5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 4 Review the Knowledge You Need to Score High
12 Reactions and Periodicity
IN THIS CHAPTER
In this chapter, the following AP topics are covered:
1.8 Valence Electrons and Ionic Compounds
3.7 Solutions and Mixtures
4.2 Net Ionic Equations
4.4 Physical and Chemical Changes
4.6 Introduction to Titration
4.7 Types of Chemical Reactions
4.8 Introduction to Acid—Base Reactions
Summary: Chemistry is the world of chemical reactions. Chemical reactions power our society, our environment, and our bodies. Some chemical species called reactants are converted into different substances called products. During this process, there are energy changes that take place. It takes energy to break old bonds. Energy is released when new bonds are formed. Does it take more energy to break the bonds than is released in the formation of the new bonds? If so, energy will have to be constantly supplied to convert the reactants into products. This type of reaction is said to be endothermic, absorbing energy. If more energy is released than is needed to break the old bonds, then the reaction is said to be exothermic, releasing energy. The chemical reactions that provide the energy for our world are exothermic reactions. In Chapter 14, Thermodynamics, you can read in more depth about the energy changes that occur during reactions.
Reactions occur because of collisions. One chemical species collides with another at the right place and transfers enough energy, and a chemical reaction occurs. Such reactions can be very fast or very slow. In Chapter 13, Kinetics, you can study how reactions occur and the factors that affect the speed of reactions.
In Chapter 15, Equilibrium, you will review the fact that reactions tend to go to equilibrium instead of completion. When the reaction is “over” both reactants and products will remain.
In Chapter 17, Electrochemistry, we will see how electricity may interact with reactions.
However, in this chapter, we will review the balancing of chemical equations, discuss the general types of chemical reactions, and describe why these reactions occur.

Keywords and Equations
There are no keywords or equations listed on the AP Exam that are specific to this chapter.
Reaction questions will always appear in the free-response section of the AP Exam.
AP Exam Format
You are expected to write a balanced chemical equation for every reaction given and answer one or more questions about each reaction. If the reaction occurs in aqueous solution, you will have to write the net ionic equation for the process. For the reactions question of the AP Exam, you will be expected not only to balance the equation but also to have an understanding of why the reaction occurs. The reactions and concepts described may also appear in other parts of the AP Exam, such as the multiple-choice sections. Again, you will need to understand why a particular reaction occurs. As you study this chapter, pay particular attention to the explanations that accompany the reactions and equations. You will be expected to demonstrate your understanding on the AP Exam.
General Aspects of Chemical Reactions and Equations
Physical and Chemical Changes
Chemistry investigates two types of changes in chemical systems. These are physical changes and chemical changes. Physical changes involve a change in one of more physical properties with no change in composition. An example is boiling water where a liquid is converted to a gas. Even though the liquid and gas phases have different physical properties, each is composed of identical H2O molecules. Physical changes are normally accompanied by changes in intermolecular interactions. The physical change may be endothermic or exothermic. Normally, a physical change is reversible. However, this is not always true. For example, crushing a rock is a physical change that is hard to reverse.
During a chemical change there is a change in composition. There may be a change in the physical properties. Burning charcoal is an example of a chemical change. Solid charcoal (mostly carbon) combines with gaseous oxygen to produce gaseous carbon dioxide. The chemical change is accompanied by the breaking and forming of chemical bonds. All chemical changes may be described with a chemical equation. Chemical changes may be endothermic or exothermic. Normally, a chemical change is not reversible. In the charcoal example, it is not possible to “unburn” the charcoal. Equilibrium processes, Chapter 15, bend this rule to a certain extent.
Some processes have characteristics of both physical and chemical changes. An example is dissolving sodium chloride in water. This is usually characterized as a physical change even though ionic bonds in the sodium chloride are broken and ion—dipole interactions between the ions and water molecules form. However, simply heating the solution until all the water evaporates reverses the process and allows the isolation of the original sodium chloride and water.
In all chemical and physical changes the Law of Conservation of Mass is obeyed.
Balancing Chemical Equations
We hope that, because you are preparing to take the AP Exam, you have already been exposed to the balancing of chemical equations. We will quickly review this topic and point out some specific aspects of balancing equations as the different types of chemical reactions are discussed. We have seen many students get into trouble because they did not pay close enough attention to the chemical equation.
A balanced chemical equation provides many types of information. It shows which chemical species are the reactants and which species are the products. It may also indicate in which state of matter the reactants and products exist. Special conditions of temperature, catalysts, etc., may be placed over or under the reaction arrow. And, very important, the coefficients (the integers in front of the chemical species) indicate the number of each reactant that is used and the number of each product that is formed. These coefficients may stand for individual atoms/molecules, or they may represent large numbers of them called moles (see Chapter 6, Stoichiometry, for a review of moles). The basic idea behind the balancing of equations is the Law of Conservation of Matter, which says that in ordinary chemical reactions matter is neither created nor destroyed. The number of each type of reactant atom must equal the number of each type of product atom. This requires adjusting the reactant and product coefficients—balancing the equation. When finished, the coefficients should be in the lowest possible whole-number ratio.
Most equations are balanced by inspection. This means basically a trial-and-error, methodical approach to adjusting the coefficients. One procedure that works well is to balance the homonuclear (same nucleus) molecule last. Chemical species that fall into this category include the diatomic elements, which you should know: H2, O2, N2, F2, Cl2, Br2, and I2. This is especially useful when you are balancing combustion reactions. If a problem states that oxygen gas was used, then knowing that oxygen exists as the diatomic element is absolutely necessary in balancing the equation correctly.
Periodic Relationships
The periodic table can give us many clues as to the type of reaction that is taking place. One general rule, covered in more detail in Chapter 8, Bonding, is that nonmetals react with other nonmetals to form covalent compounds, and that metals react with nonmetals to form ionic compounds. If the reaction that is producing the ionic compound is occurring in solution, you probably will be expected to write the net ionic equation for the reaction. Also, because of the wonderful arrangement of the periodic table, the members of a family or group (a vertical grouping) all react essentially in the same fashion. Many times, in reactions involving the loss of electrons (oxidation), as we proceed from top to bottom in a family, the reaction rate (speed) increases. Conversely, in reactions involving the gain of electrons (reduction), the reaction rate increases as we move from the bottom of a family to the top. Recall also that the noble gases (8A or 18) undergo very few reactions. Other specific periodic aspects will be discussed in the various reaction sections.
General Properties of Aqueous Solutions
Many of the reactions that you will study occur in aqueous solution. Water is called the universal solvent because it dissolves so many substances. It readily dissolves ionic compounds as well as polar covalent compounds because of its polar nature. Ionic compounds that dissolve in water (dissociate) form electrolyte solutions, which conduct electrical current owing to the presence of ions. The ions can attract the polar water molecules and form a bound layer of water molecules around themselves. This process is called solvation. Refer to Chapter 11, Solutions, for an in-depth discussion of solvation.
