5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 4 Review the Knowledge You Need to Score High
14 Thermodynamics
IN THIS CHAPTER
In this chapter, the following AP topics are covered:
6.1 Endothermic and Exothermic Processes
6.2 Energy Diagrams
6.3 Heat Transfer and Thermal Equilibrium
6.4 Heat Capacity and Calorimetry
6.5 Energy of Phase Changes
6.6 Introduction to Enthalpy of Reaction
6.7 Bond Enthalpies
6.8 Enthalpy of Formation
6.9 Hess’s Law
9.1 Introduction to Entropy
9.2 Absolute Entropy and Entropy Change
9.3 Gibbs Free Energy and Thermodynamic Favorability
9.4 Thermodynamic and Kinetic Control
9.5 Free Energy and Equilibrium
9.6 Coupled Reactions
Summary: Thermodynamics is the study of heat and its transformations. Thermochemistry is the part of thermodynamics that deals with changes in heat that take place during chemical processes. We will be describing energy changes in this chapter. Energy can be of two types: kinetic or potential. Kinetic energy is energy of motion, while potential energy is stored energy. Energy can be converted from one form to another but, unless a nuclear reaction occurs, energy cannot be created or destroyed (Law of Conservation of Energy). We will discuss energy exchanges between a system and the surroundings. The system is that part of the universe that we are studying. The system may be a beaker, or it may be Earth, or any other region. The surroundings are the rest of the universe.
The most common units of energy used in the study of thermodynamics are the joule and the calorie. The joule (J) is defined as:
1 J = 1 kg m2 /s2
The calorie was originally defined as the amount of energy needed to raise the temperature of 1 g of water 1°C. Now it is defined in terms of its relationship to the joule:
1 cal = 4.184 J

It is important to realize that this is not the same calorie that is commonly associated with food and diets. That is the nutritional Calorie, Cal, which is really a kilocalorie (1 Cal = 1,000 cal).
Most of the variables discussed in this chapter are reported under standard conditions. For thermodynamics, the standard state is the value of the variable when the pressure is 1 atmosphere (1 bar) and, if a solution is involved, the concentration is 1 M. While not required, a temperature of 25°C (298 K) is often assumed. A degree symbol (°) is normally added to the variable to indicate that it is standard.
Keywords and Equations

Energy Diagrams
The energy changes for any process may be represented with an energy diagram. Simple processes require simple diagrams and complicated processes require complicated diagrams. The complicated diagrams are only several simple diagrams stacked together. Do not be intimidated by a diagram. A simple diagram is shown in Figure 14.1. If the process involved in this figure is water melting or freezing, State 1 would be ice and State 2 would be liquid water, and the endothermic process would be melting and the exothermic process would be freezing.
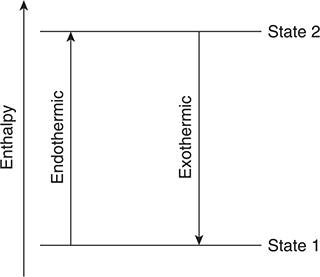
Figure 14.1 A simplified energy diagram showing an endothermic and an exothermic process.
Heat Transfer and Thermal Equilibrium
One fundamental process of thermodynamics is that heat will spontaneously flow from a hot object to a cold object. For example, an ice cube taken from a freezer and placed in a glass will spontaneously warm until it melts, and eventually reach the same temperature as the room where the glass is located.

Do not confuse exothermic and endothermic, like many other students do.

Heat and temperature are totally different concepts. They can never be interchanged. Heat always has heat units (joules, kilojoules, or even calories), while temperature always has temperature units (degrees Celsius or kelvin).
At the particle level, there is an exchange in kinetic energy between the particles. Particles at a higher temperature will have a higher average kinetic energy than particles at a lower temperature. No matter what the temperature or the state of matter, the particles present will display a Maxwell—Boltzmann distribution of energies.
Let’s reexamine the ice-cube-in-a-glass example. When the ice cube is first placed in the glass, the bottom of the ice cube touches the inside bottom of the glass (and possibly one or more sides). This results in glass particles encountering ice particles. The glass particles have a higher average kinetic energy than the water particles. (The form of the matter each is in limits the amount of motion but not the average kinetic energy.) Glass particles collide with ice particles at the interface of the two, with the higher-energy particles transferring some of their kinetic energies to the lower-kinetic-energy particles. This process is known by many names, such as “heat transfer,” “heat exchange,” or “transfer of energy as heat.” These higher-energy ice particles transfer some of this energy to ice particles in the interior of the ice. This process continues until the ice begins to melt, and after all the ice has melted, the process will continue with the liquid formed until the water and the glass are at the same temperature. When the water and the glass are at the same temperature, they are said to be in thermal equilibrium.
