5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 4 Review the Knowledge You Need to Score High
11 Solutions
IN THIS CHAPTER
In this chapter, the following AP topics are covered:
1.4 Composition of Mixtures
3.7 Solutions and Mixtures
3.9 Separation of Solutions and Mixtures Chromatography
3.10 Solubility
4.3 Representations of Reactions
Summary: A solution is a homogeneous mixture composed of a solvent and one or more solutes. The solvent is the substance that acts as the dissolving medium and is normally present in the greatest amount. Commonly the solvent is a liquid, but it doesn’t have to be. Our atmosphere is a solution with nitrogen as the solvent; it is the gas present in the largest amount (79%). Steel is a solution where iron is the solvent. Many times you will be dealing with a solution in which water is the solvent, an aqueous solution. The solute is the substance that the solvent dissolves and is normally present in the smaller amount. You may have more than one solute in a solution. For example, if you dissolved table salt (sodium chloride) and table sugar (sucrose) in water, you would have one solvent (water) and two solutes (sodium chloride and sucrose).
Some substances will dissolve in a particular solvent and others will not. There is a general rule in chemistry that states that “like dissolves like.” This general statement may serve as an answer in the multiple-choice questions; however, this simplified approach does not serve as an explanation in the free-response questions. This simply means that polar substances (salts, alcohols, etc.) will dissolve in polar solvents such as water, and nonpolar solutes, such as iodine, will dissolve in nonpolar solvents such as carbon tetrachloride. The solubility of a specific solute is normally expressed in terms of grams solute per 100 mL of solvent (g/mL) at a specified temperature. The temperature must be specified because the solubility of a substance will vary with the temperature. Normally, the solubility of solids dissolving in liquids increases with increasing temperature, while the reverse is true for gases dissolving in liquids. Pressure is important when gases are involved.
A solution in which one has dissolved the maximum amount of solute per given amount of solvent at a given temperature is called a saturated solution.
An unsaturated solution has less than the maximum amount of solute dissolved. Sometimes if the temperature, purity of the solute and solvent, and other factors are just right, you might be able to dissolve more than the maximum amount of solute, resulting in a supersaturated solution. Supersaturated solutions are unstable, and sooner or later separation of the excess solute will occur, until a saturated solution and separated solute remain.
The formation of a solution depends on many factors, such as the nature of the solvent, the nature of the solute, the temperature, and the pressure. Some of these factors were addressed in the Reactions and Periodicity chapter. In general, the solubility of a solid or liquid will increase with temperature and be unaffected by pressure changes. The solubility of a gas will decrease with increasing temperature and will increase with increasing partial pressure of the gas (Henry’s law).
Solutions will also be important in the next several chapters of this book. This is especially true of Chapter 15, Equilibrium, where many of the equilibrium constants apply to aqueous solutions.

Keywords and Equations
molarity, M = moles solute per liter solution molality
Concentration Units
There are many ways of expressing the relative amounts of solute(s) and solvent in a solution. The terms saturated, unsaturated, and supersaturated give a qualitative measure, as do the terms dilute and concentrated. The term dilute refers to a solution that has a relatively small amount of solute in comparison to the amount of solvent. Concentrated, on the other hand, refers to a solution that has a relatively large amount of solute in comparison to the solvent. However, these terms are very subjective. If you dissolve 0.1 g of sucrose per liter of water, that solution would probably be considered dilute; 100 g of sucrose per liter would probably be considered concentrated. But what about 25 g per liter—dilute or concentrated? In order to communicate effectively, chemists use quantitative ways of expressing the concentration of solutions. Several concentration units are useful, including percentage, molarity, and molality; however, only molarity appears on the AP Chemistry Exam.

Percentage
One common way of expressing the relative amount of solute and solvent is through percentage, amount per hundred. Percentage can be expressed in three ways:
• mass percent
• mass/volume percent
• volume/volume percent
Mass (Sometimes Called Weight) Percentage
The mass percentage of a solution is the mass of the solute divided by the mass of the solution, multiplied by 100% to get percentage. The mass is commonly measured in grams.
![]()
For example, a solution is prepared by dissolving 25.2 g of sodium chloride in 250.0 g of water. Calculate the mass percent of the solution.
Answer:
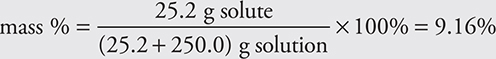
A common error is forgetting to add the solute and solvent masses together in the denominator.
When solutions of this type are prepared, the solute and solvent are weighed out separately and then mixed to form a solution. The final volume of the solution is unknown.
Mass/volume and volume/volume percentage are done in much the same fashion.
Molarity
Percentage concentration is common in everyday life (3% hydrogen peroxide, 5% acetic acid, commonly called vinegar, etc.), but the concentration unit most commonly used by chemists is molarity. Molarity (M) is the number of moles of solute per liter of solution:
M = moles solute/liter solution
In preparing a molar solution, the correct number of moles of solute (commonly converted to grams using the molar mass) is dissolved and diluted to the required volume.
Determine the molarity of ethanol, C2H5OH, in a solution produced by dissolving 25.0 g of this compound in enough water to produce 500.0 mL of solution:
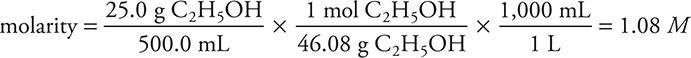

The most common error is not being careful with the units. Grams must be converted to moles, and milliliters must be converted to liters.
