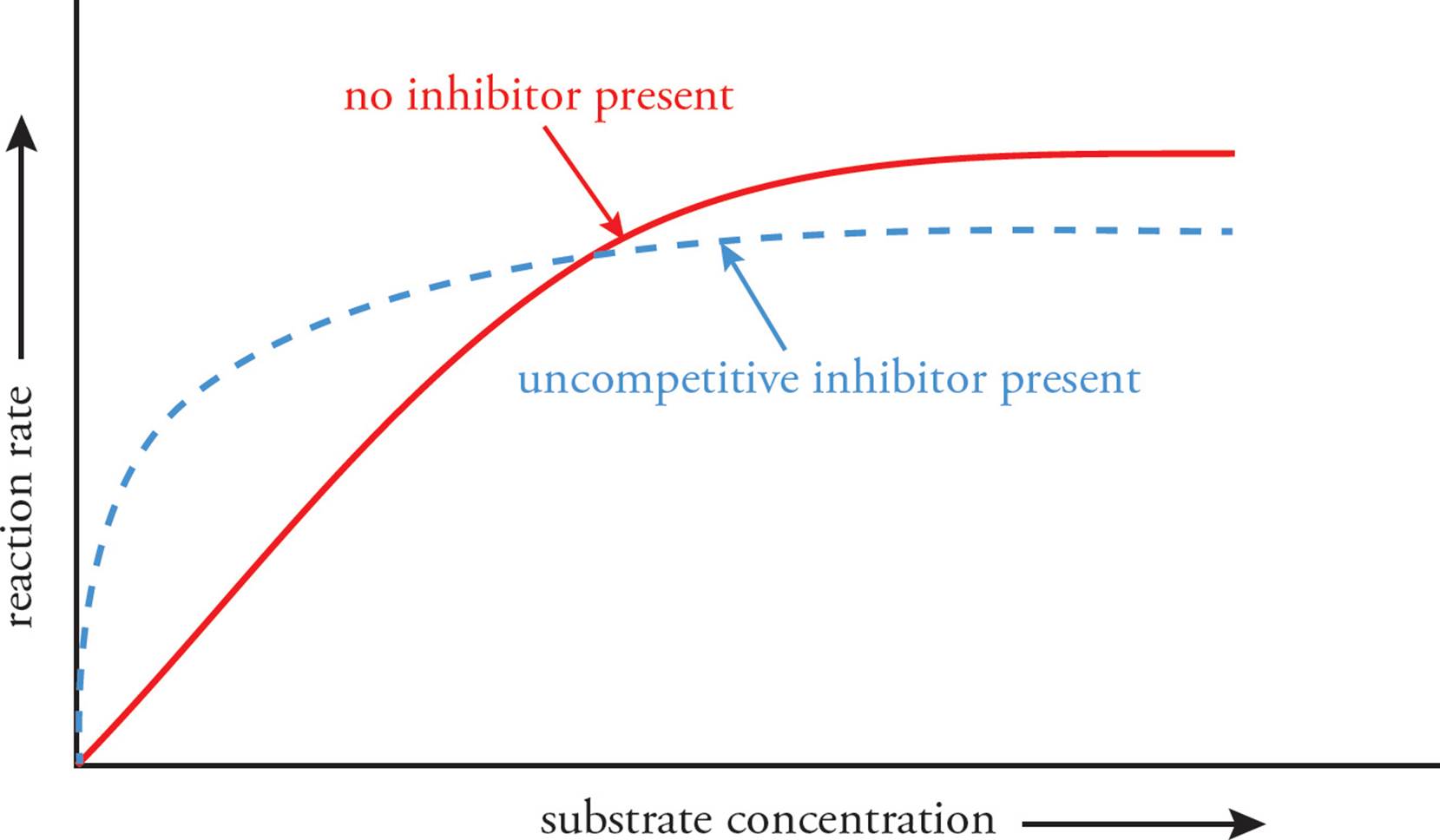
Figure 8 Uncompetitive Inhibition
Mixed-type inhibition occurs when an inhibitor can bind to either the unoccupied enzyme or the enzyme-substrate complex. If the enzyme has greater affinity for the inhibitor in its free form, the enzyme will have a lower affinity for the substrate similar to competitive inhibition (Km increases). If the enzyme-substrate complex has greater affinity for the inhibitor, the enzyme will have an apparently greater affinity (Km decreases) for the substrate similar to what we saw in uncompetitive inhibition. On the rare occasion where it displays equal affinity in both forms, it would actually be a noncompetitive inhibitor (many textbooks list noncompetitive inhibition as an example of mixed-type inhibition). In each of these situations, the inhibitor binds to an allosteric site and additional substrate cannot overcome inhibition (Vmax decreases).
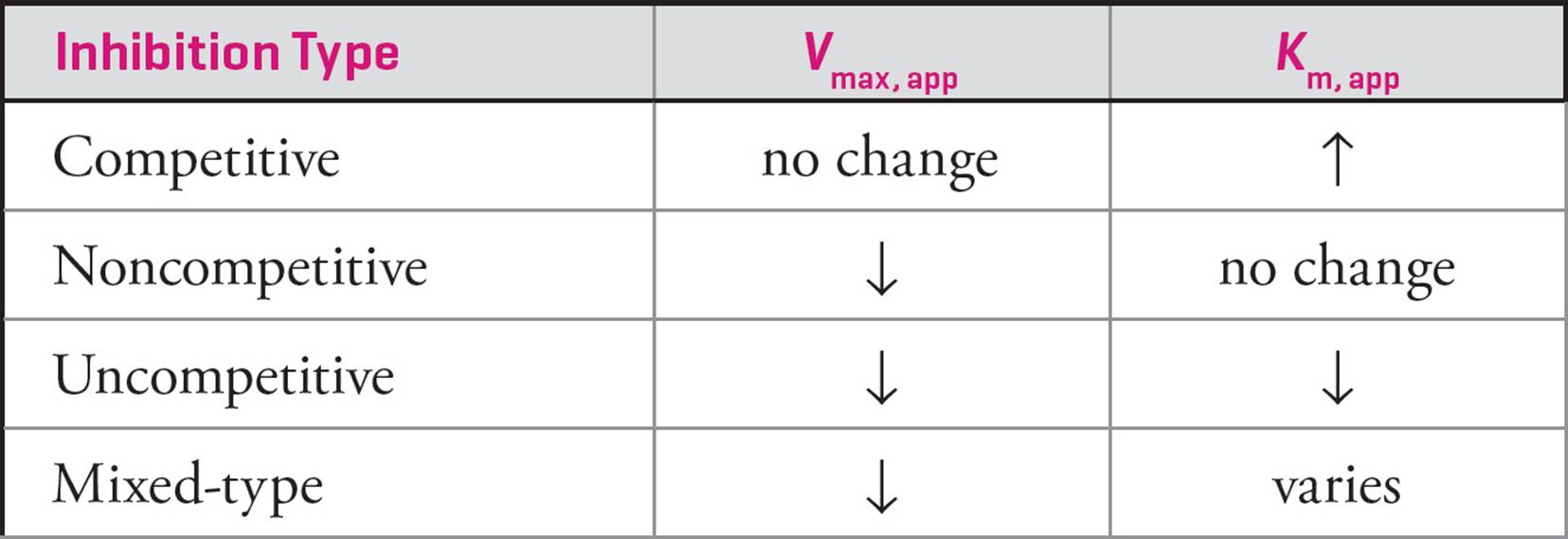
Table 2 Changes in the Apparent Vmax and Km in Response to Various Types of Inhibition
4.6 CELLULAR RESPIRATION
Energy Metabolism and the Definitions of Oxidation and Reduction
Where does the energy in foods come from? How do we make use of this energy? Why do we breathe? The answers begin with photosynthesis, the process by which plants store energy from the sun in the bond energy of carbohydrates. Plants are photoautotrophs because they use energy from light (“photo”) to make their own (“auto”) food. We are chemoheterotrophs, because we use the energy of chemicals (“chemo”) produced by other (“hetero”) living things, namely plants and other animals. Plants and animals store chemical energy in reduced molecules such as carbohydrates and fats. These reduced molecules are oxidized to produce CO2 and ATP. The energy of ATP is used in turn to drive the energetically unfavorable reactions of the cell. That’s the basic energetics of life; all the rest is detail.
In essence, the production and utilization of energy boil down to a series of oxidation/ reduction reactions. Oxidize is a chemical term meaning just what it sounds like: “bind to oxygen.” Reduce means the opposite: “remove oxygen.” In fact, there are three ways to “oxidize” (and “reduce”) an atom. Memorize them.
The Three Meanings of Oxidize:
1) attach oxygen (or increase the number of bonds to oxygen)
2) remove hydrogen
3) remove electrons
The Three Meanings of Reduce (just the opposite):
1) remove oxygen (or decrease the number of bonds to oxygen)
2) add hydrogen
3) add electrons
Though you should memorize this, it is not a subject worthy of philosophizing. If you can answer questions like the following, you’re set: Is changing CH3CH3 to H2C=CH2 an oxidation, a reduction, or neither?38 What about changing Fe3+ to Fe2+?39 What about this: O2 → H2O?40
When you reduce something, it’s like compressing a spring; you store potential energy. The reduced substance “wants” to be oxidized back to where it started. Here is one other important fact about oxidation and reduction: When one atom gets reduced, another one must be oxidized; hence the term redox pair. As you study the process of glucose oxidation, you will see that each time an oxidation reaction occurs, a reduction reaction occurs too.
Catabolism is the process of breaking down molecules. The opposite is anabolism, which is “building-up” metabolism.41 The way we extract energy from glucose is by oxidative catabolism. We break down the glucose by oxidizing it. The oxidative catabolism of glucose involves four steps: glycolysis, the pyruvate dehydrogenase complex (PDC), the Krebs cycle, and electron transport/oxidative phosphorylation. The stoichiometry of glucose oxidation looks like this:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
• What are the two members of the redox pair in this reaction?42
As we oxidize foods, we release the stored energy plants got from the sun. But we don’t make use of that energy right away. Instead, we store it in the form of ATP. Thus, cellular respiration is theoretically very simple: It’s just a big coupled reaction (described in Section 4.2). We make the unfavorable synthesis of ATP happen by coupling it to the very favorable oxidation of glucose. ATP can then be used to drive other cellular processes.
Introduction to Cellular Respiration
When glucose is oxidized to release energy, very little ATP is generated directly. Instead, the oxidation of glucose is accompanied by the reduction of high-energy electron carriers, nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD). Each of these carriers accept high-energy electrons during redox reactions (forming NADH and FADH2) and are later oxidized when they deliver the electrons to the electron transport chain. This generates the proton gradient that is used to generate ATP. Both of these carriers can serve as enzymatic cofactors and fulfill diverse roles in biological processes. For instance, NAD+ is required for activation of adenylate cyclase by cholera toxin, and FAD can associate with a protein to become a flavoprotein. Dozens of flavoproteins have been characterized and are commonly involved in redox reactions (e.g., amino acid metabolism).
Glucose is oxidized to produce CO2 and ATP in a four-step process: glycolysis, the pyruvate dehydrogenase complex (PDC), the Krebs cycle, and electron transport/oxidative phosphorylation. The first stage is glycolysis (“glucose splitting”). Here glucose is partially oxidized while it is split in half, into two identical pyruvic acid molecules. [How many carbon atoms does pyruvic acid have?43] Glycolysis produces a small quantity of ATP and a small quantity of NADH. Glycolysis occurs in the cytoplasm and does not require oxygen.
In the second stage (the pyruvate dehydrogenase complex), the pyruvate produced in glycolysis is decarboxylated to form an acetyl group. The acetyl group is then attached to coenzyme A, a carrier that can transfer the acetyl group into the Krebs cycle. A small amount of NADH is produced.
In the third stage, the Krebs cycle (also known as the tricarboxylic acid cycle (TCA cycle) or the citric
acid cycle), the acetyl group from the PDC is added to oxaloacetate to form citric acid. The citric acid is then decarboxylated and isomerized to regenerate the original oxaloacetate. A modest amount of ATP, a large amount of NADH, and a small amount of FADH2 are produced. Note that although the PDC and the Krebs cycle can only occur when oxygen is available to the cell, neither uses oxygen directly. Rather, oxygen is necessary for stage four, in which NADH and FADH2 generated throughout cellular respiration are reconverted into NAD+ and FAD. The PDC and the Krebs cycle occur in the innermost compartment of the mitochondria: the matrix.
In stage four of energy harvesting, electron transport/oxidative phosphorylation, the high-energy electrons carried by NADH and FADH2 are oxidized by the electron transport chain in the inner mitochondrial membrane. The reduced electron carriers dump their electrons at the beginning of the chain, and oxygen is reduced to H2O at the end. (The word oxidative in “oxidative phosphorylation” refers to the use of oxygen to oxidize the reduced electron carriers NADH and FADH2.) The electron energy liberated by the transport chain is used to pump protons out of the innermost compartment of the mitochondrion. The protons are allowed to flow back into the mitochondrion, and the energy of this proton flow is used to produce the high-energy triphosphate group in ATP.
Glycolysis
Glycolysis is an extremely old pathway, having evolved several billion years ago. It is the universal first step in glucose metabolism, the extraction of energy from carbohydrates. All cells from all domains (a domain is the highest taxonomic category—see Chapter 8) possess the enzymes of this pathway. In glycolysis, a glucose molecule is oxidized and split into two pyruvate molecules, producing a net surplus of 2 ATP (from ADP + Pi) and producing 2 NADH (from NAD++ H+):
Glucose + 2 ADP + 2 Pi + 2 NAD+ → 2 Pyruvate + 2 ATP + 2 NADH + 2 H2O + 2 H+
Of course it’s not quite that simple. Glycolysis involves several reactions, each of which is catalyzed by a different enzyme (see Figure 9). The general strategy is to first phosphorylate glucose on both ends and then split it into two 3-carbon units which can go on to the PDC and Krebs cycle. In the first step of glycolysis, a phosphate is taken from ATP and used to phosphorylate glucose, producing glucose 6-phosphate (G6P). This is isomerized to fructose 6-phosphate (F6P), which is then phosphorylated on carbon #1 (with the phosphate again taken from ATP) to produce fructose-1,6-bisphosphate (F1,6bP). This is split into two 3-carbon units that are oxidized to pyruvate, producing 2 ATP and 1 NADH per pyruvate, or 4 ATP and 2 NADH per glucose (since we get two 3-carbon units from each glucose). Don’t forget that each glucose gives rise to two 3-carbon units which pass through the second part of glycolysis and into the Krebs cycle.
• An extract of yeast contains all of the enzymes required for glycolysis, ADP, Pi, Mg2+, NAD+ and glucose, but when these are all combined, none of the glucose is consumed. Provided that there are no enzyme inhibitors present, why doesn’t the reaction proceed?44
Hexokinase catalyzes the first step in glycolysis, the phosphorylation of glucose to G6P. G6P feedback-inhibits hexokinase.