Even though many ionic compounds dissolve in water, many others do not. If the attraction of the oppositely charged ions in the solid for each other is greater than the attraction of the polar water molecules for the ions, then the salt will not dissolve to an appreciable amount. If solutions containing ions such as these are mixed, precipitation will occur, because the strong attraction of the ions for each other overcomes the weaker attraction for the water molecules.
As mentioned, certain covalent compounds, like alcohols, readily dissolve in water because they are polar. Since water is polar, and these covalent compounds are also polar, water will act as a solvent for them (general rule of solubility: “like dissolves like”). Compounds like alcohols are nonelectrolytes—substances that do not conduct an electrical current when dissolved in water. However, certain covalent compounds, like acids, will ionize in water, that is, form ions:
HCl(aq) → H+(aq) + Cl—(aq)
There are several ways of representing reactions that occur in water. Suppose, for example, that we were writing the equation to describe the mixing of a lead(II) nitrate solution with a sodium sulfate solution and showing the resulting formation of solid lead(II) sulfate. One type of equation that can be written is the molecular equation, in which both the reactants and products are shown in the undissociated form:
Pb(NO3)2(aq) + Na2SO4(aq) → PbSO4(s) + 2 NaNO3(aq)
Molecular equations are quite useful when you are doing reaction stoichiometry problems (see Chapter 6).
Showing the soluble reactants and products in the form of ions yields the ionic equation (sometimes called the total ionic equation):
Pb2+(aq) + 2 NO3—(aq) + 2 Na+(aq) + SO42—(aq) → PbSO4(s) + 2 Na+(aq) + 2 NO3—(aq)
Writing the equation in the ionic form shows clearly which species are really reacting and which are not. In the example above, Na+ and NO3— appear on both sides of the equation. These ions do not react and are simply there to maintain electrical neutrality of the solution. Ions like this, which are not actually involved in the chemical reaction taking place, are called spectator ions. We have seen students do this step incorrectly by separating the polyatomic ions (NO3— and SO42—) or by not including the ionic charges. Review your nomenclature to learn the polyatomic ions and the ionic charges. In an ionic equation, all strong electrolytes are written as separate ions. In water, the strong electrolytes are the strong acids, strong bases, and water-soluble ionic compounds. Any substance that is not a strong electrolyte is left in its original form.

The net ionic equation is written by dropping out the spectator ions and showing only those chemical species that are involved in the chemical reaction:
Pb2+(aq) + SO42—(aq) → PbSO4(s)
This net ionic equation focuses only on the substances that are actually involved in the reaction. It indicates that an aqueous solution containing Pb2+ (any solution, not just Pb(NO3)2(aq)) will react with any solution containing the sulfate ion to form insoluble lead(II) sulfate. If this equation form is used, the spectator ions involved will not be known, but in most cases, this is not a problem, since the focus is really the general reaction, and not the specific one. You will be expected to write the balanced net ionic equation for many of the reactions on the test. Note, you will be given hints as to what type of reaction will occur without memorizing things like solubility rules.
Precipitation Reactions
Precipitation reactions involve the formation of an insoluble compound, a precipitate, from the mixing of two soluble compounds. Precipitation reactions normally occur in aqueous solution. The example above that was used to illustrate molecular equations, ionic equations, etc., was a precipitation reaction. A solid, lead(II) sulfate, was formed from the mixing of the two aqueous solutions.
In order to predict whether or not precipitation will occur if two solutions are mixed, you must:

1. Learn to write the correct chemical formulas from the names; on the AP Exam, names are frequently given instead of formulas in the reaction section.
2. Be able to write the reactants and products in their ionic form, as in the ionic equation example above. Be sure, however, that you do not try to break apart molecular compounds such as most organic compounds, or insoluble species.
3. Know and be able to apply the following solubility rules by combining the cation of one reactant with the anion of the other in the correct formula ratio and determining the solubility of the proposed product. Then do the same thing for the other anion/cation combination.
4. On the AP Exam, you will be expected to explain why a substance is soluble/insoluble. Simply quoting the solubility rule is not sufficient.
Learn the following solubility rule:

All sodium, potassium, ammonium, and nitrate salts are soluble in water.
It is also possible to use hints to write the equation. For example, if you are given a solution, the substance is soluble. A soluble ionic compound is normally a strong electrolyte (you will be told if it is an exception). A soluble molecular compound usually is a nonelectrolyte unless it is an acid.
Although not required for the AP Exam, the following solubility rules are very useful.
• All salts containing acetate (CH3COO—) and perchlorates (ClO4—) are soluble.
• All chlorides (Cl—), bromides (Br—), and iodides (I—) are soluble, except those of Cu+, Ag+, Pb2+, and Hg22+.
• All salts containing sulfate (SO42—) are soluble, except those of Pb2+, Ca2+, Sr2+, and Ba2+.
Salts containing the following ions are normally insoluble:
• Most carbonates (CO32—) and phosphates (PO43—) are insoluble, except those of
Group 1A (or Group 1) and the ammonium ion.
• Most sulfides (S2—) are insoluble, except those of Groups 1A and 2A (or Groups 1 and 2) and the ammonium ion.
• Most hydroxides (OH—) are insoluble, except those of Group 1A (Group 1), calcium, strontium, and barium.
• Most oxides (O2—) are insoluble, except for those of Group 1A and Group 2A (Groups 1 and 2), which react with water to form the corresponding soluble hydroxides.
Let’s see how one might apply these rules. Suppose a solution of lead(II) nitrate is mixed with a solution of sodium iodide. Predict what will happen.
Write the formulas:
Pb(NO3)2 (aq) + NaI(aq) →
Convert to the ionic form:
Pb2+(aq) + 2 NO3—(aq) + Na+(aq) + I—(aq) →
Predict the possible products by combining the cation of one reactant with the anion of the other and vice versa:
PbI2 + NaNO3
Apply the solubility rules to the two possible products:

Complete the chemical equation and balance it:
Pb(NO3)2(aq) + 2 NaI(aq) → PbI2(s) + 2 NaNO3(aq)
Pb2+(aq) + 2 I—(aq) → PbI2(s)
If both possible products are soluble, then the reaction would be listed as NR (no reaction). In the reaction question part of the AP Exam, there will be a possible reaction for every part of the question. If at least one insoluble product is formed, the reaction is sometimes classified as a double displacement (replacement) or metathesis reaction.
Oxidation—Reduction Reactions
Oxidation—reduction reactions, commonly called redox reactions, are an extremely important category of reaction. Redox reactions include combustion, corrosion, respiration, photosynthesis, and the reactions involved in electrochemical cells (batteries). The driving force involved in redox reactions is the exchange of electrons from a more active species to a less active one. You can predict the relative activities from a table of activities or a half-reaction table. Chapter 17, Electrochemistry, goes into depth about electrochemistry and redox reactions.

The AP free-response booklet includes a table of half-reactions, which you may use for help during this part of the exam. A similar table can be found in the back of this book. Alternatively, you may wish to memorize the common oxidizing and reducing agents.