Another way to prepare a molar solution is by dilution of a more concentrated solution to a more dilute one by adding solvent. The following equation can be used:
![]()
In the preceding equation, before refers to before dilution and after refers to after dilution. Let’s see how to apply this relationship. Determine the final concentration when 300.0 mL of water is added to 200.0 mL of a 0.1250 M solution of HCl. Assume the volumes are additive.
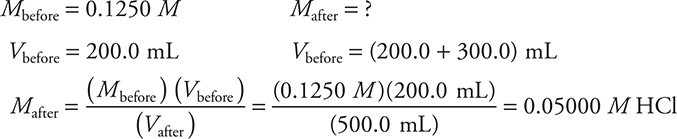
The most common error is forgetting to add the two volumes.

Molality
Sometimes the varying volumes of a solution’s liquid component(s) due to changes in temperature present a problem. Many times volumes are not additive, but mass is additive. The chemist then resorts to defining concentration in terms of the molality. Molality (m) is defined as the moles of solute per kilogram of solvent:
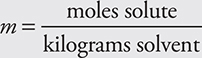
Notice that this equation uses kilograms of solvent, not solution. The other concentration units use mass or volume of the entire solution. Molal solutions use only the mass of the solvent. For dilute aqueous solutions, the molarity and the molality will be close to the same numerical value.
Molality is important when determining the efficiency of antifreeze.
For example, methanol, CH3OH, was used in antifreeze at one time. Determine the molality of methanol in a solution prepared by adding 25.00 g of methanol to 150.0 g of water.
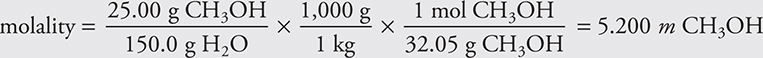
The most common error is to use the total grams in the denominator instead of just the grams of solvent.
Electrolytes and Nonelectrolytes
An electrolyte is a substance that, when dissolved in a solvent or melted, conducts an electrical current. A nonelectrolyte does not conduct a current when dissolved. The conduction of the electrical current is often determined using a light bulb connected to a power source and two electrodes. The electrodes are placed in the solution or melt, and if a conducting medium is present, such as ions, the light bulb will light, indicating the substance is an electrolyte.
The ions that conduct the electrical current can result from a couple of sources. They may result from the dissociation of a soluble ionically bonded substance (a salt). If sodium chloride (NaCl) is dissolved in water, it dissolves into sodium cations (Na+) and chloride anions (Cl—). But certain covalently bonded substances may also produce ions if dissolved in water, a process called ionization. For example, acids, both inorganic and organic, will produce ions when dissolved in water. Some acids, such as hydrochloric acid (HCl), will completely ionize. Other acids, such as acetic acid (CH3COOH), will only partially ionize. They establish an equilibrium with the ions and unionized species (see Chapter 15 for more on chemical equilibrium). Solutions of electrolytes (strong or weak) have strong ion—dipole forces.
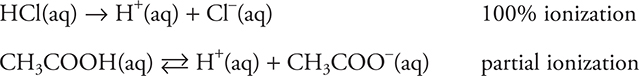
Species such as HCl that completely ionize in water are called strong electrolytes, and those that only partially ionize are called weak electrolytes. Most soluble salts also fall into the strong electrolyte category.

Colligative Properties
Some of the properties of solutions depend on the chemical and physical nature of the individual solute. The blue color of a copper(II) sulfate solution and the sweetness of a sucrose solution are related to the properties of those solutes. However, some solution properties simply depend on the number of solute particles, not the type of solute. These properties are called colligative properties and include:
• Vapor pressure lowering
• Freezing-point depression
• Boiling-point elevation
• Osmotic pressure
Vapor Pressure Lowering
If a liquid is placed in a sealed container, molecules will evaporate from the surface of the liquid and eventually establish a gas phase over the liquid that is in equilibrium with the liquid phase. The pressure generated by this gas is the vapor pressure of the liquid. Vapor pressure is temperature dependent; the higher the temperature, the higher the vapor pressure. If the liquid is made a solvent by adding a nonvolatile solute, the vapor pressure of the resulting solution is always less than that of the pure liquid. The vapor pressure has been lowered by the addition of the solute; the amount of lowering is proportional to the number of solute particles added and is thus a colligative property. When the vapor pressure equals the external pressure, the liquid or solution will boil.
Solute particles are evenly distributed throughout a solution, even at the surface. Thus, there are fewer solvent particles at the gas—liquid interface where evaporation takes place. Fewer solvent particles escape into the gas phase, and so the vapor pressure is lower. The higher the concentration of solute particles, the less solvent at the interface and the lower the vapor pressure. This relationship is referred to as Raoult’s law.