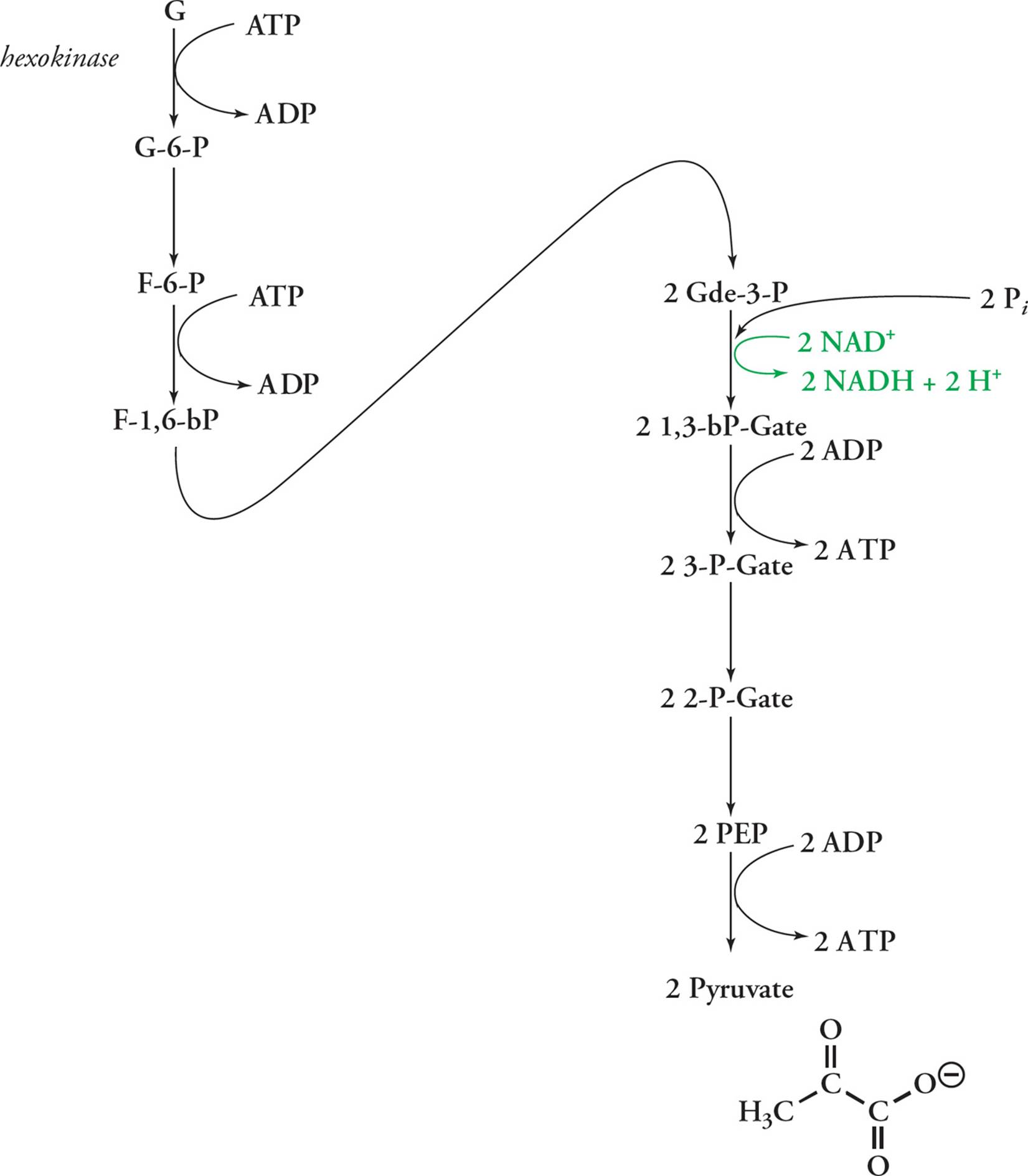
Figure 9 The 9 Reactions (Steps) of Glycolysis
Phosphofructokinase (PFK) catalyzes the third step: the transfer of a phosphate group from ATP to fructose-6-phosphate to form fructose-1,6-bisphosphate (F1,6bP). This is an important step because the reaction catalyzed by PFK is thermodynamically very favorable (like burning wood: ∆G << 0), so it’s practically irreversible. Also, G6P can be shunted to various pathways, but F1,6bP can only react in glycolysis. So once you light the PFK fire, you’re committed to glycolysis. Hence PFK is the key biochemical valve controlling the flow of substrate to product in glycolysis, and the conversion of F6P to F1,6bP is known as a committed step. In the remainder of glycolysis, F1,6bP is split into two 3-carbon molecules that are converted to pyruvate, with the production of NADH and ATP. Very favorable steps in enzymatic pathways (those with a large negative ∆G) are practically irreversible (because the back-reaction is so unfavorable). These reactions are the ones that are usually subject to allosteric regulation. Another generalization about what steps get regulated is this: early steps in a long pathway tend to be regulated. This makes sense; if you’re going from A to Z, it’s more practical to regulate the A → B reaction than the W → X one.
This is more than you need to know about glycolysis. When you get to medical school and do have to memorize the details, use an abbreviated sketch like this one. For the MCAT, know what goes in and what comes out, including energy carriers. You don’t need to memorize the following, but it should make sense:
1) NADH is produced in only one step: when an aldehyde (-de) is oxidized to a COOH (-ate).
2) ATP is converted to ADP every time a phosphate is added to a substrate, and ADP is made into ATP every time a phosphate comes off a substrate. (The only exception is an oddball HPO2– which gets picked up from the medium in Step 5.)
For example, the enzyme PFK is a key regulatory point in glycolysis. PFK is allosterically regulated by ATP. [What effect would you think a high concentration of ATP would have on PFK activity?45]
Two molecules of NAD+ are reduced in glycolysis per glucose catabolized, forming 2 NADH. As discussed above, NADH is an electron carrier, a molecule that is responsible for shuttling energy in the form of reducing power (i.e., reduction potential). Remember, these high energy electron carriers are not used directly as an energy source but are used later to generate ATP through electron transport and oxidative phosphorylation.
Fermentation
Under aerobic conditions (that is, in the presence of oxygen), the pyruvate produced in glycolysis enters the PDC and Krebs cycle to be oxidized completely to CO2. The NADH produced in glycolysis and the PDC, as well as NADH and FADH2 produced in the Krebs cycle, are all reoxidized in electron transport, where O2 is the final electron acceptor. In anaerobic conditions (without oxygen), electron transport cannot function, and the limited supply of NAD+ becomes entirely converted to NADH. [Would a limiting supply of NAD+ stimulate or inhibit glycolysis?46]
Fermentation has evolved to regenerate NAD+ in anaerobic conditions, therby allowing glycolysis to continue in the absence of oxygen. Fermentation uses pyruvate as the acceptor of the high energy electrons from NADH (see Figure 10). Two examples of this process are (1) the reduction of pyruvate to ethanol (yeast do this in the making of beer, wine, etc.) and (2) the reduction of pyruvate to lactate in human muscle cells. Lactate is thought to contribute to the “burn” that athletes encounter during anaerobic exertion, such as sprinting, when the cardiovascular system fails to deliver enough oxygen to keep the electron transport chain running in muscle cells.
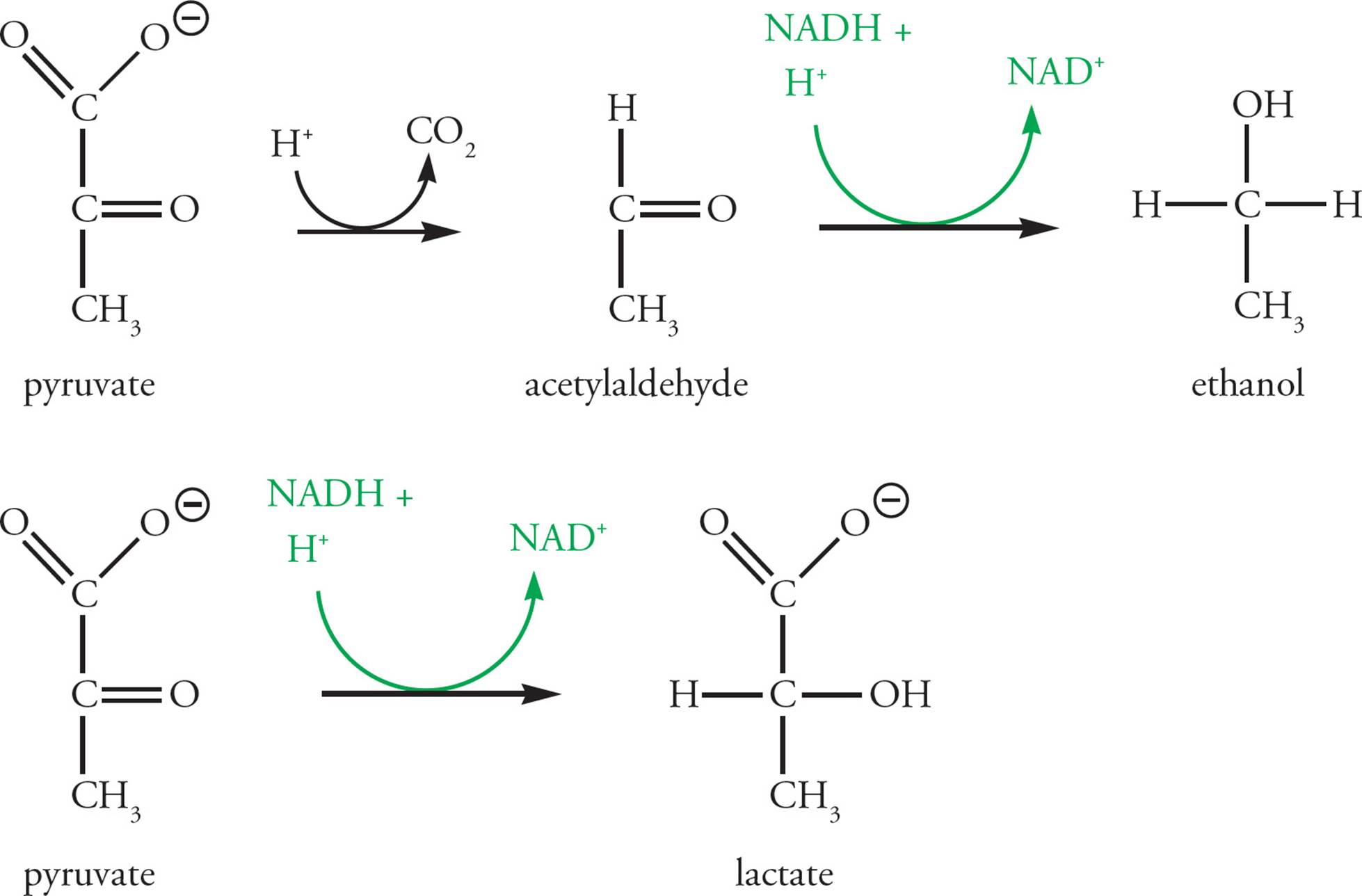
Figure 10 Anaerobic Pathways for Regeneration of NAD+ from NADH
The NAD+ produced by reducing pyruvate anaerobically is available for re-use in the glycolytic pathway, so more ATP can be produced. There is a limit to the use of anaerobic glycolysis as an energy source, however. The ethanol or lactate that is produced builds up, having no other use in the cell, and acts as a poison at high concentrations. Wine yeast die when the ethanol concentration reaches about 12 percent, and lactic acid is damaging at high concentrations in our tissues as well.
• What happens to the lactate in human muscle cells after a period of strenuous exercise?47
The Pyruvate Dehydrogenase Complex
The pyruvate produced in glycolysis in the cytoplasm is transported into the mitochondrial matrix, where it will be entirely oxidized to CO2. Pyruvate does not enter the Krebs cycle directly, however. First it is oxidatively decarboxylated by the pyruvate dehydrogenase complex (PDC; Figure 11). Oxidative decarboxylation is a reaction repeated again in the Krebs cycle, in which a molecule is oxidized to release CO2 and produce NADH. In oxidative decarboxylation, pyruvate is changed from a 3-carbon molecule to a __, while __ is given off and __ is produced.48 The PDC changes pyruvate into an activated acetyl unit. An acetyl unit is [(CH3)(O=C–)], and activatedmeans the acetyl is not floating around freely but rather is attached to a carrier, namely coenzyme A. This coenzyme is basically a long handle with a sulfur at the end, abbreviated CoA-SH. It is used in many reaction systems to pass acetyl units around (e.g., fatty acid and cholesterol synthesis and degradation). When loaded with an acetyl unit, CoA-SH is abbreviated acetyl-CoA. The bond between sulfur and the acetyl group is high energy, making it easy for acetyl-CoA to transfer the acetyl fragment into the Krebs cycle for further oxidation. Regulation of the PDC is crucial. [AMP (adenosine monophosphate) is a low-energy molecule produced by the hydrolysis of ATP during metabolism. What effect would you predict a high level of AMP to have on the activity of pyruvate dehydrogenase?49 The PDC is composed of three different enzymes. Why might a complex of three enzymes be more efficient than three independent enzymes?50]
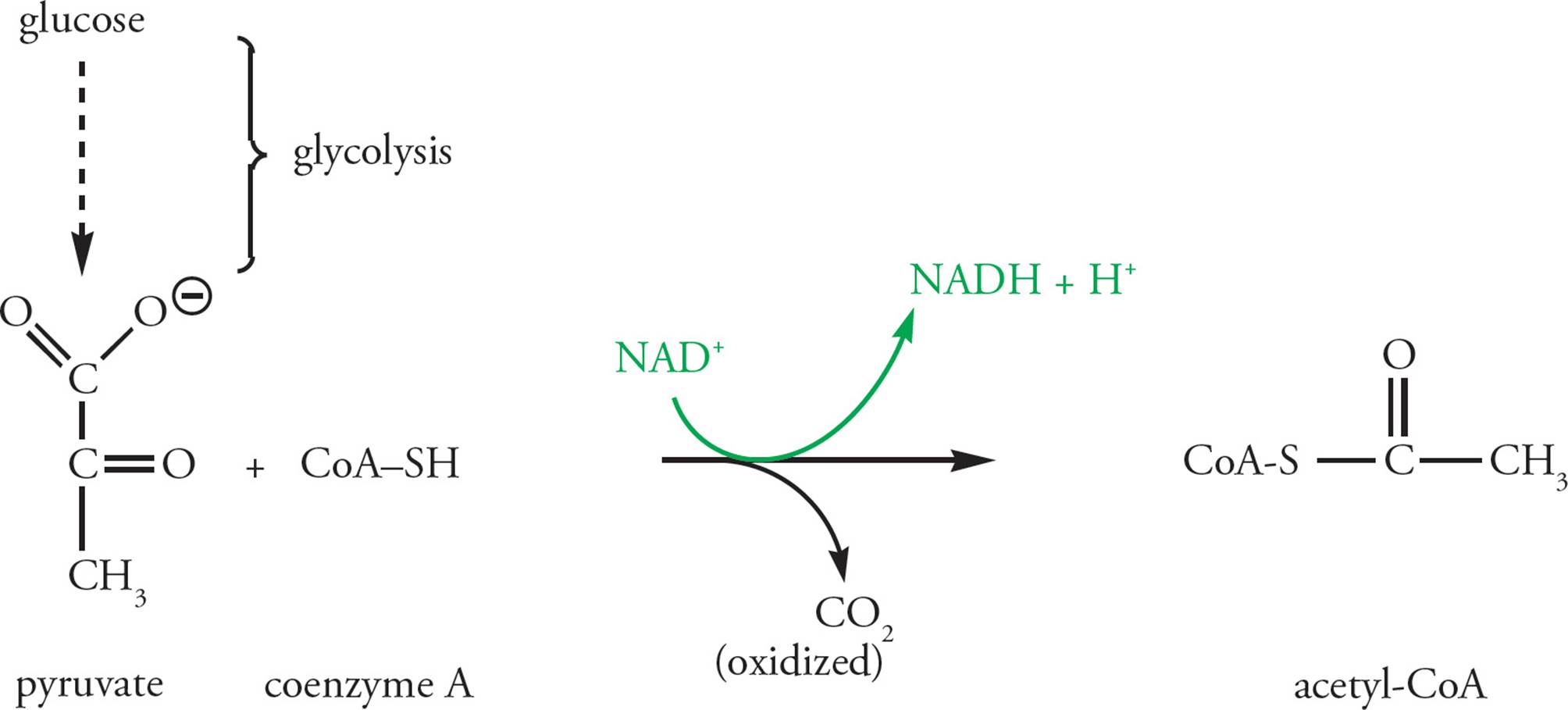
Figure 11 Oxidation of Pyruvate by Pyruvate Dehydrogenase
A prosthetic group is a nonprotein molecule covalently bound to an enzyme as part of the enzyme’s active site. The PDC contains a thiamine pyrophosphate (TPP) prosthetic group at one of its active sites. The α-ketoglutarate dehydrogenase complex, which catalyzes the third step in the Krebs cycle, is very similar to the PDC; it has a TPP prosthetic group and catalyzes an oxidative decarboxylation. The thiamine in thiamine pyrophosphate is vitamin B1. Vitamins often serve as prosthetic groups. Contrast this with NAD+, which is a cofactor. Cofactors are various organic and inorganic substances necessary to the function of an enzyme but which never actually interact with the enzyme (see Section 4.3).