Redox is a term that stands for reduction and oxidation. Reduction is the gain of electrons, and oxidation is the loss of electrons. For example, suppose a piece of zinc metal is placed in a solution containing the blue Cu2+(aq) cation. Very quickly, a reddish solid forms on the surface of the zinc metal. That substance is copper metal. As the copper metal is deposited, the blue color of the solution begins to fade. At the molecular level, the more active zinc metal is losing electrons to form the Zn2+(aq) cation, and the Cu2+(aq) ion is gaining electrons to form the less active copper metal. These two processes can be shown as:
Zn(s) → Zn2+(aq) + 2e— (oxidation)
Cu2+(aq) + 2e— → Cu(s) (reduction)
The electrons that are being lost by the zinc metal are the same electrons that are being gained by the copper(II) ion. The zinc metal is being oxidized and the copper(II) ion is being reduced. Further discussions on why reactions such as these occur can be found in the section on single displacement reactions later in this chapter.
Something must cause the oxidation (taking the electrons), and that substance is called the oxidizing agent (the reactant being reduced). In the example above, the oxidizing agent is the Cu2+(aq) ion. The reactant undergoing oxidation is called the reducing agent because it is furnishing the electrons that are being used in the reduction half-reaction. Zinc metal is the reducing agent above. The two half-reactions, oxidation and reduction, can be added together to give you the overall redox reaction. When doing this, the electrons must cancel—that is, there must be the same number of electrons lost as electrons gained:
Zn(s) + Cu2+(aq) + 2 e— → Zn2+(aq) + 2 e— + Cu(s)
or Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
In these redox reactions, there is a simultaneous loss and gain of electrons. In the oxidation reaction (commonly called a half-reaction) electrons are being lost, but in the reduction half-reaction those very same electrons are being gained. So, in redox reactions electrons being exchanged as reactants are being converted into products. This electron exchange may be direct, as when copper metal plates out on a piece of zinc, or it may be indirect, as in an electrochemical cell (battery).
Another way to determine what is being oxidized and what is being reduced is by looking at the change in oxidation numbers of the reactant species. (See Chapter 5, Basics, for a discussion of oxidation numbers and how to calculate them.) Oxidation is indicated by an increase in oxidation number. In the example above, the Zn metal went from an oxidation state of zero to +2. Reduction is indicated by a decrease in oxidation number. Cu2+(aq) went from an oxidation state of +2 to zero. In order to figure out whether a particular reaction is a redox reaction, write the net ionic equation. Then determine the oxidation numbers of each element in the reaction. If one or more elements have changed oxidation number, it is a redox reaction.
There are several types of redox reaction that are given specific names. In the next few pages, we will examine some of these types of redox reaction.
Combination Reactions
Combination reactions are reactions in which two or more reactants (elements or compounds) combine to form one product. Although these reactions may be of several different types, some types are definitely redox reactions. These include reactions of metals with nonmetals to form ionic compounds, and the reaction of nonmetals with other nonmetals to form covalent compounds.
2 K(s) + Cl2(g) → 2 KCl(s)
2 H2(g) + O2(g) → 2 H2O(l)
In the first reaction, we have the combination of an active metal with an active nonmetal to form a stable ionic compound. The very active oxygen reacts with hydrogen to form the stable compound water. The hydrogen and potassium are undergoing oxidation, while the oxygen and chlorine are undergoing reduction.
Decomposition Reactions
Decomposition reactions are reactions in which a compound breaks down into two or more simpler substances. Although not all decomposition reactions are redox reactions, many are. For example, the thermal decomposition reactions, such as the common laboratory experiment of generating oxygen by heating potassium chlorate, are decomposition reactions:
![]()
In this reaction the chlorine is going from the less stable +5 oxidation state to the more stable —1 oxidation state. While this is occurring, oxygen is being oxidized from —2 to 0.
Another example is electrolysis, in which an electrical current is used to decompose a compound into its elements:
![]()
The spontaneous reaction would be the opposite one; therefore, we must supply energy
(in the form of electricity) in order to force the nonspontaneous reaction to occur. This type of reaction will be discussed in more detail in Chapter 17, Electrochemistry.
Single Displacement Reactions

Single displacement (replacement) reactions are reactions in which atoms of an element replace the atoms of another element in a compound. All the single replacement reactions are redox reactions since the element (in a zero-oxidation state) becomes an ion. Most single displacement reactions can be categorized into one of three types of reaction:

• A metal displacing a metal ion from solution
• A metal displacing hydrogen gas (H2) from an acid or from water
• One halogen replacing another halogen in a compound
Remember: it is an element displacing another atom from a compound. The displaced atom appears as an element on the product side of the equation.
Reactions will always appear in the free-response section of the AP Chemistry Exam.
For the first two types, a table of metals relating their ease of oxidation to each other is useful in being able to predict what displaces what. Table 12.1 shows the activity series for metals, which lists the metal and its oxidation in order of decreasing ease of oxidation. An alternative to the activity series is a table of half-cell potentials, as discussed in Chapter 17, Electrochemistry. In general, the more active the metal, the lower its potential.
Table 12.1 Activity Series of Metals in Aqueous Solution
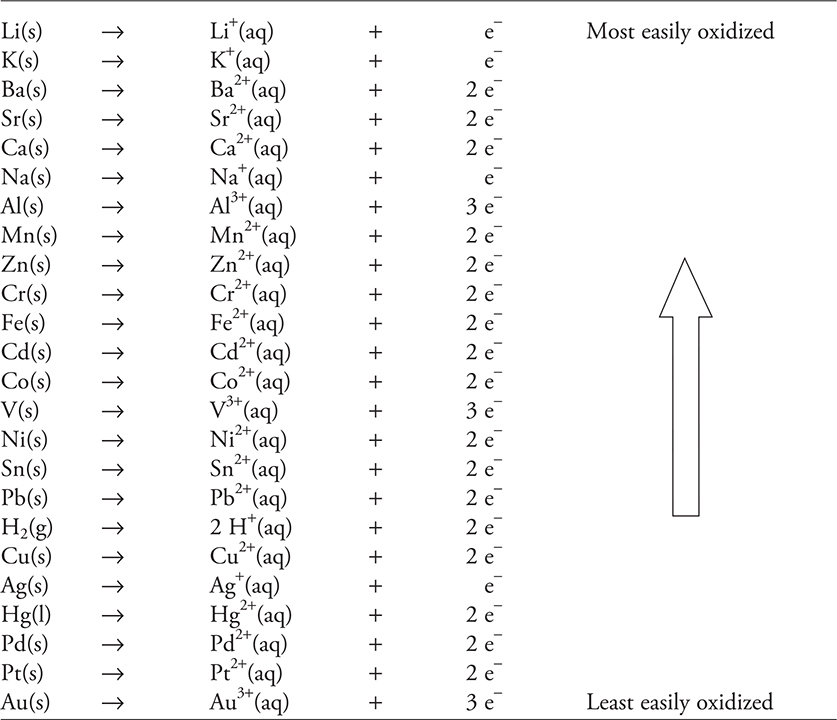

Elements on this activity series can displace ions of metals lower than themselves on the list. If, for example, one placed a piece of tin metal into a solution containing Cu(NO3)2(aq), the Sn would replace the Cu2+(aq) cation:
Sn(s) + Cu(NO3)2 (aq) → Sn(NO3)2 (aq) + Cu(s)
Sn(s) + Cu2+(aq) → Sn2+(aq) + Cu(s)
The second equation is the net ionic form that is often required on the AP Exam.