Freezing-Point Depression
The freezing point of a solution of a nonvolatile solute is always lower than the freezing point of the pure solvent. It is the number of solute particles that determines the amount of the lowering of the freezing point. The amount of lowering of the freezing point is proportional to the molality of the solute and is given by the equation:
ΔTf = iKf molality
where ΔTf is the number of degrees that the freezing point has been lowered (the difference in the freezing point of the pure solvent and the solution); Kf is the freezing-point depression constant (a constant of the solvent); the molality is the molality of the solute; and i is the van ’t Hoff factor—the ratio of the number of moles of particles released into solution per mole of solute dissolved. For a nonelectrolyte, such as ethylene glycol (antifreeze), the van ’t Hoff factor would be 1. For a strong electrolyte, such as sodium chloride, you must take into consideration that if 1 mol of NaCl dissolves, 2 mol of particles would result (1 mol Na+, 1 mol Cl—). Therefore, the van ’t Hoff factor should be 2. Let’s learn to apply the preceding equation. Determine the freezing point of an aqueous solution containing 20.50 g of calcium chloride in 250.0 g of water. The value of Kf for aqueous solutions is 1.86 K kg mol-1. In northern states, calcium chloride is sometimes used to de-ice roads and sidewalks.
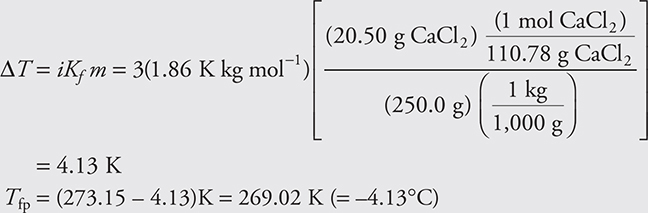
The most common mistake is to forget to subtract the ΔT value from the normal freezing point.
The freezing-point depression technique is also used to calculate the molar mass of a solute.
For example, a solution is prepared by dissolving 0.490 g of an unknown compound in 50.00 mL of water. The freezing point of the solution is —0.201°C. Assuming the compound is a nonelectrolyte, what is the molecular mass of the compound? Use 1.00 g/mL as the density of water.
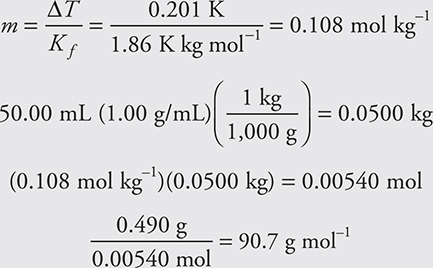
Many students make the mistake of stopping before they complete this problem. If you get an answer less than 1 g mol—1, you have made an error (that is, it is impossible to get a molar mass less than that of a hydrogen ion, H+).
Boiling-Point Elevation
Just as the freezing point of a solution of a nonvolatile solute is always lower than that of the pure solvent, the boiling point of a solution is always higher than the pure solvent. Again, only the number of solute particles affects the boiling point. The mathematical relationship is similar to the one for the freezing-point depression above and is:
ΔTb = iKb molality
where ΔTb is the number of degrees the boiling point has been elevated (the difference between the boiling point of the pure solvent and the solution); Kb is the boiling-point elevation constant; the molality is the molality of the solute; and i is the van ’t Hoff factor. You can calculate a solution’s boiling point if you know the molality of the solution. If you know the amount of the boiling-point elevation and the molality of the solution, you can calculate the value of the van ’t Hoff factor, i. Calculations involving boiling-point elevation are like those for freezing point depression.
Osmotic Pressure
If you were to place a solution and a pure solvent in the same container (such as the one in Figure 11.1) but separate them by a semipermeable membrane (which allows the passage of some particles, but not all particles), you would observe that the level of the solvent side would decrease while the solution side would increase. This indicates that the solvent molecules are passing through the semipermeable membrane, a process called osmosis. Eventually the system would reach equilibrium, and the difference in levels would remain constant. The difference in the two levels is related to the osmotic pressure. In fact, one could exert a pressure on the solution side exceeding the osmotic pressure, and solvent molecules could be forced back through the semipermeable membrane into the solvent side. This process is called reverse osmosis and is the basis of the desalination of seawater for drinking purposes. Cell membranes are common examples of semipermeable membranes.
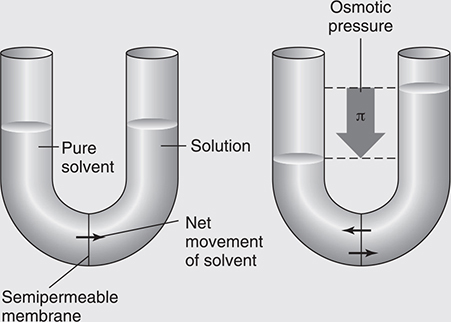
Figure 11.1 Osmotic pressure.
Colloids

If you watch a glass of muddy water, you will see particles in the water settling out. This is a heterogeneous mixture where the particles are large (in excess of 1,000 nm), and it is called a suspension. In contrast, dissolving sodium chloride in water results in a true homogeneous solution, with solute particles less than 1 nm in diameter. True solutions do not settle out because of the very small particle size. However, there are mixtures whose particle diameters fall in between solutions and suspensions. These are called colloids and have solute particles in the range of 1 to 1,000 nm diameter. Table 11.1 shows some representative colloids.
Table 11.1 Common Colloid Types
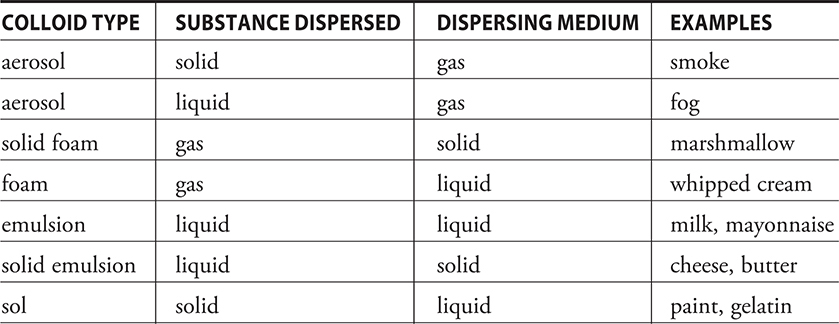
Many times it is difficult to distinguish a colloid from a true solution. The most common method is to shine a light through the mixture under investigation. A light shone through a true solution is invisible, but a light shone through a colloid is visible because the light reflects off the larger colloid particles. This is called the Tyndall effect.