• Beriberi is a disease caused by thiamine deficiency, which frequently results from a diet of white rice in underdeveloped nations. Which of the following would best describe the effect of thiamine deficiency on cellular metabolism in humans?51
A) The rate of glycolysis would increase.
B) Glycolysis would proceed anaerobically to maintain ATP production at normal levels.
C) Glucose consumption would slow, and ATP production would increase.
D) Acetyl-CoA would be provided by fatty acid metabolism, so the Krebs cycle would proceed uninhibited.
The Krebs Cycle
The Krebs cycle is a group of reactions which take the 2-carbon acetyl unit from acetyl-CoA, combine it with oxaloacetate, and release two CO2 molecules. NADH and FADH2 are generated in the process. The Figure below shows an overview of the process; note that many of the names are not necessary to know and have intentionally been left out.
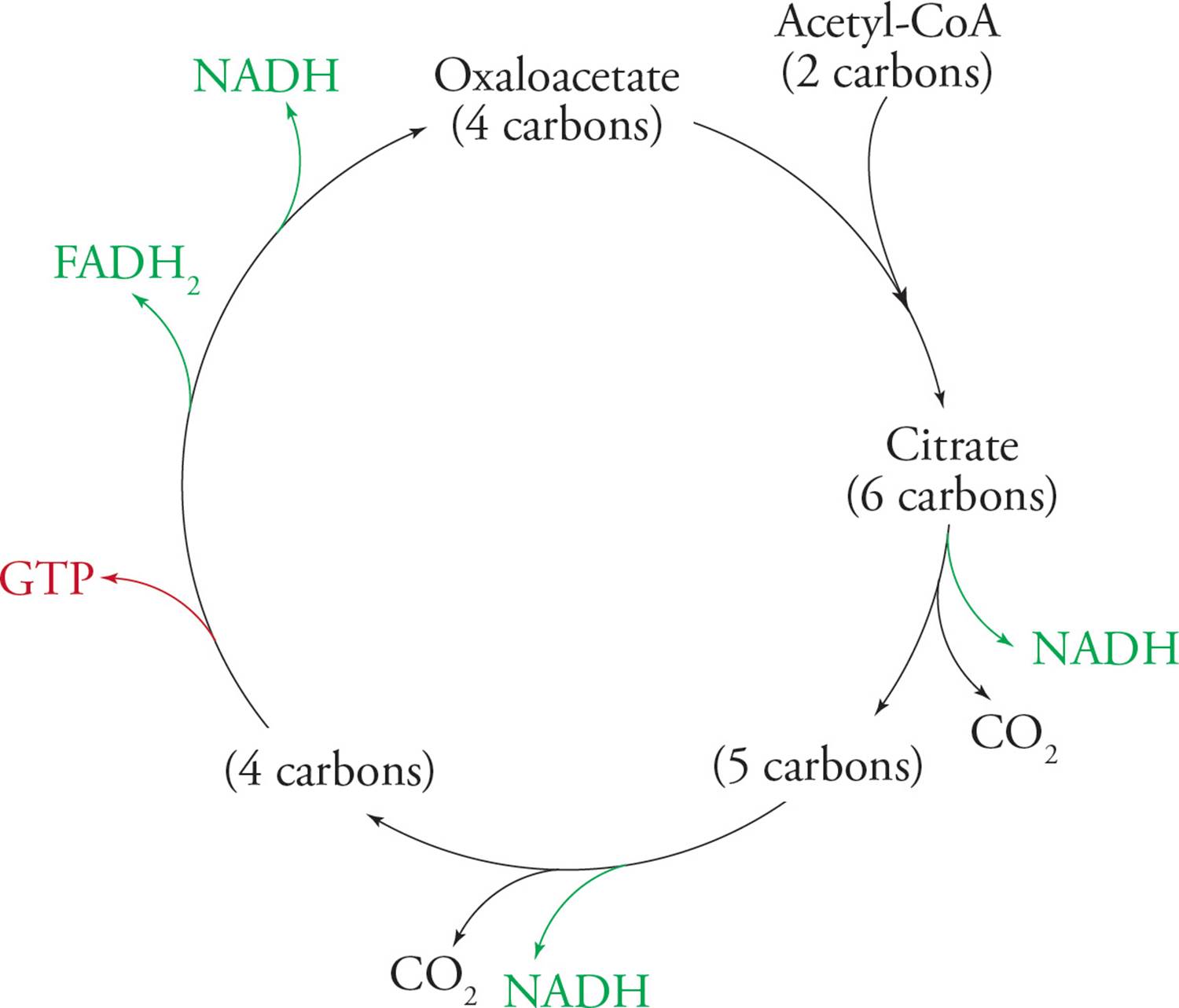
Figure 12 Overview of the Krebs Cycle
These reduced electron carriers (NADH and FADH2) go on to generate ATP in electron transport and oxidative phosphorylation. Two other names for the Krebs cycle are the tricarboxylic acid cycle (TCA cycle) and the citric acid cycle. Citrate is the first intermediate produced in the cycle, as soon as the acetyl unit is supplied. Citrate possesses three carboxylic acid functional groups, hence the term “tricarboxylic acid.” Note that a molecule with three carboxylic acids is ready to be oxidatively decarboxylated. We will now break the multistep cycle down into three general stages. The reactions are shown for conceptual understanding only; there is no need to memorize.
Krebs Stage 1: The two carbons in the acetate fragment of acetyl-CoA are condensed with the 4-carbon compound oxaloacetate (OAA; the name is worth remembering), producing citrate; see Figure 13. As you will see, the OAA is derived from the previous round of the Krebs cycle; it is recycled each time. [How many chiral carbons are present in citrate?52 If pyruvate is radiolabeled on its number one (most oxidized) carbon, where will the labeled carbon end up in the Krebs cycle?53]
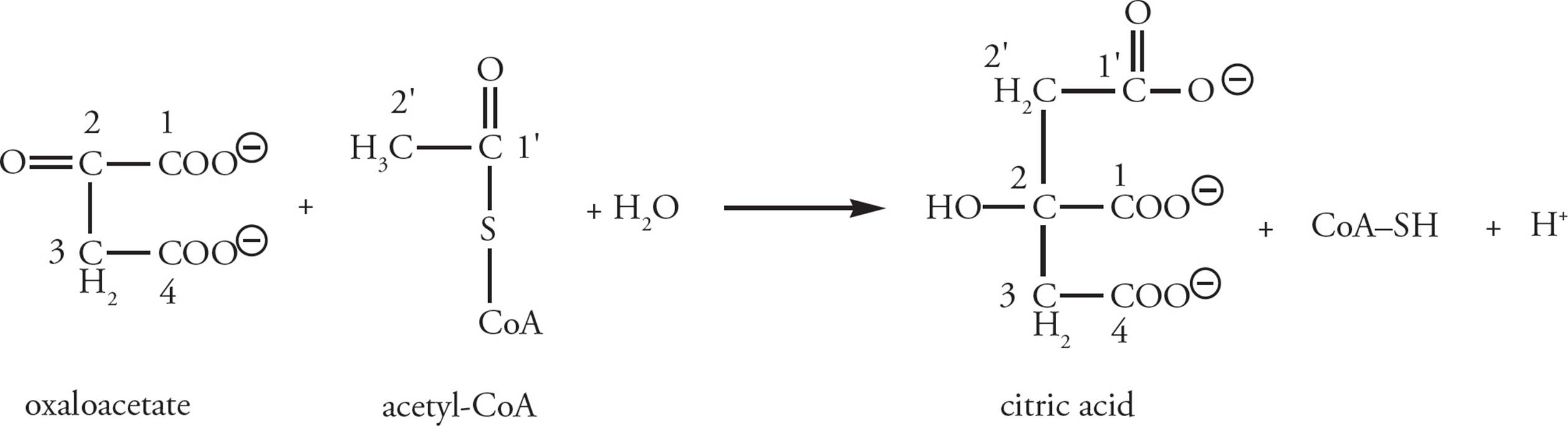
Figure 13 The Entry of Acetyl-CoA into the Krebs Cycle
Krebs Stage 2: Citrate is further oxidized to release CO2 and to produce NADH from NAD+ with each oxidative decarboxylation (Figure 14). If you’re interested in the details, citrate is first isomerized to form isocitrate, which is then oxidatively decarboxylated to yield the 5-carbon compound α-ketoglutarate, one carbon dioxide, and one NADH. Then α-ketoglutarate is oxidatively decarboxylated to produce succinyl-CoA (four carbons), releasing another CO2 and producing another NADH. The two carbons that leave as CO2 during these reactions are not the same ones that entered the cycle as acetate. Thus the two original acetyl carbons remain within the Krebs cycle. They will be lost as CO2 in later cycles. [How many carbons from the CoA component of acetyl-CoA enter into the Krebs cycle?54]
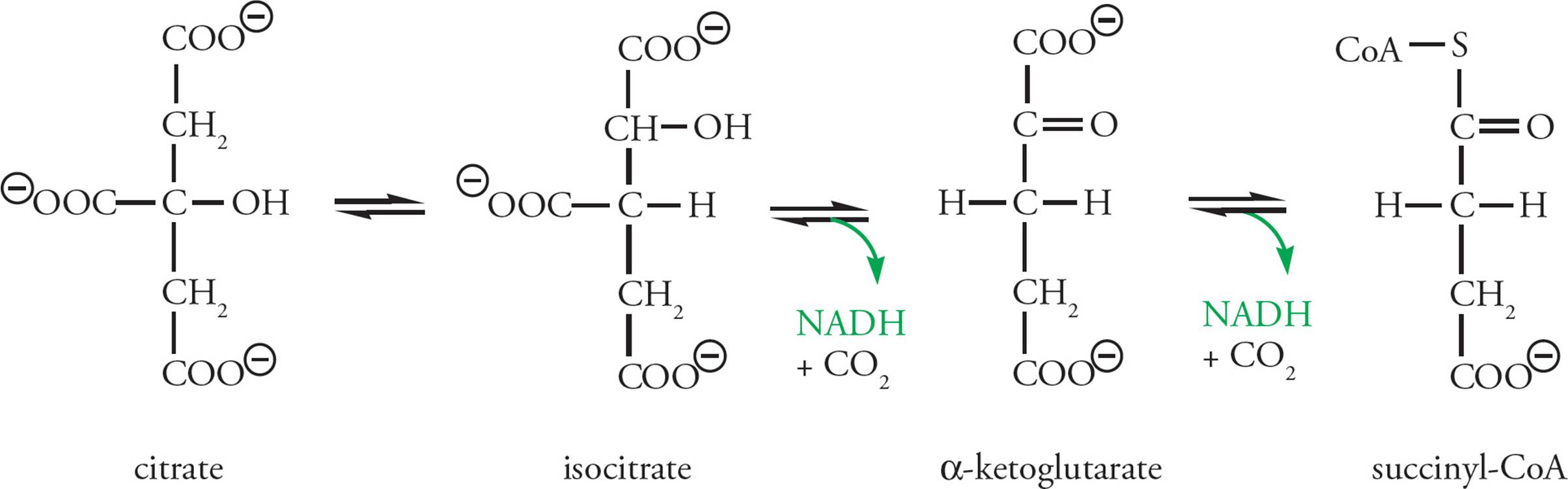
Figure 14 Oxidation of Citric Acid to Succinate
Krebs Stage 3: OAA is regenerated so that the cycle can continue. In the process, reducing power is stored in 1 NADH and 1 FADH2, and a high-energy phosphate bond is produced directly as GTP. Here GTP plays the role normally reserved for ATP. This GTP will eventually transfer its high-energy phosphate bond to ADP, converting it into ATP. FADH2 is similar to NADH, but ultimately results in the production of less ATP.
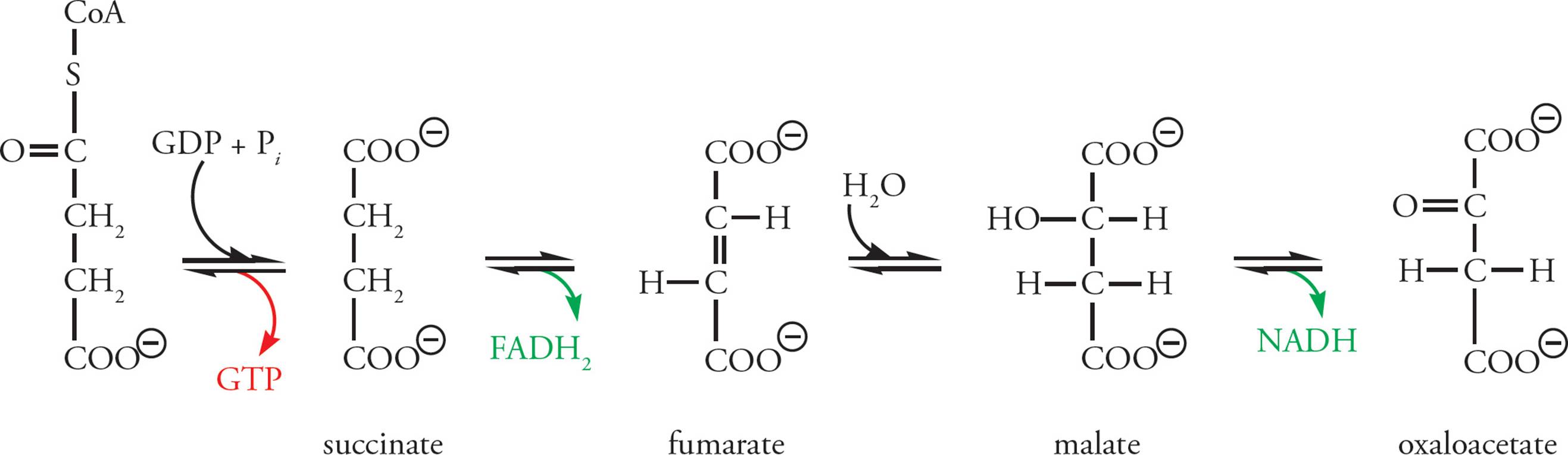
Figure 15 Succinyl CoA to OAA
To review, the oxidation of glucose has so far created:
1) 2 ATP and 2 NADH per glucose molecule in glycolysis
2) Pyruvate Dehydrogenase: 2 NADH per glucose (one per pyruvate)
3) Krebs cycle: 6 NADH, 2 FADH2, and 2 GTP per glucose
Thus, most of the energy of glucose is not extracted directly as ATP (or GTP) but in high-energy electron carriers. We will see how ATP is generated from NADH and FADH2 in electron transport/oxidative phosphorylation.