If a piece of copper metal were placed in a solution of Sn(NO3)2(aq), there would be no reaction since copper is lower than tin on the activity series. This table allows us to also predict that if sodium metal is placed in water, it will displace hydrogen, forming hydrogen gas:
2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g)
2 Na(s) + 2 H2O(l) → 2 Na+(aq) + 2 OH—(aq) + H2(g)
The Group 1A and 2A (or Group 1 and 2) elements on the activity table will displace hydrogen from water, but not the other metals shown. All the metals above hydrogen will react with acidic solutions to produce hydrogen gas:
Co(s) + 2 HCl(aq) → CoCl2(aq) + H2(g)
Co(s) + 2 H+(aq) → Co2+(aq) + H2(g)
Halogen reactivity decreases as one goes from top to bottom in the periodic table, because of the decreasing electronegativity. Therefore, a separate activity series for the halogens can be developed:
F2
Cl2
Br2
I2
The above series indicates that if chlorine gas were dissolved in a KI(aq) solution, the elemental chlorine would displace the iodide ion:
Cl2(aq) + 2 KI(aq) → 2 KCl(aq) + I2(s)
Cl2(aq) + 2 I—(aq) → 2 Cl—(aq) + I2(s)
As noted previously, you will not be expected to memorize these reactions. However, you may be expected to reaction based on information given in the question.
Combustion Reactions
Combustion reactions are redox reactions in which the chemical species rapidly combines with oxygen and usually emits heat and light. Reactions of this type are extremely important in our society as the sources of heat energy. Complete combustion of carbon yields carbon dioxide, and complete combustion of hydrogen yields water. The complete combustion of hydrocarbons, organic compounds containing only carbon and hydrogen, yields carbon dioxide and water:
2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(g)
If the compound also contains oxygen, such as in alcohols, ethers, etc., the products are still carbon dioxide and water:
2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(g)
If the compound contains sulfur, the complete combustion produces sulfur dioxide, SO2:
2 C2H6S(g) + 9 O2(g) → 4 CO2(g) + 6 H2O(g) + 2 SO2(g)
If nitrogen is present, it will normally form the very stable nitrogen gas, N2.
In these reactions, the driving force is the highly reactive oxygen forming a very stable compound(s). This is shown by the exothermic nature of the reaction.

In balancing any of these combustion reactions, it is helpful to balance the oxygen last.
Coordination Compounds
When a salt is dissolved in water, the metal ions, especially transition metal ions, form a complex ion with water molecules and/or other species. A complex ion is composed of a metal ion bonded to two or more molecules or ions called ligands. These are Lewis acid—base reactions. For example, suppose Cr(NO3)3 is dissolved in water. The Cr3+(aq)
cation attracts water molecules to form the complex ion Cr(H2O)63+(aq). In this complex ion, water acts as the ligand. If ammonia is added to this solution, the ammonia can displace the water molecules from the complex:
[Cr(H2O)6]3+(aq) + 6 NH3(aq) ⇆ [Cr(NH3)6]3+(aq) + 6 H2O(l)
In reactions involving coordination compounds, the metal acts as the Lewis acid (electron-pair acceptor), while the ligand acts as a Lewis base (electron-pair donor). In the reaction above, the ammonia ligand displaces the water ligand from the chromium complex because nitrogen is a better electron-pair donor (less electronegative) than oxygen.
The nitrogen in the ammonia and the oxygen in the water are the donor atoms. They are the atoms that actually donate the electrons to the Lewis acid. The coordination number is the number of donor atoms that surround the central atom. As seen above, the coordination number for Cr3+ is 6. Coordination numbers are usually 2, 4, or 6, but other values can be possible. Silver (Ag+) commonly forms complexes with a coordination number of 2; zinc (Zn2+), copper (Cu2+), nickel (Ni2+), and platinum (Pt2+) commonly form complexes with a coordination number of 4; most other central ions have a coordination number of 6. (Again, you are not expected to memorize these; however, knowing the possibilities may help you get the correct answer sooner.)
AgCl(s) + 2 NH3(aq) → [Ag(NH)2]+(aq) + Cl—(aq)
Zn(OH)2(s) + 2 OH—(aq) → [Zn(OH)4]2—(aq)
Fe3+(aq) + 6 CN—(aq) → [Fe(CN)6]3—(aq)
Acid—Base Reactions
Acids and bases are extremely common, as are the reactions between acids and bases. The driving force is often the hydronium ion reacting with the hydroxide ion to form water. Chapter 16, Acids and Bases, describes the equilibrium reactions of acids and bases, as well as some information concerning acid—base titrations. After you finish this section, you may want to review the acid—base part of the Equilibrium chapter.
Properties of Acids, Bases, and Salts
At the macroscopic level, acids taste sour, may be damaging to the skin, and react with bases to yield salts. Bases taste bitter, feel slippery, and react with acids to form salts.
At the microscopic level, acids are defined as proton (H+) donors (Brønsted—Lowry theory) or electron-pair acceptors (Lewis theory). Bases are defined as proton (H+) acceptors (Brønsted—Lowry theory) or electron-pair donors (Lewis theory). Consider the gas-phase reaction between hydrogen chloride and ammonia:
HCl(g) + :NH3(g) → HNH3+Cl—(s) (or NH4+Cl—(s))
HCl is the acid, because it is donating an H+ and the H+ will accept an electron pair from ammonia. Ammonia is the base, accepting the H+ and furnishing an electron pair that the H+ will bond via coordinate covalent bonding. Coordinate covalent bonds are covalent bonds in which one of the atoms furnishes both electrons for the bond. After the bond is formed, it is identical to a covalent bond formed by donation of one electron by both bonding atoms.
Acids and bases may be strong, dissociating completely, or weak, partially dissociating and forming an equilibrium system. (See Chapter 16 for the details on weak acids and bases.) Strong acids include:

1. Hydrochloric, HCl
2. Hydrobromic, HBr
3. Hydroiodic, HI
4. Nitric, HNO3
5. Chloric, HClO3
6. Perchloric, HClO4
7. Sulfuric, H2SO4
The strong acids above are all compounds that ionize completely in aqueous solution, yielding hydrogen ions and the anions from the acid.
Strong bases include:

1. Alkali metal (Group IA) hydroxides (LiOH, NaOH, KOH, RbOH, CsOH)
2. Ca(OH)2, Sr(OH)2, and Ba(OH)2
The strong bases listed above are all compounds that dissociate completely, yielding the hydroxide ion (which is really the base, not the compound).