Calorimetry
Calorimetry is the laboratory technique used to measure the heat released or absorbed during a chemical or physical change. The quantity of heat absorbed or released during a chemical or physical change (chemical reaction or phase transition) is represented as q and is proportional to the change in temperature of the system being studied. This system has what is called a heat capacity, which is the quantity of heat needed to change the temperature 1 K. It has the form:
heat capacity = q/ΔT
Heat capacity most commonly has units of J/K. The specific heat capacity (or specific heat) (c) is the quantity of heat needed to raise the temperature of 1 g of a substance 1 K:
![]()
where m is the mass of the substance.
The specific heat capacity commonly has units of J/g K. Because of the original definition of the calorie, the specific heat capacity of water is 4.184 J/g K. If the specific heat capacity, the mass, and the change of temperature are all known, the amount of energy absorbed can easily be calculated.
Another related quantity is the molar heat capacity (C), the amount of heat needed to change the temperature of 1 mole of a substance by 1 K.
Different substances have different heat capacities. Therefore, adding equal amounts of energy to two different substances will not necessarily produce the same temperature change.
Calorimetry involves the use of a laboratory instrument called a calorimeter. Two types of calorimeter, a simple coffee-cup calorimeter, and a more sophisticated bomb calorimeter, are shown in Figure 14.2. In both, a process is carried out with known amounts of substances and the change in temperature is measured.
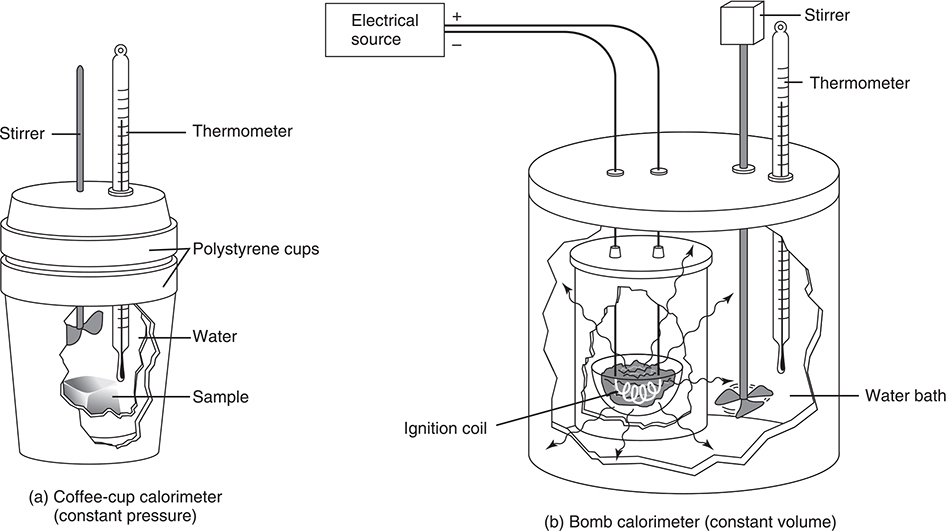
Figure 14.2 Two types of calorimeters.
The coffee-cup calorimeter can be used to measure the heat changes in reactions or processes that are open to the atmosphere: qp, constant-pressure reactions. These might be reactions that occur in open beakers and the like. This type of calorimeter is also commonly used to measure the specific heats of solids (the system). A known mass of solid is heated to a certain temperature (initial temperature of the solid) and then is added to the calorimeter containing a known mass of water at a known temperature (initial temperature of the water). The final temperature is then measured, allowing us to calculate two ΔT values, one for the solid (ΔTsolid) and one for the water (ΔTwater). (Remember, you cannot measure the change in temperature.) We know that the heat lost by the solid (the system) is equal to the heat gained by the surroundings (the water and calorimeter, although for simple coffee-cup calorimetry, the heat gained by the calorimeter is small and is normally ignored):
![]()
This is a simple expression of the First Law of Thermodynamics.
Substituting the mathematical relationship for q gives:
![]()
This equation can then be solved for the specific heat capacity of the solid.
The constant-volume bomb calorimeter is used to measure the energy changes that occur during combustion reactions. A weighed sample of the substance being investigated is placed in the calorimeter and compressed oxygen is added. The sample is ignited by a hot wire, and the temperature change of the calorimeter and a known mass of water is measured. The heat capacity of the calorimeter/water system is sometimes known.