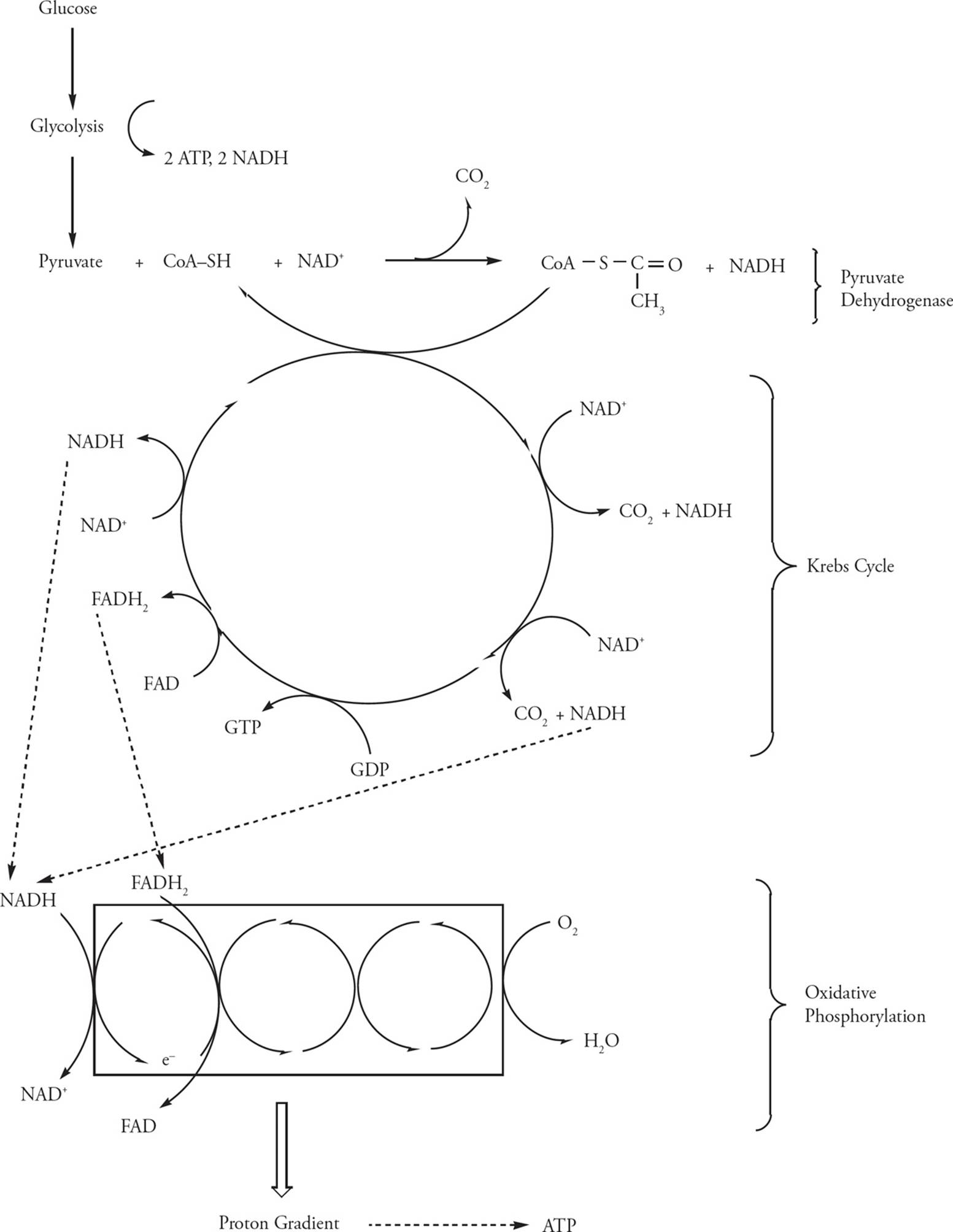
Figure 16 Cellular Respiration
Compartmentalization of Glucose Catabolism in Eukaryotes: The Mitochondria
To understand oxidative phosphorylation, you must know the structure of the mitochondrion (Figure 17). The mitochondrion contains two membranes, an outer membrane and an inner membrane, each composed of a lipid bilayer. The outer membrane is smooth and contains large pores formed by porin proteins. The inner membrane is impermeable, even to very small items like H+, and is densely folded into structures termed cristae. The cristae extend into the matrix, which is the innermost space of the mitochondrion. The space between the two membranes, the intermembrane space, is continuous with the cytoplasm due to the large pores in the outer membrane. The enzymes of the Krebs cycle and the pyruvate dehydrogenase complex are located in the matrix, and those of the electron transport chain and ATP synthase involved in oxidative phosphorylation are bound to the inner mitochondrial membrane.
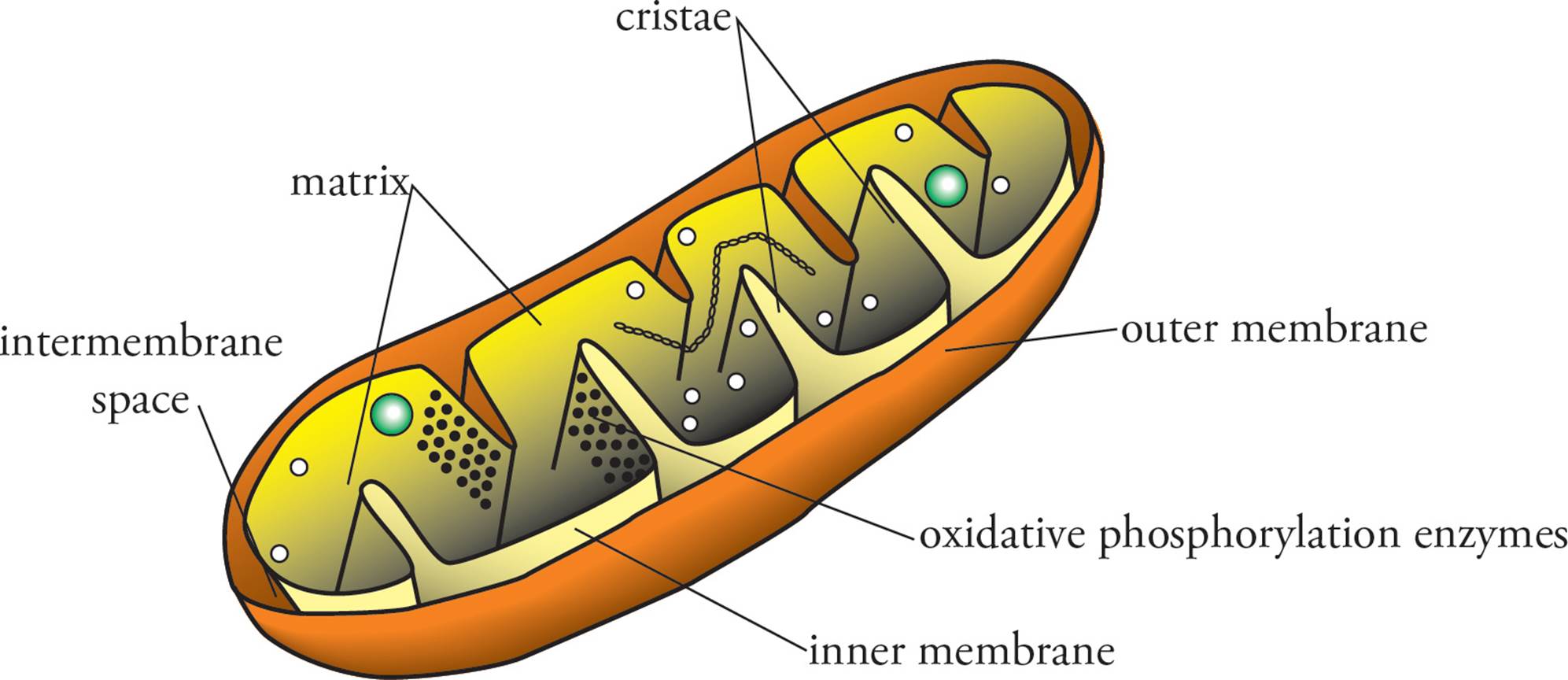
Figure 17 The Mitochondrion
The two goals of electron transport/oxidative phosphorylation are to:
1) reoxidize all the electron carriers reduced in glycolysis, PDC, and the Krebs cycle, and
2) store energy in the form of ATP in the process.
Where are all the reduced electron carriers located? Per each glucose catabolized, two NADH are created by glycolysis in the cytoplasm; the electrons from these NADH will have to be transported into the mitochondria before they can be passed along the electron transport chain. All the other NADHs and FADH2s were produced inside the mitochondrial matrix, so they are in the right place to donate electrons to the electron transport chain.
The situation in prokaryotes is a bit different: All of the reduced electron carriers are located in the cytoplasm. In fact, everything is located in the cytoplasm, since there are no membrane-bound organelles at all in prokaryotes (no mitochondria, no nucleus, no lysosomes—everything just floats around in the cytoplasm). Since they have no mitochondria, can bacteria perform oxidative phosphorylation? Yes, they can! The way the process works is that a proton gradient must be created and then used to power ATP synthesis by the membrane-bound ATP synthase. So all that’s required is a membrane impermeable to protons. Eukaryotes use the inner mitochondrial membrane; bacteria just use their cell membrane. The end result of this difference is that when eukaryotes perform aerobic respiration, they have to shuttle the electrons from cytosolic NADH into the mitochondrial matrix (at the cost of some energy) but bacteria do not. So, all things considered, prokaryotes get two more high-energy phosphate bonds from aerobic respiration than eukaryotes do (this will be discussed in more detail in just a bit). From this point forward, we will discuss the eukaryotic system. Remember that it’s the same in prokaryotes except that they do it on the cell membrane instead of on the inner mitochondrial membrane (since they have no mitochondria!).
Electron Transport and Oxidative Phosphorylation
Oxidative phosphorylation is the oxidation of the high-energy electron carriers NADH and FADH2 coupled to the phosphorylation of ADP to produce ATP. The energy released through oxidation of NADH and FADH2 by the electron transport chain is used to pump protons out of the mitochondrial matrix. This proton gradient is the source of energy used to drive the phosphorylation of ADP to ATP. The electron-transport chain is a group of five electron carriers (Figure 18). Each member of the chain reduces the next member down the line. All five are named for their redox roles. Three of them are large protein complexes found embedded in the inner mitochondrial membrane. They are classified as cytochromes due to the presence of a heme group, a porphyrin ring containing a tightly-bound iron atom. The other two members of the electron transport chain are small mobile electron carriers. The chain is organized so that the first large carrier receives electrons (reducing power) from NADH; the NADH is thus oxidized to NAD+. Hence, the first large carrier in the e– transport chain (“A” in the figure) is called NADH dehydrogenase. It passes its electrons to one of the small carriers in the transport chain, called ubiquinone, also known as coenzyme Q.55 NADH dehydrogenase is also known as coenzyme Q reductase.
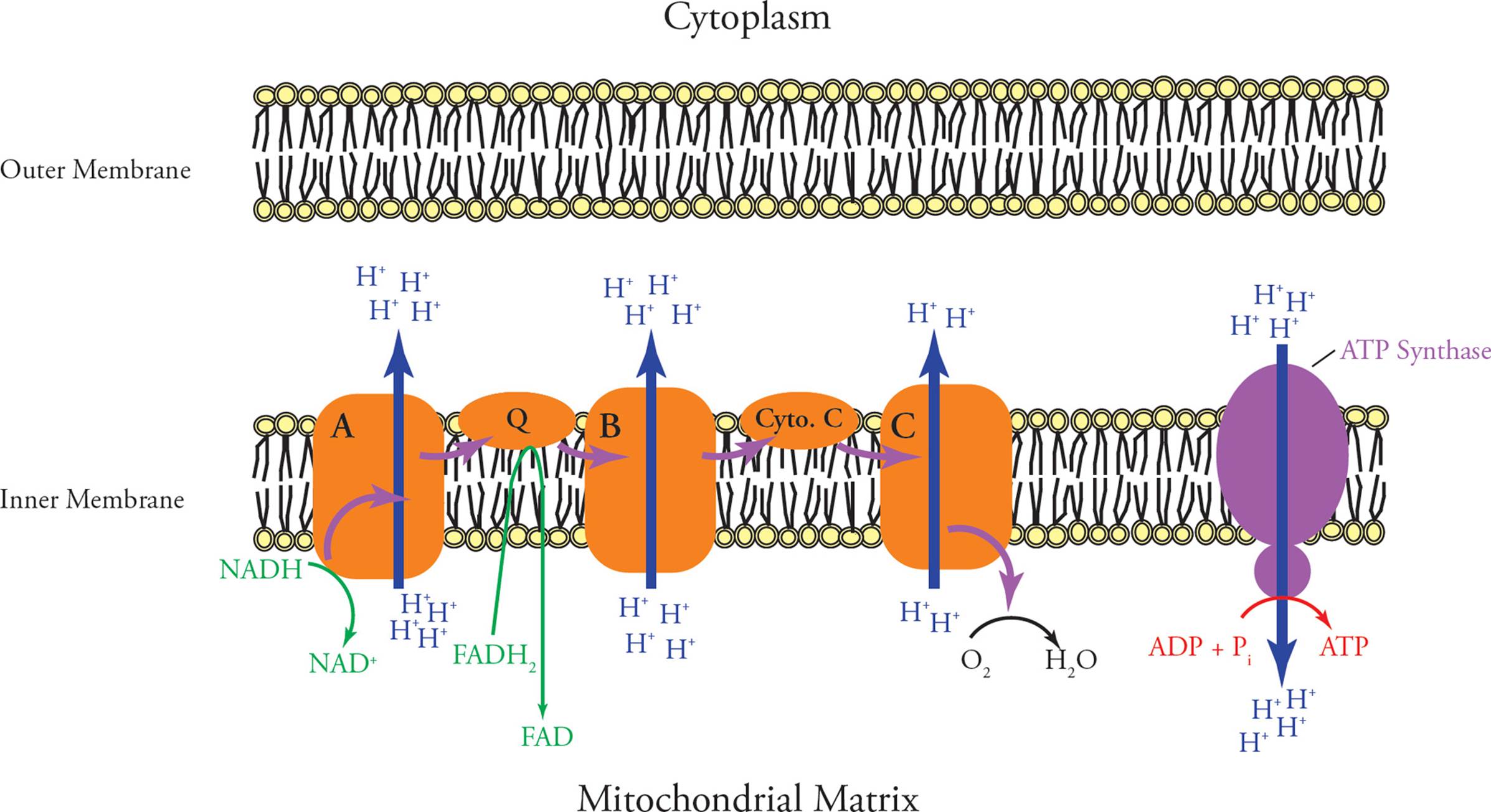
Figure 18 The Electron Transport Chain
Ubiquinone then passes its electrons to the second large membrane-bound complex in the chain (“B”), known as cytochrome C reductase. From this name, you can guess what the next carrier in the chain is called; it is cytochrome C, a small hydrophilic protein bound loosely to the inner mitochondrial membrane. The last member of the electron transport chain (“C”) is simply called cytochrome C oxidase. [Where does it pass its electrons to?56]
Each of the three large membrane-bound proteins in the electron transport chain pumps protons across the inner mitochondrial membrane every time electrons flow past. Protons are pumped out of the matrix, into the intermembrane space. The inner mitochondrial membrane is highly impermeable to protons. As a result, the electron transport chain creates a large proton gradient, with the pH being much __57 (higher/lower) inside the matrix than in the rest of the cell.