Composition of Mixtures
Mixtures, unlike elements and compounds, have a variable composition. For example, a saltwater solution may have different quantities of table salt dissolved in different quantities of water. In all cases, you have a saltwater solution; however, samples with a very low salt-to-water ratio do not taste the same as sample with a significantly higher ratio.
The composition of the mixture may be expressed in several ways. The composition of a solution is often expressed by the molarity of a solution. The composition of a mixture of elemental iron with elemental sulfur may be expressed by the mass percentages of each component. The composition of a gas mixture, such as air, may be expressed by either the mole fraction or the partial pressure of each gas. These are only a few examples.
The composition of a mixture may be known because the person creating the mixture may have carefully measured the quantity of each component before mixing the different components. However, a mixture of unknown composition will need to be analyzed. In many cases, physical processes may be involved in the analysis, while in other cases, the analysis may require a chemical process.
In the following examples, we will focus on determining the mass percentages of the components of the mixture. In each case, this begins with the determination of the masses of the component. Once the mass of a component is known, the mass may be converted to moles, atoms, and with the aid of density, the mass may be determined to the volume.
The composition of the saltwater may be determined by weighing a sample of the solution and then allowing the water to evaporate to leave the salt behind. The mass of the salt divided by the mass of the solution times 100% gives the mass percent of the salt and 100% minus the mass percent of the salt gives the mass percent of water.
The composition of the iron-sulfur mixture may be determined by weighing a quantity of the mixture and then using a magnet to remove the iron. Once the iron is removed, simply weigh the remaining sulfur and determine the percentages of the two components. However, what if the mixture was elemental iron mixed with elemental nickel? Magnetic separation will not work as both metals are attracted to a magnet.
On the other hand, what about determining the percentages of iron, manganese, and chromium in a sample of stainless steel? In this case, physical process will not work. The normal procedure is to weigh a sample of the steel into a container and to dissolve the sample in acid. Once the components are in solution, it is possible to determine the quantities of each component using a variety of titration or instrumental techniques.
Separation of Solutions and Mixtures Chromatography
The components of a solution may be separated by several techniques. Two of these techniques will be discussed here. These two techniques are chromatography and distillation. These methods are necessary in the case of a liquid mixture as filtration will not work.
There are numerous types of chromatography, each designed to deal with a specific type of solution. Chromatography will work for both liquid and gaseous solutions. In all chromatography techniques, there is a mobile phase and a stationary phase. Separation is brought about because of a competition between the intermolecular forces between the components of the sample with the mobile phase versus the stationary phase.
The types of chromatography discussed here are paper, thin-layer, and column. In paper chromatography, the stationary phase is paper. Thin-layer chromatography employs a thin layer of silica or some other solid on a glass plate as the stationary phase. In column chromatography, a glass tube is filled with silica or some other solid substance as the stationary phase. In all three cases, a solvent is used as the mobile phase. The solvent may be a pure liquid or another solution.
An example of a paper chromatography experiment is the separation of the components present in a sample of ink. Most colors of ink contain a variety of components to produce a particular color. For example, a sample of green ink will most likely contain a blue component and a yellow component. There may be other components to produce different shades of green. The separation begins by placing a small drop of the ink near the bottom of a piece of chromatography paper. Additional drops of other samples or of possible components may be placed in a line alongside the first. The paper is then placed into a container containing a small quantity of a solvent (mobile phase). The ink dots must be above the surface of this solvent. The solvent will slowly “wet” the paper. That is, the solvent will soak into the paper and slowly creep higher on the paper. As the solvent front moves past the ink dots, some of the components will be more soluble in the solvent than the others. This solubility is related to the strength of the intermolecular forces. The component will readhere to the stationary phase after traveling a short distance further along and the dissolving-readhere process will repeat over and over. The component with the weakest intermolecular attraction to the stationary phase versus the mobile phase will move farther before readhering than a component with a greater attraction. Thus, over time the one component will separate from the others because of this difference in attraction. After a certain time, there will be a series of new dots representing each component above the original dot. These may be identified by comparing the distance traveled to the distance a standard has traveled.
All other chromatography techniques may be related to this procedure. In some cases, the time required to travel a fixed distance may be measured instead of the distance traveled. Any type of intermolecular force or combination of intermolecular forces may be responsible for the separation.
Another method of separation of the components of a solution is distillation. If a volatile solvent and a nonvolatile solute are present in the solution, a simple distillation will work. However, if both the solvent and solute are volatile, fractional distillation is necessary. In both cases, the volatile components are first vaporized and then condensed back to the liquid state. This is all that is necessary in a simple distillation. The solvent is vaporized and then condensed into another container, leaving the nonvolatile phase in the first container. The setup for a simple distillation is shown in Figure 11.2.