For example, a 1.0155-g sample of ethanol (C2H6O) was ignited in a bomb calorimeter. The temperature increased by 3.889°C. The heat capacity of the calorimeter was 3.562 kJ/°C, and the calorimeter contained 1.000 kg of water (specific heat capacity = 4.184 J g—1 °C—1). Find the molar heat of reaction (i.e., kJ/mole) for:
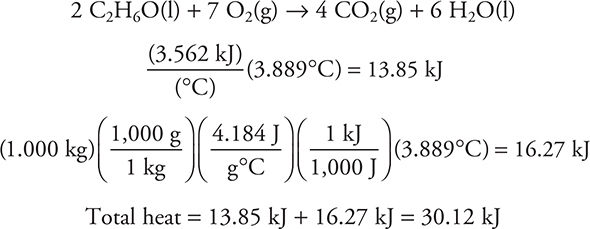
Note: The temperature increased so the reaction was exothermic (—).
![]()
This is not molar (yet); one more conversion is needed:
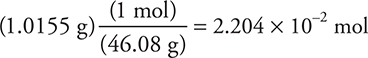
Answer:
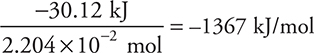
Heating increases the energy of the system and cooling lowers the energy of a system. The energy of the system may also be changed by phase transitions or chemical reactions.
Energy of Phase Changes
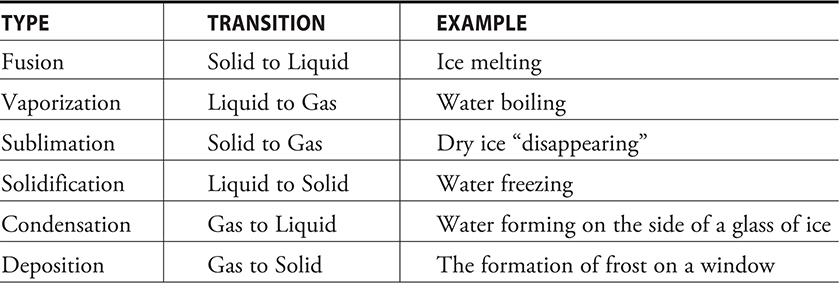
Any physical or chemical process will involve energy. The amount of energy and whether the energy enters or leaves the system depends on the process. One category of physical changes includes the phase changes. The most common phase changes are fusion (melting), vaporization, sublimation, solidification (freezing), condensation, and deposition. The processes involved are listed in the Table 14.1.
Table 14.1 Common Types of Phase Changes
There are other phase changes, such as the conversion of one solid phase into another; however, they are normally not covered on the AP Exam.
The first three processes in Table 14.1 are endothermic, and the last three processes are exothermic. The endothermic processes are usually listed in tables, while the exothermic processes are usually not tabulated. For example, it requires 0.334 kJ of energy to melt a gram of water. The energy released to freeze that same gram of water is 0.334 kJ. The two processes are simply the reverse of each other, and, as with other substances, a simple reversal of a processes only reverses the sign of the energy change. Thus, tables of the endothermic processes become tables of the exothermic processes simply by changing the signs of the values.
The energies associated with each of these processes are normally referred to as the “heat of . . .” or the “enthalpy of . . . .” Thus when water melts, the energy change is known as the heat of fusion or the enthalpy of fusion and symbolizes as qfusion or ΔHfusion. The values listed may be per gram or per mole.
In general, the amount of energy involved in a phase transition depends upon the strength of the intermolecular forces involved. For example, the very strong hydrogen bonds in water makes phase changes involving water involve much more energy than phase changes involving other substances. For example, while the heat of fusion of water is 0.334 kJ g—1, the heat of fusion of nonpolar nitrogen is 0.0129 kJ g—1. Overcoming the intermolecular forces requires energy and reforming these intermolecular forces releases that quantity of energy.
Using water as an example, when ices melts, it requires 6.01 kJ mol—1. When water vaporizes, it requires 44.0 kJ mol—1. When ice sublimes, it requires 51.1 kJ mol—1. These values will vary with temperature. Even though all of the processes involve overcoming hydrogen bonding, the value for fusion is the smallest of the three because only sufficient hydrogen bonds are overcome to break down the structure of ice; some of the hydrogen bonds are still present in water. Vaporization completely overcomes the remaining hydrogen bonds. Sublimation is the sum of these two processes.
Introduction to Enthalpy of Reaction
Any chemical reaction involves energy. This energy is referred to as the Heat of Reaction or the Enthalpy of Reaction (normally in molar terms). The quantity of energy involved is often determined using a calorimeter. It is possible to add this information to a chemical reaction to generate a thermochemical equation such as:
![]()
Thermochemical equations may be written for any reaction and are often used in Hess’s law problems.