What does this have to do with ATP synthesis? Well, there is one more very important protein embedded in the inner mitochondrial membrane: ATP synthase. It is a large protein complex which contains a proton channel that spans the inner membrane. The passage of protons from the intermembrane space through the ATP synthase channel causes it to synthesize ATP from ADP + Pi. Thus, ATP production is dependent on a proton gradient. The overall process of electron transport and ATP production is said to be coupled by the proton gradient. Together, electron transport and ATP production are known as oxidative phosphorylation. Make sure you understand these questions:
• Dinitrophenol (DNP) is an uncoupler: It destroys the proton gradient by allowing protons to flow into the matrix. Which one of the following processes does it inhibit first?58
A) Pyruvate decarboxylation by the PDC
B) The TCA cycle
C) Electron transport
D) Muscular contraction
• Which one of the following processes has a positive ∆G under normal aerobic conditions in the cell?59
A) ATP hydrolysis
B) The pumping of protons to form a pH gradient
C) The oxidation of NADH by NADH dehydrogenase
D) The folding of a protein into its correct tertiary structure
• The reason cyanide is a poison is that it inactivates cytochrome C oxidase by binding to its active site with high affinity. When a person is exposed to cyanide60
A) the difference in pH inside and outside the matrix is already as large as it can become, so no more electrons can be pumped against the gradient.
B) anaerobic glycolysis depletes pyruvate, thereby slowing the Krebs cycle and the electron transport chain and slowing the rate of proton pumping.
C) the electron transport chain ceases to transport electrons and therefore ceases to pump protons.
D) NADH becomes fully oxidized by the Krebs cycle and therefore cannot reduce NADH dehydrogenase, so no protons are pumped.
Energetics of Glucose Catabolism
How is electron transport quantitatively connected to ATP synthesis? For every NADH that is oxidized to NAD+, the three large electron transport proteins pump about ten protons across the inner mitochondrial membrane, into the intermembrane space. The ATP synthase requires three protons to generate a molecule of ATP from ADP and Pi; however, an additional proton is required to bring Pi into the matrix. This brings the “cost” of ATP synthesis up to four protons per molecule of ATP. Since NADH is responsible for the pumping of 10 protons, each molecule of NADH provides the energy to produce approximately 2.5 ATP molecules.
Even though NADH and FADH2 have similar functions, their fates are a little different. FADH2 gives its electrons to ubiquinone instead of to NADH dehydrogenase. By bypassing the first proton pump, FADH2 is only responsible for the pumping of six protons across the inner membrane.
• How many ATP are made every time an FADH2 is reoxidized to FAD?61
As mentioned earlier, the PDC, the Krebs cycle and oxidative phosphorylation all occur in mitochondria in eukaryotes, while glycolysis occurs in the cytoplasm. The electrons from the NADH generated in glycolysis must be transported into the mitochondria before they can enter the electron transport chain. In most cells, they are transported by a pathway termed the glycerol phosphate shuttle. This shuttle delivers the electrons directly to ubiquinone (just like FADH2 does), bypassing NADH dehydrogenase, and results in the production of only 1.5 molecules of ATP per cytosolic NADH, rather than the 2.5 normally formed from matrix NADH.62 Bacteria, because they lack cellular organelles, do not need to transport cytosolic electrons across any membranes; hence the discrepancy in the Table below in how much ATP is yielded from each NADH from glycolysis in eukaryotes compared to prokaryotes. All values in the following Table are per glucose molecule catabolized.
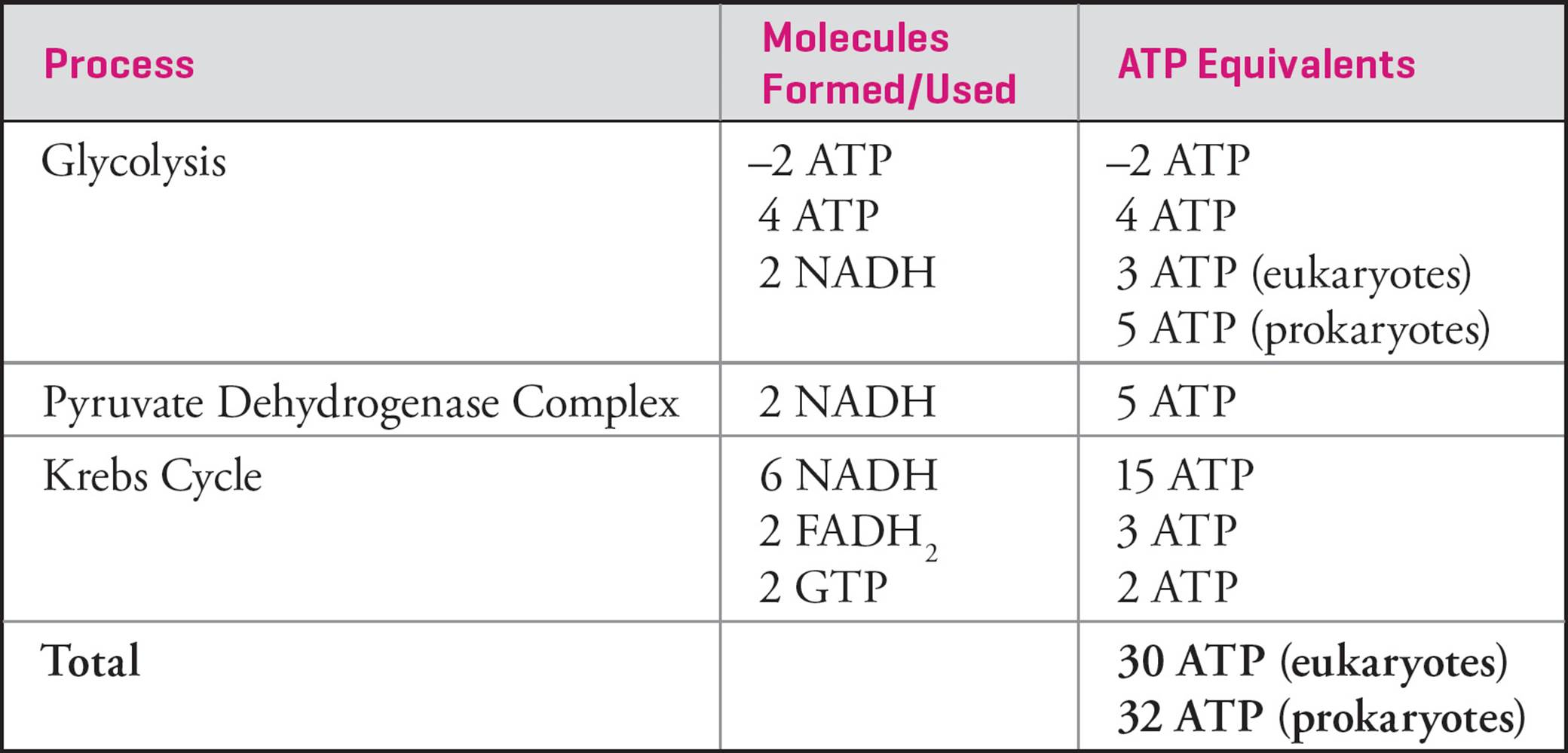
Table 3 Theoretical ATP Yield from Cellular Respiration
Notes:
1) These numbers are an estimate of the theoretical maximum amount of ATP that can be produced from a single molecule of glucose. As the proton gradient is used to transport other molecules into or out of the matrix, the actual yield may differ depending on the number of protons (i.e., the gradient) available for ATP synthesis.
2) These numbers reflect the most recent understanding of ATP synthesis, and as such, may not appear in some textbooks that still cling to the previously established counts of 36 ATP per glucose in eukaryotes and 38 ATP per glucose in prokaryotes.
Other Metabolic Pathways of the Cell
There are several other metabolic pathways that play important roles in maintaining sugar and energy levels.
Glycogenolysis
Glycogenolysis is the term for glycogen breakdown. Glycogen is a polymer of glucose that is found in muscle and liver cells, and is the main form of carbohydrate storage in animals. The synthesis of glycogen (glycogenesis) and glycogenolysis are opposing processes, controlled by hormones that regulate blood sugar levels and energy. Glycogenolysis occurs in response to the hormone glucagon, when blood sugar levels are low. It results in glucose being released into the blood where it can then be taken up in cells and enter glycolysis. A similar process occurs in higher plants where polymerized glucose, in the form of starch, can be broken down for various cellular processes including glycolysis. Both glycogen and starch are glucose polymers with a-1,4 and a-1,6 glycosidic bonds.
Gluconeogenesis
Gluconeogenesis occurs when dietary sources of glucose are unavailable, and when the liver has depleted its stores of glycogen and glucose. This process occurs primarily in the liver (and to a lesser extent in the kidneys), and involves converting non-carbohydrate precursor molecules (such as lactate, pyruvate, Krebs cycle intermediates, and the carbon skeletons of most amino acids) into intermediates of the above pathways where they ultimately become glucose. Gluconeogenesis is an 11-step pathway that uses many of the same enzymes as glycolysis. In simplified terms, it can be thought of as “glycolysis-in-reverse,” where those enzymes catalyzing the irreversible reactions have been replaced. The first of these, pyruvate carboxylase, catalyzes the conversion of pyruvate to oxaloacetate, which can be further converted into phosphoenolpyruvate, the second-to-last product in glycolysis.
While the majority of the intermediates discussed in cellular respiration can take part in gluconeogenesis, acetyl-CoA cannot. This helps explain why free fatty acids cannot be converted to glucose during periods of starvation, while the glycerol backbone of a triglyceride can.
Amino Acid Catabolism
Proteins in cells are constantly being made, kept for a certain period of time (minutes to weeks), and then degraded back into amino acids. In addition, humans absorb amino acids from dietary proteins. These free amino acids can be catabolized via several pathways. The amino group is removed and converted into urea for excretion. The remaining carbon skeleton (also called an a-keto acid) can either be broken down into water and CO2, or can be converted to glucose or acetyl-CoA.
Pentose Phosphate Pathway
The pentose phosphate pathway (PPP) diverts glucose-6-phosphate from glycolysis in order to form (among several other products) ribose-5-phosphate, which can be used to synthesize nucleotides. This cytoplasmic pathway, sometimes referred to as a shunt, is composed of an oxidative phase (that also generates NADPH) followed by a non-oxidative phase producing additional sugar precursors. NADPH, although sharing much of its structure with NADH, has a different cellular role and serves as an important reducing agent in many anabolic processes. It also aids in the neutralization of reactive oxygen species.
The first enzyme in the PPP, glucose-6-phosphate dehydrogenase (G6PDH), is the primary point of regulation and generates NADPH. A deficiency of this enzyme (which is a common heriTable disease) limits the ability of red blood cells to eliminate reactive oxygen species; this can lead to cell death and potential renal and hepatic complications.
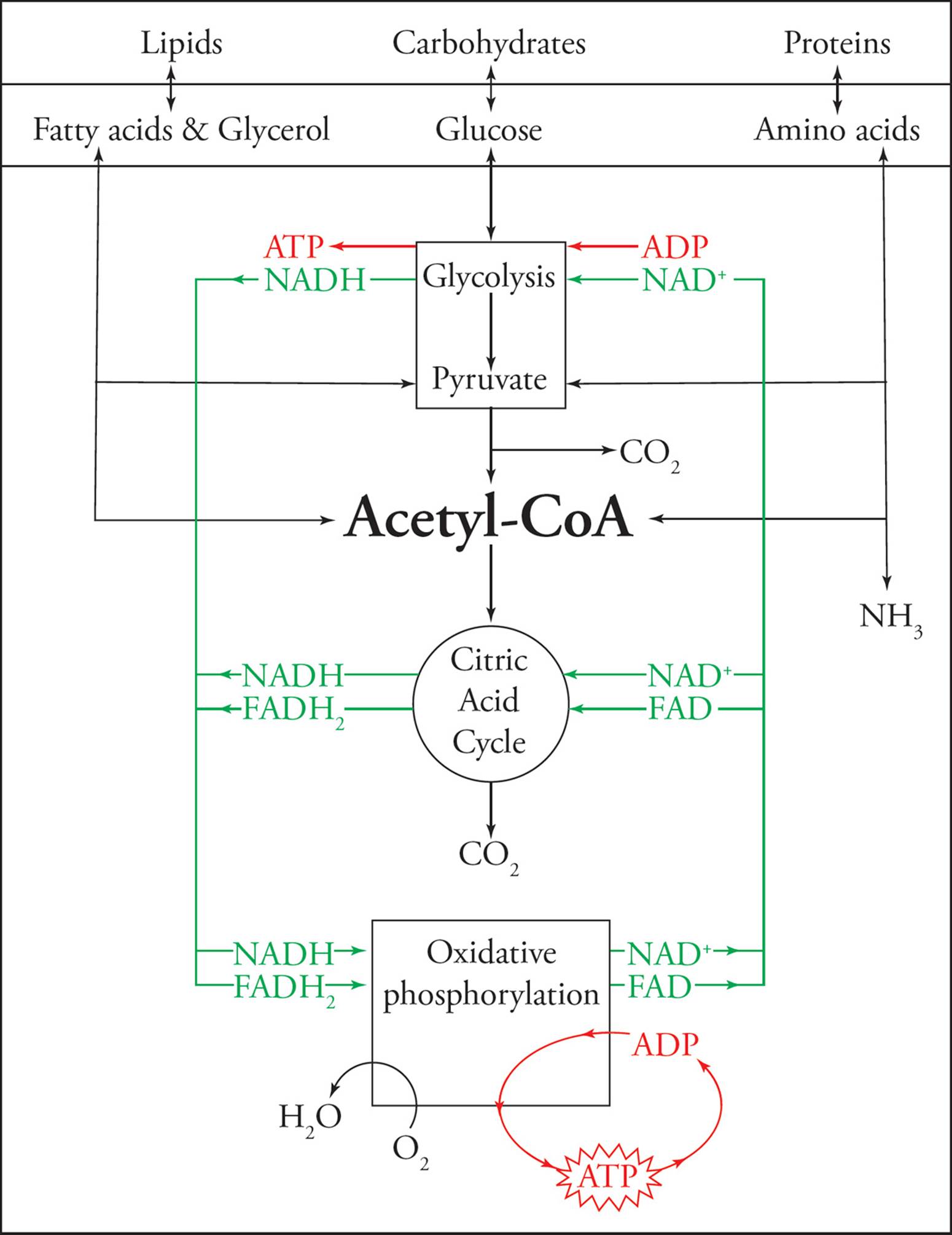
Figure 19 Metabolic Pathways
4.7 METABOLIC REGULATION
The above pathways represent a small subset of the anabolic and catabolic processes that allow cells to both consume and produce a wide array of molecules. Given that many of these pathways serve opposing roles (e.g., glycolysis and gluconeogenesis), the simultaneous activation of two such pathways would result in the net loss of energy due to futile cycling. Therefore tight regulation, resulting in reciprocal control, in response to current cellular needs is critical. Numerous strategies are used to accomplish this including compartmentalization, regulation of enzyme quantity, and regulation of enzymatic activity (as described in Section 4.4). The key regulatory processes for cellular respiration and glycogen homeostasis are considered here.