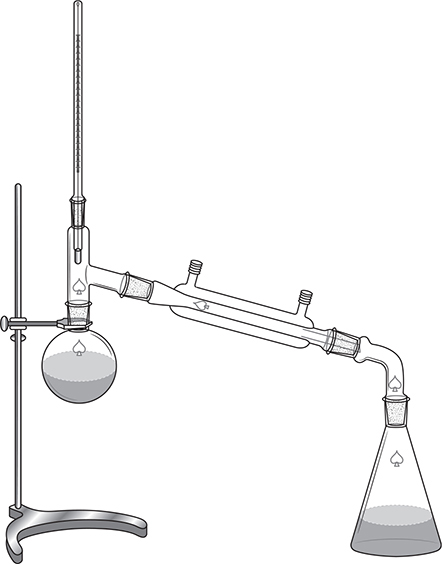
Figure 11.2 The setup for a simple distillation minus the heat source to be placed below the flask on the left and the tubing for the cooling water to enter and leave the condenser.
In fractional distillation, more than one component is volatile. Therefore, when heated each component will volatilize. The vapor phase will be enriched in the volatile component with the weaker intermolecular forces. Thus, when the vapor condenses, the resultant solution will be enriched in the more volatile component. This process may be repeated multiple times with each step producing a solution with a higher percentage of the more volatile component until the pure component is isolated. While the simple distillation apparatus shown in Figure 11.2 may be used for this, it is not practical because of the time involved. To do fractional distillation efficiently, a fractionating column is added to the simple distillation apparatus. A fractionating column provides numerous places for the components to condense and revaporize. In this way, by the time the vapor reaches the top of the column, several simple distillations have taken place; therefore, one pass through the system will accomplish a number of simple distillations and do so in much less time than repeated simple distillation. This method is used to separate the components of crude oil into its components, one of which is gasoline.
Experiments

Experimental procedures for solutions involve concentration units. Keeping close track of the units may simplify the problem. Titrations, which appear in Chapter 16, Acids and Bases, involve the concentrations of solutions.
Common Mistakes to Avoid
1. In molarity problems, be sure to use liters of solution.
2. Make sure your units cancel, leaving you with the units desired in your final answer.
3. Round off your final numerical answers to the correct number of significant figures.
4. Remember that most molecular compounds—compounds containing only nonmetals—do not ionize in solution. Acids are the most common exceptions.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. First are 20 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. There are a few questions designed to help you recall some prior knowledge. Following those are two long free-response questions like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. A solution is prepared by dissolving 1.25 g of an unknown substance in 100.0 mL of water. Which of the following procedures could be used to determine whether the solute is an electrolyte?
(A) Measure the specific heat of the solution.
(B) Measure the volume of the solution.
(C) Measure the conductivity of the solution.
(D) Determine the specific heat of the solution.
2. What is the final K+ concentration in a solution made by mixing 300.0 mL of 1.0 M KNO3 and 700.0 mL of 2.0 M K3PO4?
(A) 4.5 M
(B) 5.0 M
(C) 3.0 M
(D) 2.0 M
3. Strontium sulfate, SrSO4, will precipitate when a solution of sodium sulfate is added to a strontium nitrate solution. What will be the strontium ion, Sr2+, concentration remaining after 30.0 mL of 0.10 M Na2SO4 solution are added to 70.0 mL of 0.20 M Sr(NO3)2 solution?
(A) 0.14 M
(B) 0.15 M
(C) 0.11 M
(D) 0.20 M
4. Which of the following is a strong electrolyte when it is mixed with water?
(A) HNO2
(B) KNO3
(C) C2H5OH
(D) CH3COOH
5. A solution with a total chloride ion, Cl—, concentration of 1.0 M is needed. Initially, the solution is 0.30 M in MgCl2. How many moles of solid CaCl2 must be added to 400.0 mL of the MgCl2 solution to achieve the desired concentration of chloride ion?
(A) 0.10 mole
(B) 0.080 mole
(C) 0.20 mole
(D) 0.15 mole
6. Assuming the volumes are additive, what is the final H+(aq) concentration produced by adding 30.0 mL of 0.50 M HNO3 to 70.0 mL of 1.00 M HCl?
(A) 0.75 M
(B) 1.50 M
(C) 1.25 M
(D) 0.85 M
7. To prepare 3.0 L of a 0.20 molar K3PO4 solution (molecular weight 212 g/mole), a student should follow which of the following procedures?
(A) The student should weigh 42 g of solute and add sufficient water to obtain a final volume of 3.0 L.
(B) The student should weigh 42 g of solute and add 3.0 kg of water.
(C) The student should weigh 130 g of solute and add sufficient water to obtain a final volume of 3.0 L.
(D) The student should weigh 42 g of solute and add 3.0 L of water.
8. How many grams of MgSO4 (molecular weight 120.4 g/mol) are in 100.0 mL of a 5.00 molar solution?
(A) 602.0 g
(B) 5.00 g
(C) 12.0 g
(D) 60.2 g
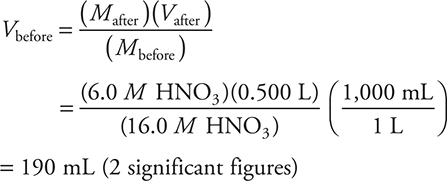
9. How many milliliters of concentrated nitric acid (16.0 molar HNO3) are needed to prepare 0.500 L of 6.0 molar HNO3?
(A) 0.19 mL
(B) 250 mL
(C) 375 mL
(D) 190 mL
10. A solution has 10 g of urea in 100 g of solution. Which of the following are needed to calculate the molarity of this solution?