Bond Enthalpies
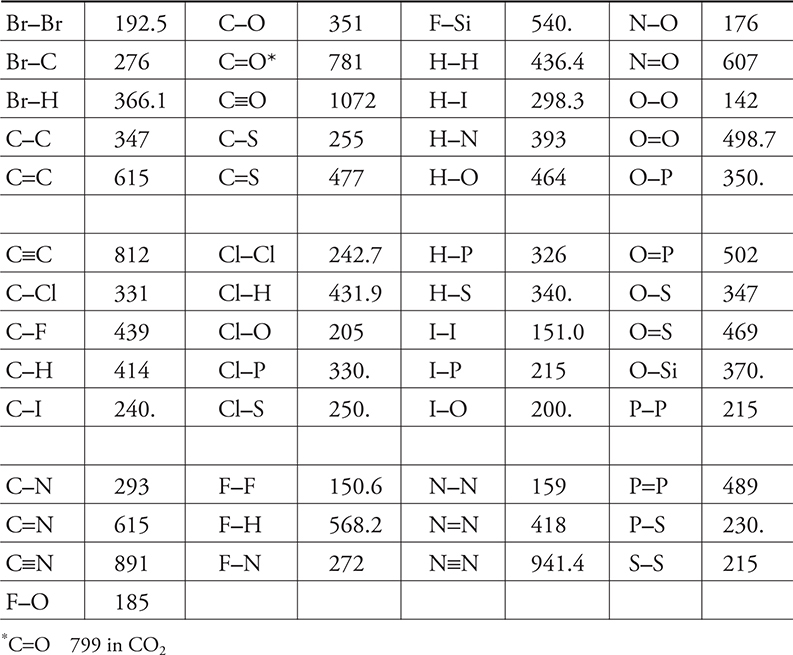
The formation of a covalent bond releases energy and breaking that bond requires an equal quantity of energy. These energies are referred to as bond enthalpies or bond energies. The energy involved depends upon the elements involved in the bond and upon whether the bond is single, double, or triple. Note: a double bond does not necessarily involve double the energy of a single bond, and a triple bond is not necessarily triple the energy of a single bond. Other factors, such a resonance, will also alter the values. Table 14.2 is a table of average bond enthalpies.
Table 14.2 Some Average Bond Enthalpies in kJ mol—1
To determine the enthalpy change from bond enthalpies, use the following equation:
![]()
Let’s use bond enthalpies in the following example.
What is the enthalpy change for the following reaction, which is used in the Born—Haber process?
![]()
Solution:
Rewriting the reaction emphasizing the bonds gives:
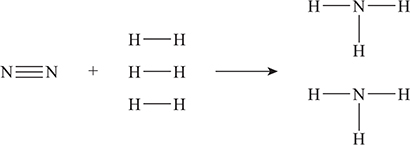
From Table 14.2 the bond enthalpies needed are: N≡N = 941.4, H—H = 436.4, and H—N = 393, all values are in kJ mol—1. Using these values in (ΔHreaction = Σ bonds broken — Σ bonds formed) gives:
![]()
In this calculation, the first 3 and the 2 are the coefficients from the balanced chemical equation. The second 3 is the number of N—H bonds in each ammonia molecule.
The enthalpy change for this reaction using standard heats of formation is —91.8 kJ mol—1. This value is different from the value calculated from the bond enthalpies. A discrepancy is not unusual. Values in bond enthalpy tables are average values, and the bonds in a particular substance may be above or below average. Also, while not present here, resonance will yield a discrepancy with resonance increasing the stability. So why use bond enthalpies to determine the enthalpy of reaction? One reason is that heat of reaction data may be lacking for one or more substances involved in a reaction; therefore, bond enthalpies are the only option. Data is often lacking for newly discovered materials.
Laws of Thermodynamics
The First Law of Thermodynamics states that the total energy of the universe is constant. This is simply the Law of Conservation of Energy. This can be mathematically stated as:
![]()
The Second Law of Thermodynamics involves a term called entropy. Entropy (S) is related to the disorder of a system. The Second Law of Thermodynamics states that all processes that occur spontaneously move in the direction of an increase in entropy of the universe (system + surroundings). Mathematically, this can be stated as:
![]() for a spontaneous process
for a spontaneous process
For a reversible process, ΔSuniverse = 0. The qualitative entropy change (increase or decrease of entropy) for a system can sometimes be determined using a few simple rules:
1. Entropy increases when the number of molecules increases during a reaction.
2. Entropy increases with an increase in temperature.
3. Entropy increases when a gas is formed from a liquid or solid.
4. Entropy increases when a liquid is formed from a solid.
5. Entropy increases when the volume increases.
6. Entropy increases when a chemical reaction increases the number of moles of gas.
Reversing any of these processes results in a decrease in entropy.
Let us now look at some applications of these first two laws of thermodynamics.