Regulation of Glycolysis and Gluconeogenesis
As we already know, glycolysis and gluconeogenesis utilize many of the same enzymes. Attempts to regulate any one of these would fail to isolate a single pathway, so regulation must focus on those enzymes catalyzing irreversible reactions. Two such heavily-regulated enzymes are phosphofructokinase (PFK) and fructose-1,6-bisphosphatase (F-1,6-BPase). These enzymes serve opposing roles in glycolysis and gluconeogenesis, respectively. Both enzymes are allosterically regulated by glycolytic intermediates that activate one enzyme while inhibiting the other. For instance, in energy-starved states, elevated cellular AMP levels activate PFK while inhibiting F-1,6-BPase, resulting in enhanced glycolysis activity and a suppression of gluconeogenesis.
Another metabolic intermediate that exerts reciprocal control on these two enzymes is fructose-2,6-bisphosphate (F-2,6-BP). Its intracellular concentration is set by a single large protein that functions as two separate enzymes: phosphofructokinase-2 (PFK-2), which synthesizes F-2,6-BP, and fructose-2,6-bisphosphatase (F-2,6-BPase), which breaks it down. Insulin and glucagon help control the concentration of intracellular F-2,6-BP by regulating the activity of PFK-2 and F-2,6-BPase. To better illustrate how this occurs, let us consider an example. When blood glucose levels fall, glucagon is released from the alpha cells in the pancreas. Glucagon then binds to cell surface receptors in the liver. This results in the production of cAMP and the activation of protein kinase A, leading to a deactivation of PFK-2 and activation of F-2,6-BPase (due to phosphorylation of the protein). Increased F-2,6-BPase activity consumes F-2,6-BP; this fall in concentration enhances the activity of F-1,6-BPase (the enzyme involved in gluconeogenesis) while decreasing the activity of PFK (the enzyme involved in glycolysis). The net result is an increase in gluconeogenesis and an inhibition of glycolysis to allow for a restoration of normal blood glucose levels. Insulin, which is released in response to elevated blood glucose, activates glycolysis while inhibiting gluconeogenesis.
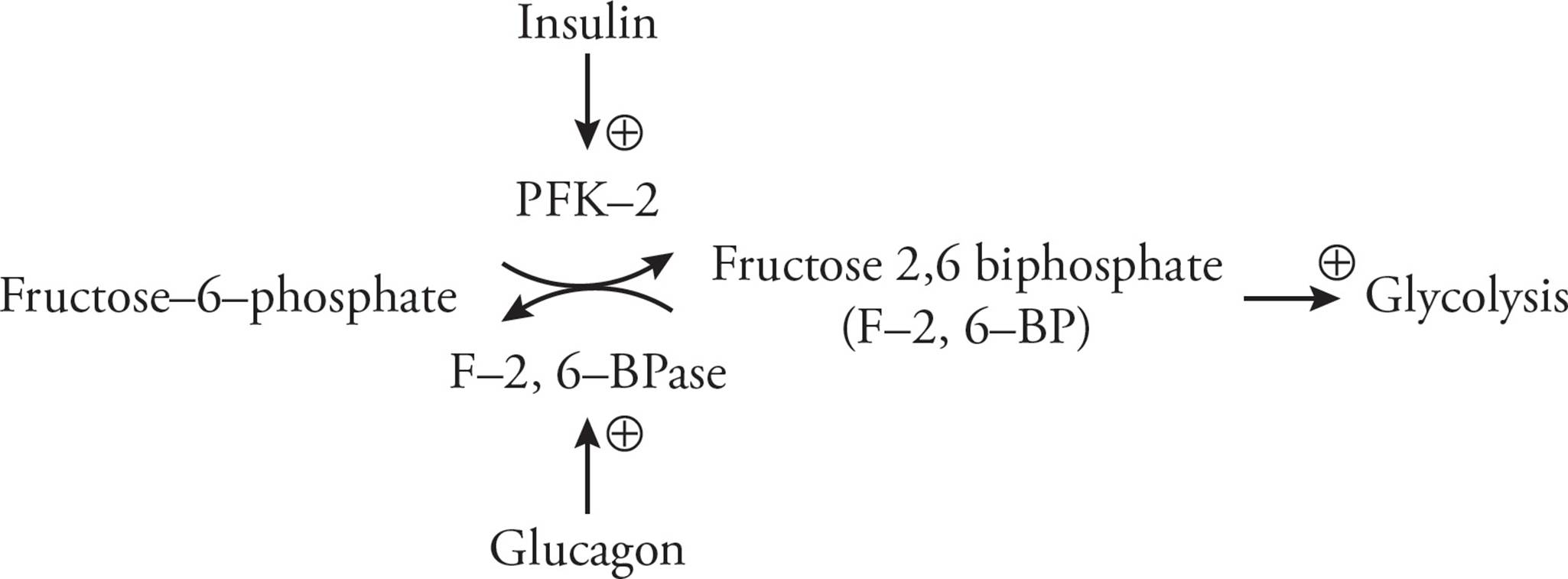
Regulation of the Krebs Cycle
Following glycolysis, pyruvate can either continue through cellular respiration or be transformed into another useful metabolic intermediate. If pyruvate continues through cellular respiration and is converted to acetyl-CoA by the pyruvate dehydrogenase complex, it can no longer be utilized by gluconeogenesis to form glucose. Therefore, this step is tightly regulated in response to the energy demands of the cell (e.g., high concentrations of NAD+ indicate an energy deficit and increase acetyl-CoA production). When acetyl-CoA enters the Krebs cycle, those enzymes that catalyze exergonic steps are also regulated. Of particular importance, the activity of isocitrate dehydrogenasechanges with the energy needs of the cell (e.g., elevated levels of ATP inhibit the enzyme).
Regulation of Glycogen Synthesis and Glycogenolysis
Glycogen, which serves as the principle storage site for glucose in the liver and muscle, must be synthesized and broken down both in response to changes in blood glucose and metabolic demand. Glycogen synthase (the principle enzyme responsible for glycogen generation from glucose-1-phosphate) and glycogen phosphorylase (which serves to catabolize glycogen) are reciprocally controlled. Immediately following a meal, elevated levels of insulin activate glycogen synthase and inhibit glycogen phosphorylase. This stimulates glycogen synthesis while inhibiting its breakdown. Conversely, glucagon results in the suppression of glycogen synthesis and the stimulation of glycogenolysis from the liver (but not muscle).
Overview
In order to meet the varied metabolic demands of the cell, many additional forms of regulation occur beyond the limited examples outlined here. The following general principles however, allow for reasonable predictions of the activity of a given pathway in response to cellular conditions:
1) In a pathway, those enzymes which catalyze irreversible (i.e., exergonic) reactions are frequently sites of regulation.
2) Increased concentrations of intermediates in a pathway generally serve to decrease the activity of that pathway (e.g., citrate decreases the activity of PFK in glycolysis).
3) Each pathway responds to the energy state of the cell. Cellular respiration is stimulated by energy deficits (e.g., high ADP:ATP or NAD+:NADH ratios) or inhibited energy surpluses (e.g., high ATP:ADP or NADH:NAD+ratios).
The Table below outlines some of the regulatory steps described in this chapter.
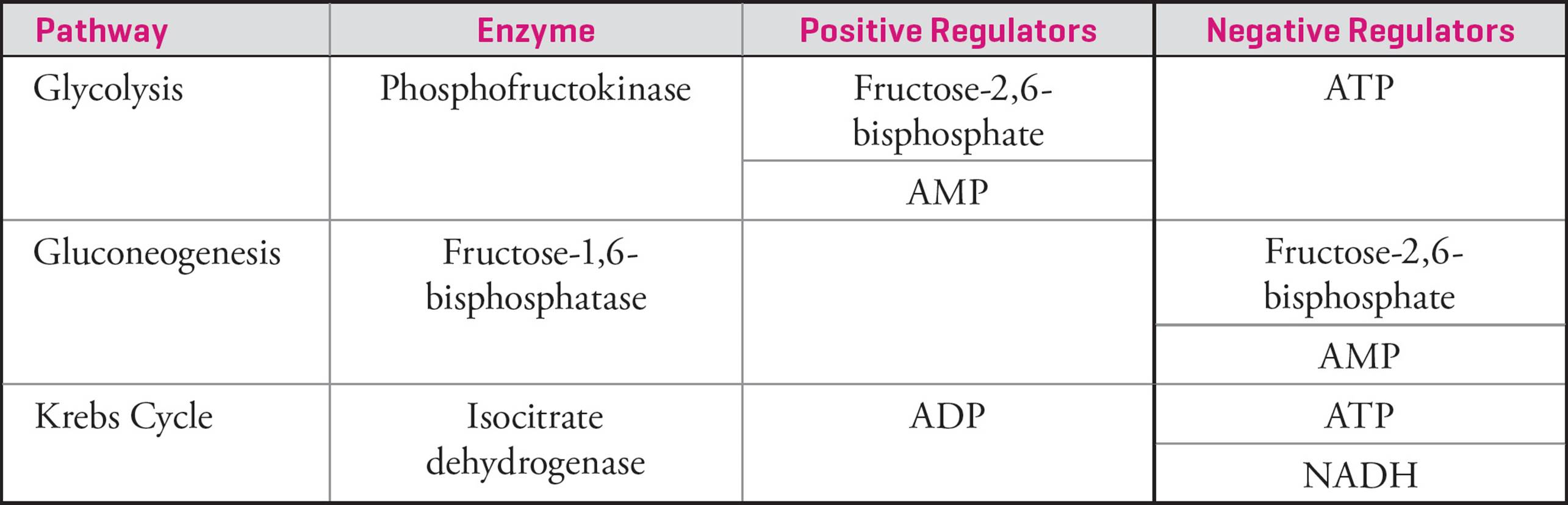
Table 4 Summary of Metabolic Regulation
4.8 FATTY ACID METABOLISM
Fatty Acid Oxidation
Following the initial steps in digestion (see Chapter 11 for more information), chylomicrons composed of fat and lipoprotein are transported via the lymphatic system and blood stream to the liver, heart, lungs, and other organs. This dietary fat, or triacylglycerol, is then hydrolyzed to liberate free fatty acids which can then undergo β-oxidation. This process begins at the outer mitochondrial membrane with the activation of the fatty acid. This reaction, catalyzed by acyl-CoA synthetase, requires the investment of two ATP equivalents to generate a fatty acyl-CoA which is then transported into the mitochondrion. Once in the matrix, the fatty acyl-CoA undergoes a repeated series of four reactions which cleave the bond between the alpha and beta carbons to liberate an acetyl-CoA in addition to generating one FADH2 and NADH.
Each round of β-oxidation cleaves a two-carbon acetyl-CoA from the molecule, however the final round cleaves a four-carbon fatty acyl-CoA to generate two acetyl-CoA. For instance, the complete β-oxidation of lauric acid (a twelve-carbon saturated fatty acid) involves the following: an investment of two ATP equivalents to convert it to a fatty acyl-CoA and then five rounds of β-oxidation. This generates five FADH2, five NADH, and six acetyl-CoA which can then enter the Krebs cycle. We then have a grand total of eleven FADH2 (five from β-oxidation and six from the Krebs cycle), 23 NADH (five from β-oxidation and 18 from the Krebs cycle), and six ATP equivalents (from the Krebs cycle). After the electron transport chain (and subtracting the two ATP equivalents required at the beginning of β-oxidation), we obtain 78 ATP from lauric acid.
The oxidation of unsaturated fatty acids, those containing double bonds, requires additional steps. For a monounsaturated fatty acid, β-oxidation proceeds normally, cleaving two-carbon subunits from the fatty acid, until the double bond is encountered. An isomerase then moves the double bond and allows the fatty acid to continue in its oxidation. If the fatty acid contains several double bonds, both the isomerase and a reductase are required to allow the fatty acid to continue through β-oxidation.
Ketogenesis
During periods of starvation, glycogen stores become exhausted and blood glucose falls significantly. To help supply the central nervous system with energy when glucose is in short supply, the liver generates ketone bodies via a process in the mitochondrial matrix known as ketogenesis. These ketone bodies include acetone, acetoacetate, and β-hydroxybutyrate which can be converted back to acetyl-CoA once they arrive at their target organ and enter the Krebs cycle. In some circumstances, ketogenesis can take place when adequate glucose is present in the blood, but cannot enter the cell. This can occur, for example, when a patient suffering from type I diabetes does not receive an insulin injection for a prolonged period of time. Without insulin, glucose cannot enter cells in order to be used for energy. This can result in diabetic ketoacidosis, which is a potentially life-threatening condition. In this pseudo-starved state, the liver begins to generate ketone bodies in an attempt to provide the body with a source of energy. Patients commonly experience fatigue, confusion, and fruity-scented breath due to the acetone present in their blood. This combination of symptoms has even led to patients being mistakenly classified as intoxicated and arrested for driving under the influence.
Fatty Acid Synthesis
The de novo synthesis of fatty acids is reminiscent of β-oxidation with several noTable exceptions. While fatty acid catabolism occurs in the mitochondrial matrix, anabolism takes place in the cytoplasm. This compartmentalization allows for easier regulation, since the enzymes required for synthesis and breakdown are separated. Much as β-oxidation involved the removal of two-carbon subunits from a fatty acid chain, the synthesis of a fatty acid involves the repeated addition of two-carbon subunits. Rather than building the nascent fatty acid directly with acetyl-CoA, acetyl-CoA is first activated in a carboxylation reaction. The activation is the committed step in fatty acid synthesis and requires the investment of ATP; it is facilitated by acetyl-CoA carboxylase to generate malonyl-CoA.
Fatty acid synthase, a single peptide with multiple catalytic domains, then catalyzes a decarboxylation reaction where malonyl-CoA provides two carbons to the growing fatty acid. This synthesis requires the reducing power of NADPH (whereas catabolism generates NADH) which is generally obtained from the pentose phosphate pathway (see Section 4.6). Once a sixteen-carbon long fatty acid is generated, additional enzymes aid in further modification of the fatty acid (e.g., addition of functional groups and elongation). This process requires no template (nor does glycogen or amino acid synthesis), which means that nothing is “read” to generate the products. This differs from the template-based syntheses of polypeptides (mRNA is “read” to generate an amino acid sequence) and nucleic acids (DNA is “read” to generate DNA during replication and RNA during transcription; for more info on these processes, see Chapter 5). The lack of a specific enzyme (usually due to genetic mutations) can result in certain fatty acids or amino acids being required in the diet, leading to their categorization as essential fatty acids or amino acids.
Chapter 4 Summary
• Enzymes are biological catalysts that increase the rate of a reaction by lowering the activation energy.
• Unfavorable reactions in the cell are performed by coupling them to favorable reactions (such as ATP hydrolysis).
• Enzyme activity can be controlled via covalent modification, proteolytic cleavage, associations, or allosteric regulation.
• Competitive inhibitors bind at the active site of an enzyme, do not affect Vmax, but increase Km.
• Noncompetitive inhibitors bind at an allosteric site of an enzyme, decrease Vmax, but do not change Km.
• Uncompetitive inhibitors bind to the enzyme-substrate complex and reduce both Km and Vmax.
• Mixed-type inhibitors can bind to either the enzyme alone or the enzyme-substrate complex. They generally reduce Vmax, but have variable effects on Km.