Unless told otherwise, assume that acids and bases not on the lists above are weak and will establish an equilibrium system when placed into water.
Some salts have acid—base properties. For example, ammonium chloride, NH4Cl, when dissolved in water will dissociate and the ammonium ion will act as a weak acid, donating a proton. We will examine these acid—base properties in more detail in the next section.
Certain oxides can have acidic or basic properties. These properties often become evident when the oxides are dissolved in water. In most case, reactions of this type are not redox reactions.
Many oxides of metals are called basic oxides (basic anhydrides) because they will react with acids.
Fe2O3(s) + 6 HCl(aq) → 2 FeCl3(aq) + 3 H2O(l)
Fe2O3(s) + 6 H+(aq) → 2 Fe3+ (aq) + 3 H2O(l)
Many oxides of metals that have a +1 or +2 charge will react with water to form a basic solution:
Na2O(s) + H2O(l) → 2 NaOH(aq)
Na2O(s) + H2O(l) → 2 Na+(aq) + 2OH—(aq)
Many nonmetal oxides are called acidic oxides (acidic anhydrides) because they react with water to form an acidic solution:
CO2(g) + H2O(l) → H2CO3(aq)
H2CO3(aq) is named carbonic acid and is the reason that most carbonated beverages are slightly acidic. It is also the reason that soft drinks have fizz, because carbonic acid will decompose to form carbon dioxide and water.
Acid—Base Reactions
In general, acids react with bases to form a salt and, usually, water. The salt will depend upon which acid and base are used:
HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq)
HNO3(aq) + KOH(aq) → H2O(l) + KNO3(aq)
HBr(aq) + NH3(aq) → NH4Br(aq)
Reactions of this type are called neutralization reactions.
The first two neutralization equations are represented by the same net ionic equation:
H+(aq) + OH—(aq) → H2O(l)
In the third case, the net ionic equation is different:
H+(aq) + NH3(aq) → NH4+(aq)
As mentioned previously, certain salts have acid—base properties. In general, salts containing cations of strong bases and anions of strong acids are neither acidic nor basic. They are neutral, reacting with neither acids nor bases. An example would be potassium nitrate, KNO3. The potassium comes from the strong base KOH and the nitrate from the strong acid HNO3.
Salts containing cations not of strong bases but with anions of strong acids behave as acidic salts. An example would be ammonium chloride, NH4Cl:
2 NH4Cl(aq) + Ba(OH)2(aq) → BaCl2(aq) + 2 NH3(aq) + 2 H2O(l)
NH4+(aq) + OH—(aq) → NH3(aq) + H2O(l)
Cations of strong bases and anions not of strong acids are basic salts. An example would be sodium carbonate, Na2CO3. It reacts with an acid to form carbonic acid, which would then decompose to carbon dioxide and water:
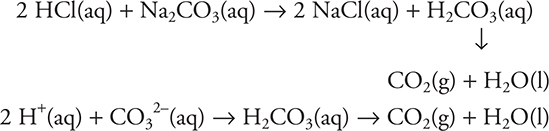
The same type of reaction would be true for acid carbonates, such as sodium bicarbonate, NaHCO3.
Another group of compounds that have acid—base properties are the hydrides of the alkali metals and of calcium, strontium, and barium. These hydrides will react with water to form the hydroxide ion and hydrogen gas:
NaH(s) + H2O(l) → NaOH(aq) + H2(g)
NaH(s) + H2O(l) → Na+(aq) + OH—(aq) + H2(g)
Note that in this case, water is behaving as H+OH—.
Acid—Base Titrations
A common laboratory application of acid—base reactions is a titration. (Note, there are other types of titrations.) A titration is a laboratory procedure in which a solution of known concentration is used to determine the concentration of an unknown solution. For strong acid/strong base titration systems, the net ionic equation is:
H+(aq) + OH—(aq) → H2O(l)
In a titration, the titrant is added to the analyte. The titrant is normally added from a buret.
For example, suppose you wanted to determine the molarity of an HCl solution. You would pipette a known volume of the acid into a flask and add a couple of drops of a suitable acid—base indicator. An indicator that is commonly used is phenolphthalein, which is colorless in an acidic solution and pink in a basic solution. You would then fill a buret with a strong base solution (NaOH is commonly used) of known concentration. The buret allows you to add small amounts of the base solution to the acid solution in the flask. See Figure 12.1 for the experimental setup for a titration. The course of the titration can also be followed using a pH meter. Initially the pH of the solution will be low since it is an acid solution. As the base is added and neutralization of the acid takes place, the pH will slowly rise. Small amounts of the base are added until one reaches the equivalence point.
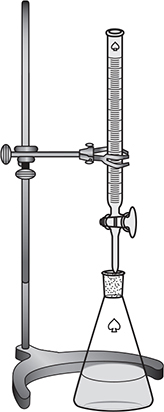
Figure 12.1 Titration setup.
The equivalence point is that point in the titration where the number of moles of H+ in the acid solution has been exactly neutralized with the same number of moles of OH—:
moles H+ = moles OH— at the equivalence point
For the titration of a strong acid with a strong base, the pH rapidly rises in the vicinity of the equivalence point. Then, as the tiniest amount of base is added in excess, the indicator turns pink. This is called the endpoint of the titration. In an accurate titration, the endpoint will be as close to the equivalence point as possible. For simple titrations that do not use a pH meter, it is assumed that the endpoint and the equivalence point are the same, so that:
moles H+ = moles OH— at the endpoint
After the equivalence point has been passed, the pH is greater than 7 (basic solution) and begins to level out somewhat. Figure 12.2 shows the shape of the curve for this titration.
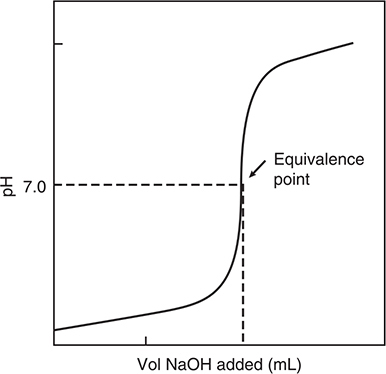
Figure 12.2 Titration of a strong acid with a strong base.
Reaction stoichiometry can then be used to solve for the molarity of the acid solution.
See Chapter 6, Stoichiometry, for a discussion of solution stoichiometry. In addition, you will see more on titrations in Chapter 20, Experimental Investigations.
An unknown base can be titrated with an acid solution of known concentration. One major difference is that the pH will be greater than 7 initially and will decrease as the titration proceeds. The other major difference is that the indicator will start pink, and the color will vanish at the endpoint.
Experiments

Laboratory experiments involving reactions are usually concerned with both the reaction and the stoichiometry. You need some idea of the balanced chemical equation. In the case of an acid—base reaction, an acid reacts with a base. The acid supplies H+ and the base accepts the H+. If the acid is diprotic, such as H2SO4, it can donate two H+.