Hess’s Law
Enthalpies

Chemists study reactions under a variety of conditions, such as reactions that occur at constant pressure. During the discussion of the coffee-cup calorimeter, the heat change at constant pressure was defined as qp. Because this constant-pressure situation is so common in chemistry, a special thermodynamic term is used to describe this energy: enthalpy. The enthalpy change, ΔH, is equal to the heat gained or lost by the system under constant-pressure conditions. The following sign conventions apply:
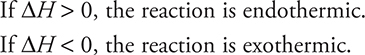
If a reaction is involved, ΔH is sometimes indicated as ΔHreaction. ΔH is often given in association with a specific process. For example, the enthalpy change associated with the formation of water from hydrogen and oxygen gases can be shown in this fashion:
![]()
The negative sign indicates that this reaction for the formation of water is exothermic. This value of ΔH is for the formation of 1 mol of water. If 2 mol were produced, ΔH would be twice this value or —483.6 kJ. The techniques developed in working reaction stoichiometry problems (see Chapter 6, Stoichiometry) also apply here. In addition, thermochemical equations, such as this one, may contain fractions because these equations are restricted to moles and never molecules. A fraction of a molecule is not possible, but a fraction of a mole is allowed.
If the previous reaction for the formation of water were reversed, the sign of ΔH would be reversed. That would indicate that it would take 483.6 kJ of energy to decompose 2 moles of water. This would then become an endothermic process.
ΔH is dependent upon the state of matter. The enthalpy change would be different for the formation of liquid water instead of gaseous water and a third value would apply if ice were formed instead of liquid or gaseous water.
ΔH can also indicate whether a reaction will be spontaneous. A negative (exothermic) value of ΔH is associated with a spontaneous reaction. However, in many reactions this is not the case. There is another factor to consider in predicting a reaction’s spontaneity. We will cover this other factor a little later in this chapter.
Enthalpies of reaction can be measured using a calorimeter. However, they can also be calculated in other ways. Hess’s law states that if a reaction occurs in a series of steps, then the enthalpy change for the overall reaction is simply the sum of the enthalpy changes of the individual steps. If, in adding the equations of the steps together, it is necessary to reverse one of the given reactions, then the sign of ΔH must also be reversed. Also, be very careful of the reaction stoichiometry. The value of an individual ΔH may need to be adjusted.
It doesn’t matter whether the steps used are the actual steps in the mechanism of the reaction, because ΔHreaction (ΔHrxn) is a state function, a function that doesn’t depend on the pathway, but only on the initial and final states.
Let’s see how Hess’s law can be applied, given the following information:
One step in the Ostwald process to produce nitric acid is the combustion of ammonia in the presence of a platinum catalyst. The reaction is:
![]()
Use the following thermodynamic equations and Hess’s law to determine the heat of reaction for this process.
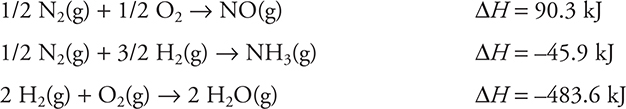
Answer:
It is necessary to manipulate the three given equations so that they sum to the desired equation. To do this, the first equation must be multiplied by 4, which will also multiply the enthalpy change by 4. The second equation will need to be reversed (reversing the sign of its ΔH), and it will also be necessary to multiply the reaction and enthalpy change by 4. Finally, multiply the third equation and its enthalpy change by 3. In terms of the reactions, these steps are:

Equal amounts of anything appearing on opposites sides of the reaction arrow will cancel, and if they are on the side, they will sum. This gives:
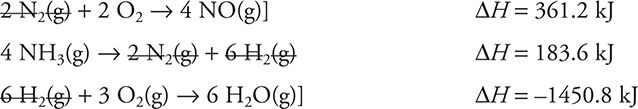
Summing the equations and the enthalpy values leaves:
![]()

Enthalpies of Formation
Enthalpies of reaction can also be calculated from individual enthalpies of formation (or heats of formation), ΔHf for the reactants and products. Because the temperature, pressure, and state of the substance will cause these enthalpies to vary, it is common to use a standard state convention. For gases, the standard state is 1 atm pressure. For a substance in an aqueous solution, the standard state is 1 molar concentration. And, for a pure substance (compound or element), the standard state is the most stable form at 1 atm pressure and 25°C. A degree symbol to the right of the H indicates a standard state, ΔH°. The standard enthalpy of formation of a substance (ΔHf°) is the change in enthalpy when 1 mole of the substance is formed from its elements when all substances are in their standard states. There are tables containing this information in the form of standard heats of formation (![]() ). These values are for the formation of ONE mole of a substance from the ELEMENTS under standard conditions. In a table of
). These values are for the formation of ONE mole of a substance from the ELEMENTS under standard conditions. In a table of ![]() values, there would be an entry such as H2O(g) = —241.8 kJ. If the conditions are not standard, the enthalpy will be different than the standard value.
values, there would be an entry such as H2O(g) = —241.8 kJ. If the conditions are not standard, the enthalpy will be different than the standard value.