• Cellular respiration is the oxidation of carbohydrates, reduction of electron carriers, and generation of ATP.
• Glycolysis occurs in the cytoplasm and generates two pyruvate molecules, two ATP, and two NADH per glucose.
• Under anaerobic conditions, the cell performs fermentation to regenerate NAD+ so that glycolysis can continue.
• The pyruvate dehydrogenase complex (PDC) functions in the mitochondrial matrix, converts pyruvate into acetyl-CoA, and generates an NADH.
• The Krebs cycle in the mitochondrial matrix generates six NADH, two FADH2, and two GTP per glucose.
• The electron transport chain in the inner mitochondrial membrane starts with the oxidation of the electron carriers NADH and FADH2, and ends with the reduction of oxygen and the generation of a proton gradient across the inner mitochondrial membrane.
• ATP synthase in the inner mitochondrial membrane uses the proton gradient to generate ATP (2.5 ATP per NADH from the mitochondrial matrix, 1.5 ATP per NADH from the cytoplasm, and 1.5 ATP per FADH2).
• Both eukaryotes and prokaryotes perform cellular respiration, but prokaryotes use their plasma membrane for the electron transport chain and generate two more ATP per glucose than eukaryotes.
• The pentose phosphate pathway generates ribose-5-phosphate (necessary for nucleotide synthesis) and NADPH (necessary as a reducing agent during anabolic pathways).
• During times of starvation glycogenolysis in the liver produces glucose that can be released into the blood. When the glycogen is depleted, gluconeogenesis can generate glucose from precursors such as pyruvate, lactate, and Krebs cycle intermediates.
• β-oxidation breaks down fatty acids two carbons at a time (liberates acetyl-CoA). Each turn of the cycle generates one NADH and one FADH2. The acetyl-CoA can then go through the Krebs cycle to generate more energy.
• If starvation is prolonged and glucose levels are extremely reduced, the liver can generate ketone bodies for fuel. This also happens during uncontrolled diabetes mellitus.
• Fatty acid, amino acid, and glycogen synthesis are non-template driven processes, unlike protein or nucleic acid synthesis.
CHAPTER 4 FREESTANDING PRACTICE QUESTIONS
1. In eukaryotes, the ultimate yield of ATP from NADH is lower when the NADH is produced by:
A) glycolysis.
B) pyruvate dehydrogenase complex (PDC).
C) Krebs cycle.
D) electron transport and oxidative phosphorylation.
2. Some inhibitors bind irreversibly to enzymes by covalent attachment. Would the kinetics seen under these conditions (V vs. [S] curve) be similar to those seen with a reversible noncompetitive inhibitor?
A) Yes, because it would reduce the Km of the reaction.
B) Yes, because the net effect would be a loss of active enzyme available for the reaction.
C) No, because if enough substrate binds to the active site the reaction will reach Vmax.
D) No, because Km will increase and Vmax will stay the same, similar to competitive inhibition.
3. Some enzymes can modify their substrate, and by this means, regulate its activity. In many instances, these modifications are not permanent since other enzymes can reverse them. Which of the following category of enzymes will irreversibly modify their substrate?
A) A kinase
B) A protease
C) A phosphatase
D) An acetylase
4. Salicylic acid (aspirin) if taken in excess may act as an uncoupling agent. Uncoupling agents increase the permeability of the inner mitochondrial membrane, resulting in the dissipation of the proton gradient. Which of the following would most likely be true in the presence of an uncoupling agent?
A) Electron transport at the inner mitochondrial membrane would cease.
B) The energy from the proton-motive force would likely be dissipated as heat rather than in producing ATP from ADP.
C) H+ ions would flow through the inner membrane into the intermembrane space.
D) There would be an increase in biosynthesis.
5. For a given enzyme concentration at a low substrate concentration, how does reaction rate change as the substrate concentration increases?
A) Logarithmically
B) Linearly
C) Exponentially
D) Indirectly
6. Glycogen metabolism is regulated by different hormones that activate reciprocal pathways. After several hours of fasting, a person eats a large meal. Which of the following statements is correct?
A) Elevated glucagon levels deactivate glycogen phosphorylase and activate glycogen synthase.
B) Elevated insulin levels deactivate glycogen phosphorylase and activate glycogen synthase.
C) Elevated glucagon levels activate glycogen phosphorylase and deactivate glycogen synthase.
D) Elevated insulin levels activate glycogen phosphorylase and deactivate glycogen synthase.
7. Which of the following is a product of the pentose phosphate pathway?
A) NADH
B) NADPH
C) Succinyl-CoA
D) Fructose-6-phosphate
CHAPTER 4 PRACTICE PASSAGE
The human body has one main goal during a fasted state: to supply the body with energy. Since the main source of energy for the body is glucose, there is an initial activation in biochemical processes that lead to the production of glucose. Among the major pathways activated are glycogenolysis and gluconeogenesis, both of which occur in the liver. If the starved state is not overcome, these pathways become depleted and insufficient, forcing the body to increase the breakdown of protein and begin relying on an additional energy source—ketone bodies.
Protein catabolism involves the breakdown of amino acids, many of which can serve as gluconeogenic substrates. In order to make use of the carbon skeleton of an amino acid, the amino group must be removed. If this newly liberated free ammonia is allowed to accumulate in the body, it can be highly toxic. To combat this, the urea cycle consumes ammonia to produce urea, which is considerably less toxic. The urea produced can subsequently be excreted by the kidneys. The urea cycle, which occurs primarily in the hepatocytes, is outlined in Figure 1.
Ketogenesis, the production of ketone bodies, also occurs principally in the liver. Ketone bodies are generated from acetyl-CoA molecules, which are largely supplied by the breakdown of fatty acids via b-oxidation. One of the main ketone bodies produced is acetoacetate, generated by the enzyme thiolase via the condensation of two acetyl-CoA molecules. Acetoacetate is then spontaneously decarboxylated in a nonenzymatic reaction, forming acetone. Acetone is the source of sweetness on the breath of a patient with Diabetes Mellitus Type I in a state of ketogenesis. More important than the sweet breath, these patients also experience hyperventilation (secondary to the acidosis caused by ketogenesis).
Figure 1 The Urea Cycle
(CPS = carbamoyl phosphate synthase,
OTC = ornithine transcarbamoylase,
AS = argininosuccinate synthetase,
AL = argininosuccinate lyase)
1. According to Figure 1, the enzyme ornithine transcarbamoylase (OTC) condenses carbamoyl phosphate and ornithine into citrulline. Which of the following would be true in a patient with OTC deficiency?
A) Decreased urea in the blood and decreased ammonia in the blood
B) Decreased urea in the urine and increased ammonia in the blood
C) Increased urea in the urine and increased ammonia in the blood
D) Increased urea in the blood and decreased ammonia in the urine
2. Which of the following is true in a starved state?
A) Decreased lipolysis
B) Deactivation of ketone body production
C) Increased glycolysis
D) Increased protein catabolism
3. The urea cycle is located in both the cytosol and mitochondria. Which of the following correctly matches the biochemical pathway to its location?
I. Krebs cycle — mitochondrial matrix
II. Glycolysis — cytosol
III. Electron transport chain — outer mitochondrial membrane
A) I only
B) I and II only
C) I and III only
D) II and III
4. Which of the following describes a potential method of how muscle may be able to use ketone bodies as energy?
A) Ketone bodies create a state of intracellular acidosis in the muscle cells, which is vital to the production of ATP.
B) Ketone bodies stimulate the activation of glycogenolysis in muscle cells, allowing the production of acetyl-CoA.
C) The ketone bodies are cleaved into acetyl-CoA and utilized in the Krebs cycle.
D) Gluconeogenesis uses ketone bodies to generate glucose.
5. Glucagon is an example of a hormone active in a fasted state. Which of the following glands release glucagon?
A) Adrenal cortex
B) Posterior pituitary
C) Pancreas
D) Adrenal medulla
6. The passage states that the hyperventilation in a diabetic patient is due to acidosis. Which of the following explains why acidosis triggers hyperventilation?
A) The patient is able to take in a greater amount of oxygen with hyperventilation.
B) Hyperventilation allows increased expiration of carbon dioxide.
C) The increased contractions of respiratory muscles used during hyperventilation decreases the amount of acid produced in the body.
D) Hyperventilation has no effect on acid-base homeostasis and is only used as a clinical sign.
7. Which of the following enzymatic activities would occur in a liver cell during a fasted state?
A) Increased thiolase activity and increased hexokinase activity
B) Decreased thiolase activity and increased hexokinase activity
C) Increased thiolase activity and decreased hexokinase activity
D) Decreased thiolase activity and decreased hexokinase activity
SOLUTIONS TO CHAPTER 4 FREESTANDING PRACTICE QUESTIONS
1. A In order for the NADH produced during glycolysis to be utilized by the electron transport chain (ETC) in ATP formation, the electrons must be shuttled into the mitochondria (remember, glycolysis occurs in the cytosol and the ETC occurs along the inner mitochondrial membrane). When shuttled in, the electrons are typically used to reduce Coenzyme Q, and bypass NADH dehydrogenase. This results in the pumping of fewer protons out of the mitochondrial matrix, and thus, ultimately, in fewer ATP being formed. The NADH produced by the PDC or Krebs cycle is energetically equivalent because both occur in the mitochondrial matrix. Thus, this NADH is immediately accessible to the ETC (choices B and C are wrong). Finally, note that the ETC regenerates NAD+ rather than producing NADH (choice D is wrong).
2. B An irreversible inhibitor (regardless of where it binds) will permanently deactivate some enzyme, reducing effective enzyme concentration. If the enzyme concentration is effectively lowered, Vmax will be reduced (choices C and D are wrong). This is similar to what is seen in noncompetitive inhibition, where the inhibitor binds to an allosteric site and turns the enzyme off. Even if the noncompetitive inhibitor is reversible, because at any given time some enzyme is “off,” the effective enzyme concentration is lowered and Vmax is reduced. If Km were affected, it would increase, not decrease; an increase in Km indicates that the substrate-enzyme interaction has been compromised in some way (choice A is wrong).
3. B Proteases are enzymes that cleave their substrates at specific sites, permanently removing a part of the protein. This modification is practically irreversible—there are no enzymes that can reconnect proteins split by a protease. Answers A, C, and D are wrong because these categories of enzymes can reversibly modify their substrates. A kinase adds a phosphate to its substrate, but this modification can be reversed by a phosphatase that will remove the phosphate. An acetylase will add an acetyl group, while a deacetylase will remove it.
4. B The proton gradient obtained by the electron transport chain (which pumps H+ ions across the inner membrane into the intermembrane space) is necessary in order to create ATP at the ATP synthase. This enzyme allows the protons to move through its channel back into the matrix, and thus harnesses the energy created by the gradient to create ATP from ADP + Pi. If the inner membrane was made more permeable by uncoupling agents, then the H+ ions would naturally move down their gradient, from the intermembrane space back to the mitochondrial matrix (choice C is wrong). This unharnessed energy would be dissipated as heat (known as non-exercise activity thermogenesis [NEAT]), and since the ions would not pass through the ATP synthase, less ATP would be created (choice B is correct). Although the utility of the electron transport chain would be compromised, the uncoupling agent would not inhibit electron transport itself (choice A is wrong); in fact this would lead to the rapid oxidation of Krebs cycle substrates and would promote the mobilization of carbohydrates and fats. Since the energy is lost as heat, biosynthesis is not promoted (choice D is wrong), and weight loss can be dramatic. Experimental uncoupling agents have been used in the past as effective diet pills; however, their use is very dangerous and thankfully this practice has fallen out of use.
5. B At low substrate concentrations, the reaction rate increases linearly as the substrate concentration increases (see curve below). At or near saturation levels, the reaction rate begins to level off and does not change regardless of how much substrate it added. This is called the maximum velocity of reaction rate or Vmax.
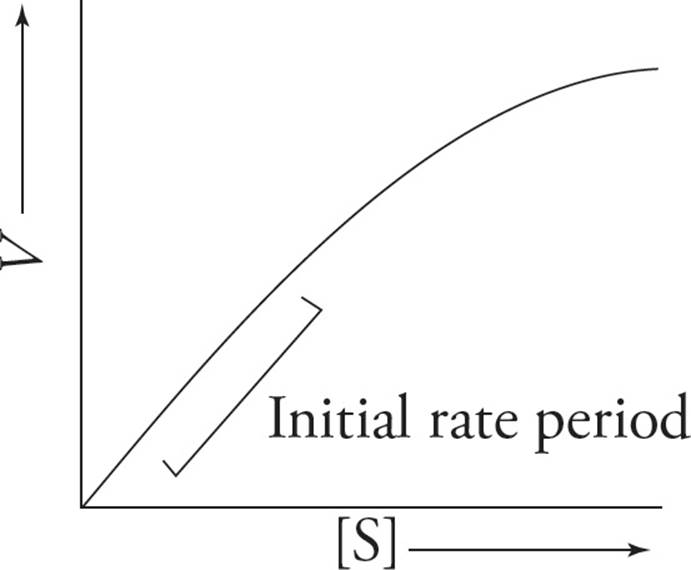
6. B Insulin levels are elevated when blood glucose levels rise, such as after consuming a large meal. Glucagon levels are elevated during fasting (choices A and C can be eliminated). Insulin allows cells to take up glucose and activates the pathways that store it as glycogen (as well as those that convert it into energy). Therefore, high insulin levels would lead to activation of the enzyme that synthesizes glycogen, glycogen synthase and deactivation of the enzyme that breaks glycogen down, glycogen phosphorylase (choice D can be eliminated and choice B is correct).
7. B The pentose phosphate pathway produces ribose, which acts as a backbone for nucleotide synthesis, as well as NADPH (choice B is correct). NADH is made in catabolic reactions, such as glycolysis and the Krebs cycle (choice A is wrong). Succinyl-CoA is an intermediate product of the Krebs cycle, not the pentose phosphate pathway (choice C is wrong). Fructose-1-phosphate is one of the intermediate products of glycolysis (choice D is wrong).
SOLUTIONS TO CHAPTER 4 PRACTICE PASSAGE
1. B According to the passage, the urea cycle rids the body of toxic ammonia and produces urea. OTC is required to run the urea cycle, so an OTC deficiency would lead to a decrease in urea (both blood and urine, choices C and D are wrong) and an increase in free ammonia in the blood (choice B is correct and choice A is wrong).
2. D Protein catabolism, as mentioned in the passage, is activated in a starved state to allow generation of glucose via gluconeogenesis using the carbon skeletons of certain amino acids (choice D is correct). Lipolysis (the breakdown of fat) and ketogenesis (formation of ketone bodies) are also activated when the body is in need of additional energy (choices A and B are wrong). To prevent a further fall in blood glucose levels, sugar breakdown via glycolysis is inhibited (choice C is wrong).
3. B Item I is true: The Krebs cycle occurs in the matrix of the mitochondria (choice D can be eliminated). Item II is true: glycolysis occurs in the cytosol of cells (choices A and C can be eliminated and choice B is correct). Note that Item III is false: the enzymes of the electron transport chain are found in the inner mitochondrial membrane not the outer.