The key to any reaction experiment is moles. The numbers of moles may be calculated from various measurements. A sample may be weighed on a balance to give the mass, and the moles calculated with the formula weight. Or the mass of a substance may be determined using a volume measurement combined with the density. The volume of a solution may be measured with a pipette or calculated (NOT measured) from the final and initial readings from a buret. This volume, along with the molarity, can be used to calculate the moles present. The volume, temperature, and pressure of a gas can be measured and used to calculate the moles of a gas. You must be extremely careful on the AP Exam to distinguish between those values that you measure and those that you calculate.
The moles of any substance in a reaction may be converted to the moles of any other substance through a calculation using the balanced chemical equation. Other calculations are presented in Chapter 6, Stoichiometry.
Common Mistakes to Avoid
1. In balancing chemical equations don’t change the subscripts in the chemical formula, just the coefficients.
2. Molecular compounds ionize as do ionic compounds.
3. In writing ionic and net ionic equations, show the chemical species as they actually exist in solution (i.e., strong electrolytes as ions, etc.).
4. In writing ionic and net ionic equations, don’t break apart covalently bonded compounds unless they are strong acids that are ionizing.
5. Know the solubility rules as guidelines.
6. Oxidizing and reducing agents are reactants, not products.
7. The products of the complete combustion of a hydrocarbon are carbon dioxide and water. This is also true if oxygen is present as well; but if some other element, like sulfur, is present you will also have something else in addition to carbon dioxide and water.
8. If a substance that does not contain carbon, like elemental sulfur, undergoes complete combustion, no carbon dioxide can be formed.
9. If an alcohol like methanol, CH3OH, is dissolved in water, no hydroxide ion, OH—, will be formed.
10. Know the strong acids and bases; assume all other acids and bases are weak.
11. HF is not a strong acid.
12. In titration calculations, you must consider the reaction stoichiometry.
13. Be sure to indicate the charges on ions correctly.
14. The common coordination numbers of complex ions are 2, 4, and 6.
15. Do not confuse measured values and calculated values.
![]() Review Questions
Review Questions
Here are questions you can use to review the content of this chapter and practice for the
AP Chemistry Exam. First are 20 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. Following those is a short free-response question like ones in Section II of the exam. To make these review questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. Fe(OH)2(s) + _____ H3PO4(aq) → _____ Fe3(PO4)2(s) + _____ H2O(l)
After the above chemical equation is balanced, the lowest whole-number coefficient for water is:
(A) 3
(B) 1
(C) 9
(D) 6
2. This ion will generate gas bubbles upon the addition of hydrochloric acid.
(A) Cu2+
(B) CO32—
(C) Fe3+
(D) Al3+
3. Aqueous solutions of this ion are blue.
(A) Cu2+
(B) CO32—
(C) Fe3+
(D) Al3+
4. Which of the following best represents the balanced net ionic equation for the reaction of lead(II) carbonate with concentrated hydrochloric acid? In this reaction, all lead compounds are insoluble.
(A) Pb2CO3(s) + 2 H+(aq) + Cl—(aq) → Pb2Cl(s) + CO2(g) + H2O(l)
(B) PbCO3(s) + 2 H+(aq) + 2 Cl—(aq) → PbCl2(s) + CO2(g) + H2O(l)
(C) PbCO3(s) + 2 H+(aq) → Pb2+(aq) + CO2(g) + H2O(l)
(D) PbCO3(s) + 2 Cl—(aq) → PbCl2(s) + CO32—(aq)
5. A sample of copper metal is reacted with concentrated nitric acid in the absence of air. After the reaction, which of these final products are present?
(A) CuNO3 and H2O
(B) Cu(NO3)3, NO, and H2O
(C) Cu(NO3)2, NO, and H2O
(D) CuNO3, H2O, and H2
6. Which of the following is the correct net ionic equation for the reaction of acetic acid with potassium hydroxide?
(A) HC2H3O2(aq) + OH—(aq) → C2H3O2—(aq) + H2O(l)
(B) HC2H3O2(aq) + K+(aq) → KC2H3O2(aq) + H+(l)
(C) HC2H3O2(aq) + KOH(aq) → KC2H3O2(s) + H2O(l)
(D) H+(aq) + OH—(aq) → H2O(l)
7. Which of the following is the correct net ionic equation for the addition of aqueous ammonia to a precipitate of silver chloride?
(A) AgCl(s) + 2 NH3(aq) → [Ag(NH3)2]+(aq) + Cl—(aq)
(B) AgCl(s) + 2 NH4+(aq) → [Ag(NH4)2]3+(aq) + Cl—(aq)
(C) AgCl(s) + NH4+(aq) → Ag+(aq) + NH4Cl(s)
(D) AgCl(s) + NH3(aq) → Ag+(aq) + NH3Cl(aq)
8. Potassium metal will react with water to release a gas and form a potassium compound. Which of the following is true?
(A) The final solution is basic.
(B) The gas is oxygen.
(C) The potassium compound precipitates.
(D) The potassium compound will react with strong bases.
9. It is possible to analyze an iron sample for percent iron by dissolving the sample in sulfuric acid and titrating the solution with standard potassium dichromate solution. The balanced molecular equation for the titration is:
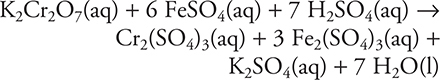
Which of the following is the correct net ionic equation for this reaction?
(A) 2 Cr6+(aq) + 6 Fe2+(aq) → 2 Cr3+(aq) + 6 Fe3+(aq)
(B) Cr2O72—(aq) + 6 Fe2+(aq) + 14 H+(aq) → 2 Cr3+(aq) + 6 Fe3+(aq) + 7 H2O(l)
(C) Cr2O72—(aq) + 6 FeSO4(aq) + 14 H+(aq) + 6 SO42—(aq) → Cr2(SO4)3(aq) + 3 Fe2(SO4)3(aq) + 7 H2O(l)
(D) K2Cr2O7(aq) + 6 FeSO4(aq) + 7 H2SO4(aq) →
Cr2(SO4)3(aq) + 3 Fe2(SO4)3(aq) + K2SO4(aq) + 7 H2O(l)
10. How many moles of Pb(NO3)2 must be added to 0.10 L of a solution that is 1.0 M in MgCl2 and 1.0 M in KCl to precipitate all the chloride ion? The compound PbCl2 precipitates.
(A) 1.0 mole
(B) 0.20 mole
(C) 0.50 mole
(D) 0.15 mole
11. When 50.0 mL of 1.0 M AgNO3 is added to 50.0 mL of 0.50 M HCl, a precipitate of AgCl forms. After the reaction is complete, what is the concentration of silver ions in the solution?
(A) 0.50 M
(B) 0.0 M
(C) 1.0 M
(D) 0.25 M
12. A student mixes 50.0 mL of 0.10 M Pb(NO3)2 solution with 50.0 mL of 0.10 M KCl. A white precipitate forms, and the concentration of the chloride ion becomes very small. Which of the following correctly places the concentrations of the remaining ions in order of decreasing concentration?