![]() of an element in its standard state is zero.
of an element in its standard state is zero.
![]() can be determined from the tabulated
can be determined from the tabulated ![]() of the individual reactants and products. It is the sum of the
of the individual reactants and products. It is the sum of the ![]() of the products minus the sum of the
of the products minus the sum of the ![]() of the reactants:
of the reactants:
![]()
In using this equation be sure to consider the number of moles of each, because ![]() for the individual compounds refers to the formation of 1 mole.
for the individual compounds refers to the formation of 1 mole.
For example, let’s use standard enthalpies of formation to calculate ΔHrxn for:
![]()
Answer:
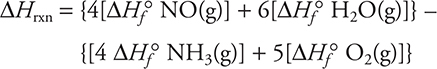
Using tabulated standard enthalpies of formation gives:
![]()
People commonly forget to subtract all the reactants from the products.
The values of ![]() will be given to you on the AP Exam, or you will be asked to stop before putting the numbers into the problem. To save time, the value for all elements in their standard states is defined as zero.
will be given to you on the AP Exam, or you will be asked to stop before putting the numbers into the problem. To save time, the value for all elements in their standard states is defined as zero.
An alternative means of estimating the heat of reaction is to take the sum of the average bond energies of the reactant molecules and subtract the sum of the average bond energies of the product molecules.
Entropies
In much the same way as ΔHf was determined, the standard molar entropies (S°) of elements and compounds can be tabulated. The standard molar entropy is the entropy associated with 1 mole of a substance in its standard state. Entropies are also tabulated, but unlike enthalpies, the entropies of elements are not zero. For a reaction, the standard entropy change is calculated in the same way as the enthalpies of reaction:
![]()

Calculate ΔS° for the following reaction. If you do not have a table of S° values, just set up the problems.
Note: these are thermochemical equations, so fractions are allowed.
![]()
Answer:
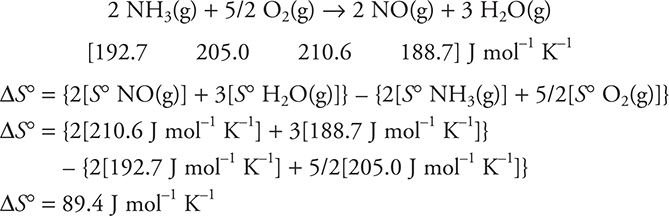
One of the goals of chemists is to be able to predict if a reaction will be spontaneous or nonspontaneous. Some general guidelines for a spontaneous reaction have already been presented (negative ΔH and positive ΔS ), but neither is a reliable predictor by itself. Temperature also plays a part. A thermodynamic factor that combines the entropy, enthalpy, and temperature of a process is the best indicator of spontaneity. This factor is called the Gibbs free energy.
Gibbs Free Energy
The Gibbs free energy (G) is a thermodynamic function that combines the enthalpy, entropy, and temperature:
G = H — TS, where T is the Kelvin temperature
Like most thermodynamic functions, only the change in Gibbs free energy can be measured, so the relationship becomes:
ΔG = ΔH — TΔS
• If ΔG > 0, the reaction is not spontaneous (thermodynamically unfavored); energy must be supplied to cause the reaction to occur.
• If ΔG < 0, the reaction is spontaneous (thermodynamically favored).
• If ΔG = 0, the reaction is at equilibrium (neither spontaneous nor nonspontaneous).
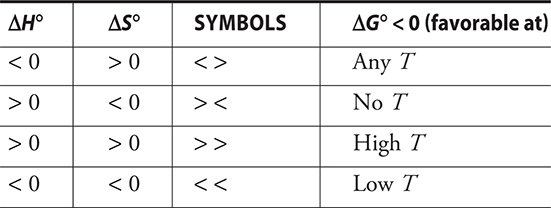
Thermodynamic favorability may also be predicted by using the following table.
If there is a ΔG associated with a reaction and that reaction is then reversed, the sign of ΔG also reverses.
Just like with the enthalpy and entropy, the standard Gibbs free energy change, ΔG°, is calculated:
![]()
ΔGf° of an element in its standard state is zero.
ΔG° for a reaction may also be calculated by using the standard enthalpy and standard entropy of reaction:
![]()
Calculate ΔG° for:
![]()
(If you do not have a table of ΔG° values, just set up the problems.)