(A) the density of the solution and the molecular weight of urea
(B) the density of urea and the molecular weight of urea
(C) the density of the solvent and the density of the solute
(D) the molecular weight of urea and the density of the solvent
11. Which of the following aqueous solutions would have the greatest conductivity?
(A) 0.2 M NaOH
(B) 0.2 M RbCl
(C) 0.2 M K3PO4
(D) 0.2 M H2SO3
12. How many milliliters of water must be added to 50.0 mL of 10.0 M HNO3 to prepare 4.00 M HNO3, assuming that the volumes of nitric acid and water are additive?
(A) 50.0 mL
(B) 125 mL
(C) 500 mL
(D) 75.0 mL
13. The best method to isolate pure MgSO4 from an aqueous solution of MgSO4 is to:
(A) evaporate the solution to dryness
(B) titrate the solution
(C) electrolyze the solution
(D) use paper chromatography
14. Pick the conditions that would yield the highest concentration of N2(g) in water.
(A) partial pressure of N2 = 1.0 atm; temperature of water = 25°C
(B) partial pressure of N2 = 0.50 atm; temperature of water = 55°C
(C) partial pressure of N2 = 2.0 atm; temperature of water = 25°C
(D) partial pressure of N2 = 2.0 atm; temperature of water = 85°C
15. A student prepares 100 mL of each of the following solutions by dissolving the appropriate solute in water. He then proceeds to measure the electrical conductivity of each solution. Which of the following aqueous solutions has the greatest electrical conductivity?
(A) 0.01 M Ba(OH)2
(B) 0.01 M KCl
(C) 0.01 M Na3PO4
(D) 0.01 M HC2H3O2
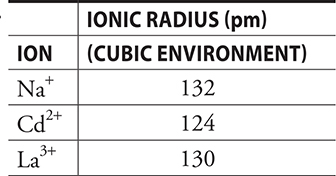
16.
The energy change in the following reaction is the heat of hydration:
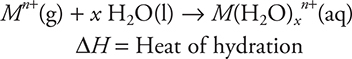
In general, the heat of hydration is exothermic. Assuming the value of x is the same in all cases, which of the following correctly predicts the relative order of the heats of hydration for the ions listed in the table and gives a correct explanation?
(A) ![]() because ions of elements lower on the periodic table have lower hydration energies.
because ions of elements lower on the periodic table have lower hydration energies.
(B) ![]() because smaller radii lead to higher hydration energies.
because smaller radii lead to higher hydration energies.
(C) ![]() because higher charges lead to higher hydration energies.
because higher charges lead to higher hydration energies.
(D) All are about the same because their ionic radii are similar.
17. Bronze is an alloy of copper and tin. What would be the most likely first step required to determine the mass percentage of tin in a sample of bronze?
(A) Distill the bronze to separate the two metals.
(B) Use paper chromatography to separate the two metals.
(C) React the bronze with an acid.
(D) Once mixed to form bronze, it is no longer possible to separate the components.
18. The separation of the components in a mixture by paper chromatography depends on what property?
(A) Intermolecular forces
(B) Melting point
(C) Molar mass
(D) Boiling point
19. Mercury(II) chloride, HgCl2, dissolves in water as discrete molecules. What is a simple procedure for determining that this is true?
(A) Observe the presence of molecules with an optical microscope.
(B) Use paper chromatography.
(C) Measure the electrical conductivity of the solution.
(D) Add silver nitrate solution to precipitate the chloride ion as AgCl.
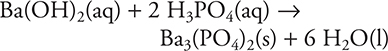
20. Which of the following techniques will allow you to determine if the above reaction is complete (with no excess of either reactant)?
(A) Determine the density of the solution.
(B) Determine the electrical conductivity of the solution.
(C) Determine volume of the solution.
(D) Determine the mass of the solution.
![]() Answers and Explanations
Answers and Explanations
To simplify some of the calculations, molarity will be expressed as 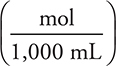 instead of
instead of 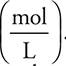 . This eliminates one or two conversions in the problems where this conversion is used.
. This eliminates one or two conversions in the problems where this conversion is used.
1. C—If the solute is an electrolyte, the solution will conduct electricity. Nonelectrolytes do not conduct electricity. Strong electrolytes conduct electricity extremely well. Weak electrolytes also conduct electricity, but not as well as a strong electrolyte.
2. A—The potassium ion contribution from the KNO3 is:
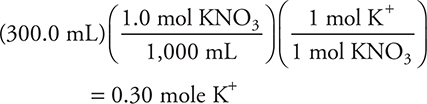
The potassium ion contribution from K3PO4 is:

The total potassium is 4.5 moles in a total volume of 1.000 L. Thus, the potassium concentration is 4.5 M.