(B) [NO3—] > [Pb2+] > [K+]
(B) [NO3—] > [K+] > [Pb2+]
(C) [K+] > [NO3—] > [Pb2+]
(D) [Pb2+] > [NO3—] > [K+]
13. A solution is prepared for qualitative analysis. The solution contains the following ions: Co2+, Pb2+, and Al3+. Which of the following will cause no observable reaction?
(A) Dilute NH3(aq) is added.
(B) Dilute K2CrO4(aq) is added.
(C) Dilute HNO3(aq) is added.
(D) Dilute K2S(aq) is added.
14. Chlorine gas is bubbled through a colorless solution, and the solution turns reddish. Adding a little methylene chloride to the solution extracts the color into the methylene chloride layer. Which of the following ions may be present in the original solution?
(A) Cl—
(B) I—
(C) SO42—
(D) Br—
15. The addition of excess concentrated NaOH(aq) to a 1.0 M (NH4)2SO4 solution will result in which of the following observations?
(A) The solution becomes neutral.
(B) The formation of a brown precipitate takes place.
(C) Nothing happens because the two solutions are immiscible.
(D) The odor of ammonia will be detected.
16. _____ C4H11N(l) + _____ O2(g) → _____ CO2(g) + _____ H2O(l) + _____ N2(g)
When the above equation is balanced, the lowest whole number coefficient for CO2 is:
(A) 4
(B) 16
(C) 27
(D) 32
Pb2+(aq) + SO42—(aq) → PbSO4(s)
17. A student mixes 50.00 mL of a 0.1000 M Pb(NO3)2(aq) solution with 50.00 mL of a 0.1000 M Na2SO4(aq) solution. The net ionic equation for the reaction that occurs is given above. After the reaction has gone to completion and all the precipitate has settled, the student tests the solution with an electrical conductivity meter. Which of the following results does the student observe?
(A) The solution is conducting because not all of the Pb2+(aq) has precipitated.
(B) The solution is nonconducting because all the ions have precipitated.
(C) The solution is nonconducting because PbSO4(s) is not an electrical conductor.
(D) The solution is conducting because of the spectator ions remaining.
2 CuI2(s) → 2 CuI(s) + Cu(s) + I2(s)
18. Which of the following best describes what type of reaction the above equation represents?
(A) It is a precipitation reaction.
(B) It is an oxidation—reduction reaction.
(C) It is a neutralization reaction.
(D) It is a combination reaction.
19. It is possible to represent the formula of any strong acid as HX and to represent the formula of any strong base as MOH. Using these two representations, which of the following is the net
ionic equation for strong acid—strong base reactions in aqueous solution?
(A) HX(aq) + MOH(aq) → MX(aq) + H2O(l)
(B) H+(aq) + OH—(aq) → H2O(l)
(C) HX(aq) + MOH(aq) → XOH(aq) + MH(aq)
(D) H+(aq) + X(aq) + M+(aq) + OH—(aq) → X—(aq) + M+(aq) + H2O(l)
20. A chemist titrates a Ba(OH)2 solution with an HF solution. BaF2 precipitates during the titration. Which of the following is the net ionic equation for the titration reaction?
(A) 2 H+(aq) + 2 F—(aq) + Ba2+(aq) + 2 OH—(aq) → BaF2(s) + 2 H2O(l)
(B) 2 HF(aq) + Ba(OH)2(aq) → BaF2(s) + 2 H2O(l)
(C) 2 HF(aq) + Ba2+(aq) + 2 OH—(aq) → BaF2(s) + 2 H2O(l)
(D) H+(aq) + OH—(aq) → H2O(l)
![]() Answers and Explanations
Answers and Explanations
1. D—The balanced equation is 3 Fe(OH)2(s) +
2 H3PO4(aq) → Fe3(PO4)2(s) + 6 H2O(l). If you had trouble balancing this equation, you need more practice.
2. B—Carbonates produce carbon dioxide gas in the presence of an acid. None of the other ions will react with hydrochloric acid to produce a gas.
3. A—Aqueous solutions of Cu2+ are normally blue. Iron ions give a variety of colors but are normally colorless, or nearly so, in the absence of complexing agents. The other ions are colorless.
4. B—Lead(II) carbonate is insoluble (given), so its formula should be written as PbCO3. Hydrochloric acid is a strong acid, so it should be written as separate H+ and Cl— ions. Lead(II) chloride, PbCl2, is insoluble (given), and carbonic acid, H2CO3, quickly decomposes to CO2 and H2O. Also notice that A cannot be correct because the charges do not balance. For practice, analyze the other answers and determine why they are wrong.
5. C—The balanced chemical equation is 3 Cu(s) +
8 HNO3(aq) → 3 Cu(NO3)2(aq) + 2 NO(g) +
4 H2O(l). The copper is below hydrogen on the activity series, so H2 cannot form by this acid—metal reaction. Nitric acid causes oxidation, which will oxidize copper to Cu2+, giving Cu(NO3)2. Some of the nitric acid reduces to NO. An oxidation and a reduction must ALWAYS be together, so if Cu is oxidized, HNO3 must be reduced. If air had been present, the NO would be converted to NO2.
6. A—Acetic acid is a weak acid; as such, it should be written as HC2H3O2. Potassium hydroxide is a strong base, so it will separate into K+ and OH— ions. Any potassium compound that might form is soluble and will yield K+ ions. The potassium ions are spectator ions and are left out of the net ionic equation.
7. A—Aqueous ammonia contains primarily NH3, which eliminates answers B and C. Ammonia is NH3 not NH4+. NH3Cl does not exist, which eliminates answer D. The reaction produces the silver—ammonia complex, [Ag(NH3)2]+. Notice that the key here is not knowing what the reaction is but being able to eliminate impossible answers because of the nomenclature.
8. A—The reaction of potassium to produce a potassium compound is an oxidation; therefore, there must be a reduction, and the only species available for reduction is hydrogen. The reaction is 2 K(s) + 2 H2O(l) → 2 KOH(aq) + H2(g). KOH is a water-soluble strong base, which will not react with other strong bases.
9. B—All the compounds in the molecular equation except H2O ionize in aqueous solution. [We know they are in aqueous solution because their chemical formulas are followed by (aq).] Answer (A) incorrectly breaks up the dichromate ion (Cr2O72—). Answer (C) incorrectly leaves the FeSO4(aq), Cr2(SO4)3(aq), and Fe2(SO4)3(aq) undissociated. Answer (D) does not separate any of the strong electrolytes [everything but H2O(l)] in solution, which is an error.
10. D—The magnesium chloride gives 0.20 mole of chloride ion, and the potassium chloride gives 0.10 mole of chloride ion. A total of 0.30 mole of chloride will react with 0.15 mole of lead, because two Cl— require one Pb2+. You may wish to do the actual calculations to see how the moles were determined.
11. D—The HCl is the limiting reagent. The HCl will react with one-half the silver to halve the concentration. The doubling of the volume (50 mL + 50 mL) halves the concentration a second time.