Answer:
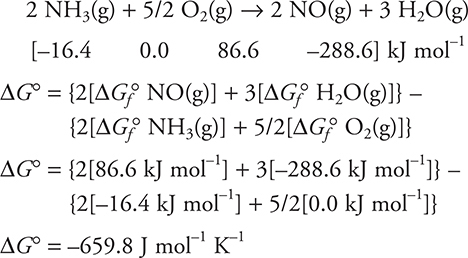
Thermodynamics and Equilibrium
Thus far, we have considered only situations under standard conditions. But how do we cope with nonstandard conditions? The change in Gibbs free energy under nonstandard conditions is:
![]()
Q is the activity quotient, products over reactants (see Chapter 15, Equilibrium). This equation allows the calculation of ΔG in those situations in which the concentrations or pressures are not 1.
Note that Q, when at equilibrium, becomes K, and ΔG = 0. This equation gives us a way to calculate the equilibrium constant, K, from a knowledge of the standard Gibbs free energy of the reaction and the temperature. Substituting K for Q, entering ΔG = 0, and rearranging converts the preceding equation to:
![]()
This equation sometimes appears in another form:
![]()
For example, calculate ΔG° for:
![]()
Note: ° = 298 K
Answer:
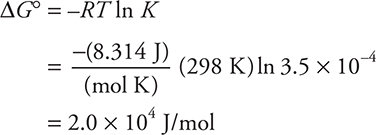
Thermodynamic and Kinetic Control
Why do some reactions not occur when they are thermodynamically favored? The energy diagram in Figure 13.3 for an exothermic reaction offers an explanation. This reaction is thermodynamically favored; however, the reactants must get over the barrier (activation energy) to reach the products. The higher the barrier is, the slower the reaction will be. An extremely high barrier will lead to an extremely slow reaction. A reaction with an extremely high activation energy is considered to be under kinetic control.
A high activation energy is the primary reason for a reaction to be under kinetic control. It is improper to assume the system is in equilibrium simply because there is no noticeable change in a reasonable time.
Coupled Reactions
A thermodynamically unfavorable process may be forced to occur through coupled reactions. There are many examples of coupled reactions in biological systems.
The following example illustrates coupled reactions.
The decomposition of PbCO3(s) has a positive ΔG°reaction; therefore, it is not thermodynamically favored.
![]()
The combustion of carbon has a negative ΔG°reaction; therefore, it is a thermodynamically favored process.
![]()
If these two processes are coupled, a thermodynamically favored reaction results.
![]()
A coupled reaction may also come from combining a thermodynamically unfavored process with some other energy source. Examples are using an external power source to charge a battery or using sunlight to drive photosynthesis.
Experiments

The most common thermodynamic experiment is a calorimetry experiment. In this experiment, the heat of transition or heat of reaction is determined.
The experiment will require a balance to determine the mass of a sample and possibly a pipet to measure a volume, from which a mass may be calculated using the density. A calorimeter, usually a polystyrene (Styrofoam) cup, is needed to contain the reaction. Finally, a thermometer is required. Tables of heat capacities or specific heats may be provided.
Mass and possible volume measurements, along with the initial and final temperatures, are needed. Remember: you measure the initial and final temperature so you can calculate the change in temperature.
After the temperature change is calculated, there are several ways to proceed. If the calorimeter contains water, the heat may be calculated by multiplying the specific heat of water (normally given) by the mass of water by the calculated temperature change. The heat capacity of the calorimeter may be calculated by dividing the heat by the temperature change. If a reaction is carried out in the same calorimeter, the heat from that reaction is the difference between the heat with and without a reaction.

Do not forget, the thermometer is part of the surroundings, so if the temperature increases, heat has gone from the system to the thermometer (surroundings), making the process exothermic, and the heat has a negative sign. The opposite is true if the temperature drops.
Common Mistakes to Avoid

1. Be sure your units cancel, giving you the unit desired in the final answer.
2. Check your significant figures.
3. Don’t mix energy units, joules, and calories.
4. Watch your signs in all the thermodynamic calculations. They are extremely important.
5. Don’t confuse enthalpy, ΔH, entropy, ΔS, and ΔG.
6. Pay close attention to the state of matter for your reactants and products and choose the appropriate value for use in your calculated entropies and enthalpies.
7. Remember: products minus reactants.
8. ΔHf and ΔGf are for 1 mole of substance. Use appropriate multipliers if needed.
9. ΔGf and ΔHf for an element in its standard state are zero.
10. All temperatures are in kelvin.
11. When using ΔG ° = ΔH°rxn — T ΔS°rxn, pay attention to your enthalpy and entropy units. Commonly, enthalpies will use kJ and entropies J. This means that you will be required to make a J to kJ conversion or a kJ to J conversion.