4. C The passage explains that ketone bodies are generated from acetyl-CoA obtained from metabolic pathways such as fatty acid metabolism. It is important to recall that acetyl-CoA is a substrate for the Krebs cycle, which would allow the muscle cells to obtain ATP. The logical and correct answer would be that the ketone bodies are split into their acetyl-CoA subunits and utilized in the Krebs cycle by the muscle cells (choice C is correct). Several of the ketone bodies produced during ketogenesis are acidic molecules, however acidosis does not activate energy production in muscle cells. In fact, lactic acid is produced by muscle cells as a byproduct of ATP production during anaerobic fermentation, not as a stimulator of ATP production (choice A is wrong). Glycogen stores are usually depleted by the time ketone bodies are used as source of energy as mentioned in the first paragraph (choice B is wrong). Ketone bodies cannot be used in gluconeogenesis. If this were the case, the body would use them as gluconeogenic substrates rather than relying on them as a source of energy and risking acidosis (choice D is wrong).
5. C Glucagon is produced in the alpha cells found in the Islets of Langerhans of the pancreas (choice C is correct). The adrenal cortex produces three important steroid hormones: aldosterone, cortisol and sex steroid (choice A is wrong). The posterior pituitary only releases two peptides: ADH and oxytocin (choice B is wrong). The adrenal medulla produces catecholamines: epinephrine and norepinephrine (choice D is wrong).
6. B Acid-base shifts in the body stimulate responses from both the lungs and kidneys in order to maintain pH homeostasis. Acidosis triggers an increase in ventilation, resulting in increased carbon dioxide expiration. This shifts the equilibrium of the respiratory equation to the left, pulling free H+ ions out of the blood and increasing the pH:
CO2 + H2O ![]() H2CO3
H2CO3 ![]() H+ + HCO3–
H+ + HCO3–
(choice B is correct and D is wrong). Oxygen has no effect on neutralization of acids in the body during ketoacidosis (choice A is wrong). Increased use of respiratory muscles would not alleviate the acidosis and in extreme circumstances may result in the production of lactic acid which could exacerbate the acidotic state (choice C is wrong).
7. C In the third paragraph, thiolase is described as one of the important enzymes of ketogenesis, which is activated in the fasting state (choices B and D can be eliminated). Hexokinase is the important first enzyme of glycolysis, however glycolysis would be down-regulated in a fasted state, since there is a preference for creating glucose in liver cells rather than breaking it up (choice A can be eliminated and choice C is correct).
1 ATP, which stores energy in the ester bonds between its phosphate groups.
2 If ∆S is negative, then the system lost entropy, which means that disorder decreased.
3 As in ∆S, the Greek letter ∆ (delta) indicates “the change in.” For example, ∆Grxn = Gproducts – Greactants.
4 H ≈ E, since the change in volume is negligible (∆V ≈ 0).
5 Favorable reactions have ∆G < 0. We can deduce this from the second law and Equation 1 because the second law states that increasing entropy is favorable, and the equation has ∆G directly related to –T∆S.
6 Yes. If ∆S > 0 and ∆H = 0, then according to the second law of thermodynamics, the reaction is spontaneous; see Equation 1.
7 Equation 3 says that ∆G°′ = 0 when K′eq = 1 since ln 1 = 0. Note: for more information about MCAT Math, see MCAT Physics and Math Review.
8 No. You need to know the concentrations of A, B, C, and D in the human cell. For example ∆G°′ might be +14.8 kcal/mol, indicating that the reaction is very unfavorable under standard conditions. But, if the concentration of reactants is much higher than the concentration of products in the cell, the reaction may be favorable in the cell since Q < 1 and ∆G may be less than zero. (Although ∆G could still be positive if ∆G°′ is very large.) The significance of Q as an independent variable in Equation 4 is that it accounts for Le Châtelier’s principle: A high concentration of reactants will drive a reaction forward and a high concentration of products will drive it backward, regardless of the intrinsic thermodynamics (∆G°′) of the reaction.
9 The reaction may be favorable (∆G < 0) if the ratio of the concentrations of reactants to products is sufficiently large to drive the reaction forward (that is, if RT ln Q is more negative than ∆G°′ is positive, which would make their sum (which, by Equation 4, is ∆G) negative).
10 Keq indicates only the relative concentrations of reagents once equilibrium is reached, not the reaction rate (how fast equilibrium is reached).
11 A large Keq means that more products are present at equilibrium. Remember that equilibrium tends towards the lowest energy state. Hence, when Keq is large, products have lower free energy than reactants.
12 The size of Q says nothing about the properties of the reactants and products. Q is calculated from whatever the initial concentrations happen to be. It is Keq that says something about the nature of reactants and products, since it describes their concentrations after equilibrium has been reached.
13 If ∆G is 0, then neither the forward nor the reverse reaction is favored. Look at Equations 3 and 4. Note that when ∆G = 0, Equation 4 reduces to Equation 3, and thus Q = Keq (which means Q at this moment is the same as Keq, measured after the reaction system is allowed to reach equilibrium). When Q = Keq, we are by definition at equilibrium. Understand and memorize the following: When ∆G = 0, you are at equilibrium; forward reaction equals back reaction, and the net concentrations of reactants and products do not change.
14 The reaction is in dynamic equilibrium where reactions are occurring in both directions, but at an equal rate. Because ∆G = 0, we know that the forward reaction and the reverse reaction proceed at equal rates, even though we don’t know the actual value. Therefore, after a period of time, the radiolabel will be present in both A and B.
15 The ∆G is the same in both cases. ∆G does not depend on the pathway, only on the different energies of the reactants and products.
16 The rate would be increased, since lowering Ea is tantamount to reducing the energy required to achieve the transition state. The more transition state intermediates that are formed, the greater the amount of product produced, i.e., the more rapid the rate of reaction.
17 No. It will only affect the rate at which the reactants and products reach equilibrium.
18 ATP hydrolysis!
19 Q(cell) ≠ Keq. This means that the relative concentrations of ATP and ADP + Pi are not at equilibrium levels in the cell. Actually, Q(cell) << Keq because the cell keeps a high concentration of ATP around.
20 quaternary
21 Globular. Structural proteins such as collagen tend to be fibrous, but proteins that act as catalysts tend to be roughly spherical to form an active site in a cleft in the sphere.
22 A positive charge would stabilize the negative charge in the intermediate. Such a charge might be contributed by His, Arg, or Lys. Alternatively, the hydrogen of the –NH2 group in glutamine or asparagine could hydrogen bond with the negative charge.
23 Yes, the amino acids at the active site may be distant from each other in a polypeptide’s primary sequence but be near each other in the final folded protein. This is why protein folding is crucial for enzyme function.
24 It must occur at a particular serine residue which sticks out into the active site.
25 You don’t need to calculate ∆G°′; all you need to know is that with a Keq of 1000, there will be 1000 times more B than A in solution at equilibrium. If we create a solution with only A, the reaction must move spontaneously toward B.
26 If only B exists in solution, then the back-reaction producing A will predominate until equilibrium is reached (ΔG = 0), regardless of the presence or absence of enzyme (choice D is correct and choice B is wrong). According to the Keq given, at equilibrium there will be 1000 times more B than A, not the other way around (choice A is wrong). Note that enzymes do not act on the transition state, they act to produce the transition state (choice C is wrong).
27 L amino acids and D sugars. Remember the L in aLanine.
28 Beware of long, complex-sounding questions! They may not be as bad as they look; for instance, the phrase “assuming that the reactants are present in excess compared to the enzyme” adds nothing to the substance of this question. If His (which is positive or neutral at pH 7) is replaced by Glu (negatively charged at pH 7), this could decrease the effectiveness—or destroy altogether—the active site of the enzyme. This means the transition state would not be effectively stabilized, and the rate of the reaction would simply reduce to that of the uncatalyzed reaction (choice C is correct). The rate would not proceed more slowly than the uncatalyzed reaction (i.e., “in solution without enzyme,” choice A is wrong), and remember that enzymes do not alter reaction equilibria (Keq will be unaffected, choices B and D are wrong).
29 Usually the concentration of enzyme is kept fixed, and [S] is taken as the only independent variable (the one the rate depends on). This is applicable to biological systems, where substrate concentrations change much more than enzyme concentrations.
30 If the enzyme is acting at Vmax, it is saturated with substrate; adding more substrate will not increase the reaction rate; the rate is still Vmax.
31 Imagine a group of people who can’t get any dates. They are all depressed about it, and they keep each other depressed, which makes it even less likely that any will get a date. They are tense, “turned off,” and inactive. Then one of the depressed group gets a date and gets so excited about it that all the other friends in the group get so enthusiastic that they get dates too. They are “turned on,” relaxed, hip, groovy, and active.
32 Saturation, just as in the case of a noncooperative enzyme.
33 Cooperativity is a special kind of allosteric interaction. One active site acts like an allosteric regulatory site for the other active sites. Secondly, cooperative enzyme complexes are often allosterically regulated also. Hb is an excellent example. Not only does O2 binding to one subunit increase the other subunits’ affinities, but also several other molecules can bind to various sites to change the affinity of the complex. For example, CO2 stabilizes tense Hb, causing each of the four binding sites to have a lower affinity for oxygen. As a result, in the presence of CO2, Hb tends to give up whatever O2 it has bound. The most important thing to remember, though, is that the binding in cooperativity takes place at the active site, while the binding in allosteric regulation takes place at “other sites.”
34 Structurally, competitive inhibitors must at least resemble the substrate; however, the most effective competitive inhibitors resemble the transition state which the active site normally stabilizes.
35 No. The rate increase is greater than linear, indicating that the effect is caused by cooperativity.
36 Item I: False. The question states that CO can be displaced by oxygen. Item II: True. The question states that it dissociates easily. Item III: True. The question states it binds allosterically, which means “at another site” (not the active site).
37 Since Curve 3 and Curve 1 have the same Vmax, but Curve 3 has a reduced rate of product formation, it suggests that Curve 3 represents competitive inhibition of the enzyme in Curve 1 (choice D is correct). If Curve 3 represented noncompetitive inhibition, its Vmax would be reduced compared to Curve 1 (choice A is wrong), and in no case would an inhibitor have a higher Vmax than an uninhibited reaction (choice B is wrong). Lastly, it can be seen on the graph that Curve 2 has a reduced Vmax compared to Curve 3 (choice C is wrong).
38 It’s an oxidation, because hydrogens have been removed.
39 It’s a reduction, because an electron has been added.
40 It’s a reduction, because bonds to oxygen have been replaced by bonds to hydrogen.
41 The mnemonics are cata = breakdown, as in catastrophe, and ana = buildup, sounds like “add-a.” (Think of anabolic steroids, which weightlifters use to bulk up.)
42 The carbons in the sugar are oxidized (to CO2), and oxygen is reduced (to H2O).
43 The text states that glucose is split in half in the formation of pyruvate. Since glucose has six carbons, pyruvate must have three.
44 Although glycolysis results in a net ATP production, ATP is initially required to drive the reaction forward in the phosphorylation of glucose to glucose-6-phosphate and the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate. Without ATP to “prime the pump,” there is no way to start the pathway. In case you’re wondering about the Mg2+, it’s necessary for all reactions involving ATP.
45 When energy (ATP) is abundant, the cell should slow glycolysis. High concentrations of ATP inhibit PFK activity by binding to an allosteric regulatory site. It is interesting to note that since ATP is a reactant in the reaction catalyzed by PFK, you would expect a high concentration of ATP to increase the rate of the reaction (Le Châtelier’s principle). However the inhibitory allosteric effects of ATP on PFK outweigh this thermodynamic consideration. So lowering the concentration of ATP will increase the reaction rate, even though ATP is a reactant. Of course, if the ATP level went too low, the reaction could not proceed at all.
46 If NAD+ has all been converted to NADH, then the step in glycolysis that produces NADH (catalyzed by glyceraldehyde 3-phosphate dehydrogenase) cannot occur because it requires NAD+ as a substrate. Thus, a lack of NAD+ will inhibit glycolysis.
47 The lactate is exported from the muscle cell to the liver. When oxygen becomes available, the liver cell will convert the lactate back to pyruvate, while making NADH from NAD+. Then the liver will utilize this excess NADH to make ATP in oxidative phosphorylation. This pyruvate can enter gluconeogenesis or the Krebs cycle in the liver, or it can be sent back to the muscle. (This cycle, whereby the liver deals with lactate from muscle, is known as the Cori Cycle.)
48 Pyruvate is converted to a 2-carbon molecule, CO2 is given off, and NADH is made from NAD+. You can Figure all of this out based on your knowledge of oxidative decarboxylation. Also, note the name of the enzyme, “dehydrogenase.” To remove a hydrogen (dehydrogenate) is to oxidize. So the name of the enzyme also tells us that pyruvate is oxidized.
49 A high ratio of AMP or ADP to ATP is described as low-energy charge. A low-energy charge will stimulate the PDC, increasing the rate of entry of pyruvate into the Krebs cycle.
50 Simply because intermediates are passed directly from active site to active site, without having to diffuse.
51 Thiamine deficiency would effectively shut down both the PDC and the Krebs cycle (choice D is wrong), since both of these processes require thiamine in their TPP prosthetic group. In the absence of PDC and Krebs, the amount of NADH and FADH2 provided to the electron transport chain would be reduced, and ATP production would fall (choice C is wrong). In order to compensate and maintain ATP levels as close to normal as possible, the rate of glycolysis would increase (choice A is correct). Note that this would not happen anerobically, as conditions are not anaerobic (choice B is wrong).
52 None, since none of the six carbons has four unique substituents.
53 In CO2. Pyruvate’s most oxidized carbon is a carboxylic acid which is removed by the PDC.
54 None. CoA assists in catalysis, which means that it is not consumed in the reaction, but regenerated as CoA-SH.
55 A quinone is a particular type of aromatic molecule, and the prefix “ubi” indicates that this molecule is ubiquitous, i.e., present in all cells.
56 If it’s the last member of the chain, it must pass its reducing power to O2, reducing it to H2O, an end product of electron transport. This is the only reason we breathe and the only reason we evolved with lungs, RBCs, etc.
57 higher (remember, high pH = low [H+])
58 If the proton gradient is destroyed, the processes in A, B, and C will continue unabated, because NADH will be reoxidized to NAD+ at a normal rate, or perhaps faster than normal. The problem will be that without a proton gradient no ATP will get made from all this glucose breakdown. The answer is D because this will be the first problem encountered from running out of ATP.
59 A, C, and D are all thermodynamically favorable processes that occur spontaneously without any external energy input. Thus, all of these processes have a negative ∆G. However, the large positive ∆G of the process in B makes undoing it favorable enough for it to power ATP synthesis. Creation of the proton gradient is dependent upon the very negative ∆G of electron transport.
60 If the active site of cytochrome C oxidase is occupied with cyanide, then oxygen cannot bind there to be reduced to water; in other words, cytochrome C oxidase will be unable to get rid of its electrons and will remain reduced. Therefore it will be unable to accept electrons from cytochrome C, which will be unable to accept electrons from cytochrome C reductase, which will be unable to accept electrons from coenzyme Q, etc., etc., all the way back up the electron transport chain. The end result will be a cessation of all electron transport chain activity (choice C is correct). Note that protons, not electrons, are pumped against their gradient (choice A is wrong), and this will stop completely, not just be slowed down (choice B is wrong). Also, NAD+ is reduced to NADH in the Krebs cycle, not the other way around (choice D is wrong).
61 Only 1.5 ATP are made as a result of the reoxidation of FADH2. Six protons divided by four protons per ATP equals 1.5 ATP.
62 Some high energy-requiring tissues (such as liver and cardiac muscle cells) utilize a different shuttle (the malate-aspartate shuttle) to bring the electrons to NADH hydrogenase, thus getting the full 2.5 ATP from those electrons. But this is the exception, and generally the MCAT does not test exceptions.