12. B—Since the Cl— became very small, it must have combined with a cation and precipitated. You have a KCl solution, so KCl will not precipitate This leaves PbCl2 as the only possible precipitate. Equal volumes of equal concentrations give the same number of moles of reactants; however, two nitrate ions are produced per solute formula as opposed to only one potassium ion. Initially, the lead and potassium would be equal, but some of the lead is precipitated as PbCl2.
13. C—Ammonia, as a base, will precipitate the metal hydroxides since the only soluble hydroxides are the strong bases. Chromate, sulfide, and chloride ions might precipitate one or more of the ions. Nitrates, from nitric acid, are soluble; therefore, this is the solution that is least likely to cause an observable change.
14. D—Chlorine causes oxidation. It is capable of oxidizing both B and D. Answer B gives I2, which is brownish in water and purplish in methylene chloride. Bromine solutions are reddish in both.
15. D—Excess strong base will ensure the solution is basic and not neutral. Both ammonium and sodium salts are soluble; therefore, no precipitate will form. One aqueous solution will mix with another aqueous solution. The following acid—base reaction occurs to release ammonia gas: NH4+(aq) + OH—(aq) → NH3(g) + H2O(l).
16. B—The balanced equation is 4 C4H11N(l) +
27 O2(g) → 16 CO2(g) + 22 H2O(l) + 2 N2(g). While organic chemistry is not part of the
AP curriculum, you should know how to balance any equation given to you.
17. D—The solutions have equal volumes with equal molarities; therefore, they contain equal moles (no need to calculate this). The reaction has 1:1 stoichiometry, so equal moles of Pb2+ and SO42— will react, and since they have equal moles, both are limiting, which means all the Pb2+ and SO42— has precipitated. This means that any answer with either of these ions in solution cannot be correct. In general, ionic solids, such as PbSO4(s), are nonconductors; however, since the PbSO4(s) is no longer in solution, this has nothing to do with the solution being conducting or nonconducting. While the net ionic equation shows that everything should precipitate, it is better to examine the complete ionic equation which is:
Pb2+(aq) + 2 NO3—(aq) + 2 Na+(aq) + SO42—(aq) → PbSO4(s) + 2 NO3—(aq) + 2 Na+(aq)
The NO3—(aq) + Na+(aq) are the spectator ions and remain in solution to conduct electricity.
18. B—One substance going to more than one product is a decomposition reaction; however, since this is not one of the choices, it is necessary to remember that decomposition reactions are a subcategory of oxidation—reduction reactions. The type of reaction could be determined by determining the oxidation number of the elements (actually only Cu or I needs to be determined). On the reactant side, the oxidation numbers are Cu = +2 and I = —1. On the product side, the oxidation numbers are Cu = +1 and 0, while I = —1 and 0. Any change in the oxidation states makes this an oxidation reduction reaction. A precipitation reaction requires a sold forming from a solution, and there is no solution present. A neutralization reaction requires an acid and a base, acids contain H+, and there is no hydrogen present. A combination reaction (also a subcategory of oxidation—reduction reactions) requires multiple reactants producing a single product.
19. B—If the symbolism (HX and MOH) confuses you, pick a specific strong acid and strong base, such as HCl and NaOH. Do not confuse the issue by choosing more complicated acids or bases, such as H2SO4 and Ba(OH)2. Both net ionic and complete ionic equations require ions to be present on at least one side of the reaction arrow. Equations containing either of the spectator ions (M+ and X—) on both sides of the reaction arrow are not net ionic equations. Note, the compounds XOH and MH could not possibly exist since each contains only ions with like charges (not + and —).
20. C—An ionic equation must have ions on at least one side of the reaction arrow. Since hydrofluoric acid, HF, is a weak acid, it is a weak electrolyte, which means it is not ionized in an ionic equation. Barium hydroxide, Ba(OH)2, is a strong base; therefore it is completely ionized in an ionic equation. The reaction H+(aq) + OH—(aq)
→ H2O(l) applies only to strong acids reacting with strong bases, which is not the case here.
![]() Free-Response Question
Free-Response Question
On the AP Exam, there will be both long-answer and short-answer free-response questions. The following is an example of a short-answer question.
You have 5 minutes to answer the following question. You may use a calculator and the tables in the back of the book.
Question
An iron(III) nitrate, Fe(NO3)3, solution is mixed with a potassium phosphate, K3PO4, solution, and a precipitate forms. What is the precipitate, and which ions, if any, are spectator ions in this reaction? Explain how you arrived at your answers.
![]() Answer and Explanation
Answer and Explanation
There are four ions present. These ions are K+, PO43—, Fe3+, and NO3—. The starting materials are in solution; therefore, they are soluble. The compounds that might form in the reaction are KNO3 and FePO4. Since potassium (and nitrate) salts are normally soluble, the precipitate must be FePO4. The spectator ions are the nitrate ions (NO3—) and the potassium ions (K+), the ions not in the precipitate.
Total your points. There are 4 points possible. You get 1 point for correctly identifying the precipitate. You get an additional 1 point for identifying the spectator ions and 2 points for the explanation.
![]() Rapid Review
Rapid Review
• Reaction questions will always appear in the free-response section of the AP Exam. This may not be true in the multiple-choice part.
• Energy will be released in a reaction (exothermic) or absorbed (endothermic).
• Chemical equations are balanced by adding coefficients in front of the chemical species until the number of each type of atom is the same on both the right and left sides of the arrow.
• The coefficients in the balanced equation must be in the lowest whole-number ratio.
• Water is the universal solvent, dissolving a wide variety of both ionic and polar substances.
• Electrolytes are substances that conduct an electrical current when dissolved in water; nonelectrolytes do not.
• Most ions in solution attract and bind a layer of water molecules in a process called solvation.
• Some molecular compounds, like acids, ionize in water, forming ions.
• In the molecular equation, the reactants and products are shown in their undissociated/ un-ionized form; the ionic equation shows the strong electrolytes in the form of ions; the net ionic equation drops out all spectator ions and shows only those species that are undergoing chemical change.
• Precipitation reactions form an insoluble compound, a precipitate, from the mixing of two soluble compounds.
• It will help if you have some idea of which ionic compounds are soluble.
• Redox reactions are reactions where oxidation and reduction take place simultaneously.
• Oxidation is the loss of electrons, and reduction is the gain of electrons.
• Combination reactions are usually redox reactions in which two or more reactants (elements or compounds) combine to form one product.
• Decomposition reactions are usually redox reactions in which a compound breaks down into two or more simpler substances.
• Single displacement reactions are redox reactions in which atoms of an element replace the atoms of another element in a compound.
• Combustion reactions are redox reactions in which the chemical species rapidly combine with diatomic oxygen gas, emitting heat and light. The products of the complete combustion of a hydrocarbon are carbon dioxide and water.
• Indicators are substances that exhibit different colors under acidic or basic conditions.
• Acids are proton donors (electron-pair acceptors).
• Bases are proton acceptors (electron-pair donors).
• Coordinate covalent bonds are covalent bonds in which one atom furnishes both electrons for the bond.