3. C—The net ionic equation for the reaction is ![]() . (You do not need to worry about solubility rules since you are specifically told that strontium sulfate precipitates.) Two reactants are given; therefore, you need to determine which is the limiting reactant. The strontium nitrate solution contains:
. (You do not need to worry about solubility rules since you are specifically told that strontium sulfate precipitates.) Two reactants are given; therefore, you need to determine which is the limiting reactant. The strontium nitrate solution contains:
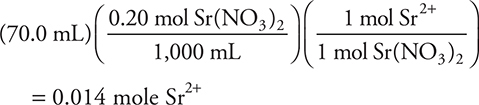
The sodium sulfate solution contains:
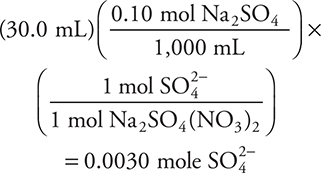
The sulfate ion is the limiting reactant. The strontium and sulfate ions react in a 1:1 ratio, so 0.0030 mole of sulfate ion will combine with 0.0030 mole of strontium ion, leaving 0.011 mole of strontium in a total volume of 100.0 mL. The final strontium ion concentration is:
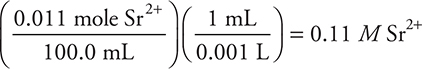
4. B—A (nitrous acid) and D (acetic acid) are weak acids. Weak acids and bases are weak electrolytes. C (ethanol) is a nonelectrolyte. Potassium nitrate is a water-soluble ionic compound; such compounds are normally strong electrolytes.
5. B—The number of moles of chloride ion needed is
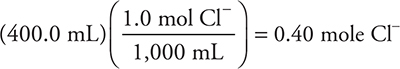
The initial number of moles of chloride ion in the solution is:
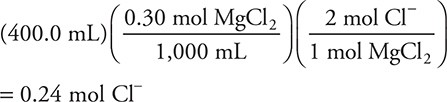
The number of moles needed
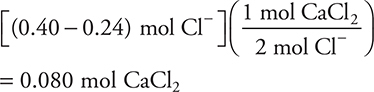
6. D—Both acids are strong acids and yield 1 mole of H+ each. Calculate the number of moles of H+ produced by each acid. Divide the total number of moles by the final volume (100.0 mL). The calculation is:
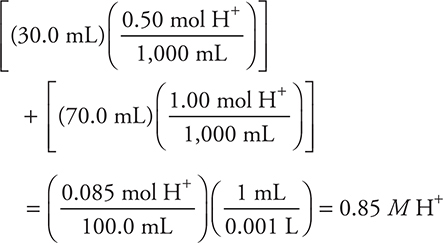
7. C—To produce a molar solution of any type, the final volume must be the desired volume. This eliminates answer D. B involves mass of water instead of volume. A calculation of the required mass will allow a decision between A and C:
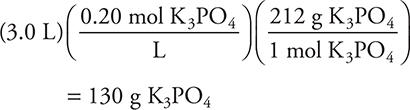
8. D—The calculation is:
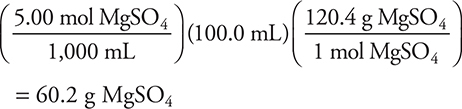
9. D—This is a dilution problem:
10. A—To calculate the molarity, the moles of urea and the volume of the solution are necessary. The density of the solution and the mass of the solution give the volume of the solution (it may be necessary to convert to liters). The mass of urea and the molar mass of urea give the moles of urea.
11. C—The strong electrolyte with the greatest concentration of ions is the best conductor. D is a weak electrolyte, not a strong electrolyte. The number of ions for the strong electrolytes may be found by simply counting the ions: A, 2; B, 2; C, 4. The best conductor has the greatest value when the molarity is multiplied by the number of ions. Also, since A and B both produce the same number of ions, they both cannot be right; therefore, neither can be right.
12. D—This is a dilution problem:
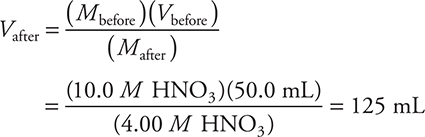
This is the final volume; since you already had 50.0 mL, you need an additional 75.0 mL.
13. A—Solutions cannot be separated by titrations or filtrations. Electrolysis of the solution would produce hydrogen and oxygen gas. Chromatography might achieve a minimal separation (so it cannot be the best method).
14. C—The solubility of a gas is increased by increasing the partial pressure of the gas and by lowering the temperature. Pick the answer with the highest nitrogen pressure and the lowest temperature.
15. C—Three of the compounds are strong electrolytes and one is a weak electrolyte. The weak electrolyte, HC2H3O2, will not be as good a conductor as are the strong electrolytes (eliminating D). The strong electrolyte that produces the highest concentration of ions will be the best conductor. The number of ions produced by the strong electrolytes may be found by simply counting the ions: A, 3 (Ba2 + 2 OH—); B, 2 (K+ + Cl-); C, 4 (3 Na+ + PO43—). The ion concentration for the strong electrolytes is the molarity of the solution times the number of ions present (van’t Hoff factor). Answer C gives the highest concentration.
16. C—The ionic radii are too alike to make a significant difference; therefore, it is necessary to focus on the charge differences. The greater the charge, the greater the attraction of the ion for the polar water molecules (ion—dipole forces). The greater the attraction is, the greater the energy change is.
17. C—To begin the analysis it is necessary to place at least one of the two components in solution. Reacting with acid is one way of doing this. Distillation requires an unreasonably high temperature. Paper chromatography depends upon the ability of at least one of the components to be at least partially soluble in the mobile phase, which requires the bronze be dissolved in acid or some other substance first. There is always a method of separating the components.
18. A—Intermolecular forces are the key. The separation depends upon the relative strengths of the intermolecular forces of the components and the stationary phase (paper) and the relative intermolecular forces between the components and the mobile phase. The component held more strongly to the stationary phase moves more slowly than the component held less strongly. Intermolecular forces between the components and the mobile phase aids in this separation.
19. C—If the HgCl2 dissolves as discrete molecules, it is a nonelectrolyte, which means there are no ions in solution. If ions are present in solution, the solution will conduct electricity, which is easily determined by measuring the electrical conductivity of the solution. Molecules are far too small to be observed by an optical isotope. Paper chromatography will not separate the cations from the anions as electrical neutrality must be maintained. A reaction to precipitate the chloride ions does not necessarily mean that they were initially present as separate ions before the reaction.
Note there are a variety of other techniques involving colligative properties (freezing point depression, boiling point depression, and osmosis) these topics only appear in college general chemistry courses and not on the AP Exam.
20. B—The reactants are electrolytes (Ba(OH)2(aq) is a strong electrolyte and H3PO4(aq) is a weak electrolyte), and neither of the products is an electrolyte. Any excess reactant will yield ions making the solution conducting, while the lack of an excess reactant will leave a nonconducting mixture (note, no aqueous solution has absolutely no conductivity). Measuring the electrical conductivity of the solution is a simple method of determining if there is no excess reactant. The other methods present will not distinguish an incomplete reaction from a complete reaction.
![]() Free-Response Questions
Free-Response Questions
Question 1
You have 5 minutes to answer the following two-part question. You may use a calculator and the tables in the back of the book.
Five beakers each containing 100.0 mL of an aqueous solution are on a lab bench. The solutions are all at 25°C. Solution 1 contains 0.20 M KNO3. Solution 2 contains 0.10 M BaCl2. Solution 3 contains 0.15 M C2H4(OH)2. Solution 4 contains 0.20 M (NH4)2SO4. Solution 5 contains 0.25 M KMnO4.
(a) Which solution has the lowest pH? Explain.
(b) Which solution would be the poorest conductor of electricity? Explain.
Question 2
You have 15 minutes to answer the following four-part question. You may use a calculator and the tables in the back of the book.
Five beakers are placed in a row on a countertop. Each beaker is half-filled with a 0.20 M aqueous solution. The solutes, in order, are (1) potassium sulfate, K2SO4; (2) methyl alcohol, CH3OH; (3) sodium carbonate, Na2CO3; (4) ammonium chromate, (NH4)2CrO4; and (5) barium chloride, BaCl2. The solutions are all at 25°C. Answer the following questions with respect to the five solutions.
(a) Which solution will form a precipitate when ammonium chromate is added to it?
(b) Which solution is the most basic? Explain.
(c) Which solution would be the poorest conductor of electricity? Explain.
(d) Which solution is colored?
![]() Answers and Explanations
Answers and Explanations
Question 1
(a) Solution 4, because the ammonium ion is a weak acid.
You get 1 point for picking solution 4 and 1 point for saying the ammonium ion (NH4+) is a weak acid or that it undergoes hydrolysis.
(b) Solution 3, because the solute is a nonelectrolyte.
Give yourself 1 point for picking solution 3 and 1 point for saying it is a nonelectrolyte or that it does not ionize.
Total your points. There are 4 points possible.
Question 2
(a) The ammonium ion, from the ammonium chromate, will not form a precipitate since most ammonium compounds are water soluble. Therefore, the precipitate must contain the chromate ion combined with a cation from one of the solutions. Solution (2) is a nonelectrolyte; therefore, there are no cations present to combine with the chromate ion. The potassium and sodium ions, from solutions (1) and (3), give soluble salts like the ammonium ion. This leaves only solution (5), barium chloride, which will give a precipitate. The formula of the precipitate is BaCrO4.
You get 1 point for picking the correct solution.
(b) Solution (3), sodium carbonate, is the most basic. Since the carbonate ion is the conjugate base of a weak acid, it will undergo significant hydrolysis to produce a basic solution.
You get 1 point for picking the correct solution and giving the correct explanation.
(c) Methyl alcohol is a nonelectrolyte, so its solutions do not conduct electricity. The remaining solutions contain ionic salts, which, in general, are electrolytes in solution.
You get 1 point for picking the correct solution and giving the correct explanation.
(d) Solution (4), ammonium chromate, is yellow. Most solutions containing a transition metal ion are colored.
You get 1 point for picking the correct solution.
Total your points. There are 4 points possible.
![]() Rapid Review
Rapid Review
• A solution is a homogeneous mixture composed of a solvent and one or more solutes. A solute is a substance that dissolves in the solvent and is normally present in smaller amount.
• The general rule of solubility is “like dissolves like.” This means that polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes. Remember, however, that simply quoting this rule will not be sufficient as an explanation in the free-response section.
• A saturated solution is one in which the maximum amount of solute is dissolved for a given amount of solvent at a given temperature. Any solution with less than the maximum solute is called unsaturated. A solution with greater than maximum solute is supersaturated (an unstable state).
• For the chemist, the most useful unit of concentration is molarity (M ), which is the moles of solute per liter of solution. Know how to work molarity problems. Be careful not to confuse molarity, M or [ ], with moles, n or mol.
• Electrolytes conduct an electrical current when melted or dissolved in a solvent, whereas nonelectrolytes do not.
• A colloid is a mixture in which the solute particle size is intermediate between a true solution and a suspension. If a light is shone through a colloid, the light beam is visible. This is the Tyndall effect.