12. Be careful when calculating ΔT. The Δ means final — initial. For example: What is ΔT when a sample of water changes from 0°C to 25°C? Therefore, 0°C (273 K) is the initial temperature, and 25°C (298 K) is the final temperature. So:
![]()
When calculating a ΔT, it does not matter if you are in °C or K; the numerical values will be the same. In addition, if the change was from 25°C to 0°C, the answer would be —25°C (—25 K).
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. First are 24 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. This list includes questions to help you review prior knowledge. Following those is a long free-response question like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 35 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. Which of the following is the minimum energy required to initiate a reaction?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) activation energy
2. What is the minimum energy required to force a nonspontaneous reaction to occur?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) activation energy
3. A student heats a 25-g sample of lead metal to 65°C. This sample is immediately clamped in contact with a 47-g sample of magnesium metal. The original temperature of the magnesium was 25°C. No heat was lost to the surroundings during the experiment. The specific heat of lead metal is 0.127 J/g °C, and the specific heat of magnesium metal is 1.024 J/g °C. The final temperature of both metals was 27°C. What is one possible conclusion the student might postulate from this experiment?
(A) The lead lost more heat than the magnesium gained.
(B) The lead lost the same amount of heat as the magnesium gained.
(C) The lead lost less heat than the magnesium gained.
(D) The student made an error because the final temperature should be the average (45°C).
4. What is the energy released when gaseous ions combine to form an ionic solid?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) activation energy
5. 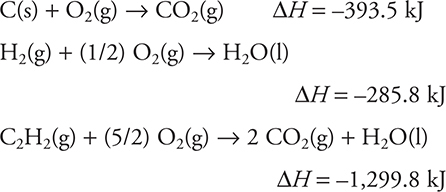
Given this information, find the enthalpy change for 2 C(s) + H2(g) → C2H2(g).
(A) 454.0 kJ
(B) —227.0 kJ
(C) 0.0 kJ
(D) 227.0 kJ
6. A 10-g sample of solid mercury metal is sealed inside a well-insulated, rigid container. The temperature inside the container is at the melting point of mercury metal (—38.8°C). The system is well insulated, so it is possible to assume the insulation prevents any energy change with the surroundings. Which of the following is true about the total energy and the entropy of the system after equilibrium has been established in the system?
(A) The total energy increases. The total entropy will increase.
(B) The total energy is constant. The total entropy is constant.
(C) The total energy is constant. The total entropy will decrease.
(D) The total energy is constant. The total entropy will increase.
7. When ammonium chloride dissolves in water, the temperature drops. Which of the following conclusions may be related to this?
(A) Ammonium chloride is more soluble in hot water.
(B) Ammonium chloride produces an ideal solution in water.
(C) The heat of solution for ammonium chloride is exothermic.
(D) Ammonium chloride has a low lattice energy.
8. Which of the following reactions is expected to have the greatest increase in entropy?
(A) H2O(g) → H2O(l)
(B) 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
(C) Ca(s) + H2(g) → CaH2(s)
(D) N2(g) + 3 H2(g) → 2 NH3(g)
9. Magnesium metal reacts readily with liquid bromine under standard conditions. Which of the following conclusions may be drawn from this fact?
(A) Keq < 1 and ΔG° > 0
(B) Keq > 1 and ΔG° = 0
(C) Keq < 1 and ΔG° < 0
(D) Keq > 1 and ΔG° < 0
10. Which of the following combinations is true when sodium chloride melts?
(A) ΔH > 0 and ΔS > 0
(B) ΔH = 0 and ΔS > 0
(C) ΔH > 0 and ΔS < 0
(D) ΔH < 0 and ΔS < 0
11. Which of the following reactions has a negative entropy change?
(A) 2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(g)
(B) 2 NH3(g) → N2(g) + 3 H2(g)
(C) CaCl2(s) → Ca(s) + Cl2(g)
(D) 2 H2(g) + O2(g) → 2 H2O(l)
12. A certain reaction is nonspontaneous under standard conditions but becomes spontaneous at higher temperatures. What conclusions may be drawn under standard conditions?
(A) ΔH < 0, ΔS > 0, and ΔG > 0
(B) ΔH > 0, ΔS < 0, and ΔG > 0
(C) ΔH > 0, ΔS > 0, and ΔG > 0
(D) ΔH < 0, ΔS < 0, and ΔG > 0
13. 2 H2(g) + O2(g) → 2 H2O(l)
From the table below, determine the enthalpy change for the above reaction.
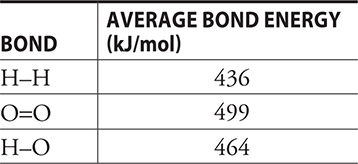
(A) 0 kJ
(B) 485 kJ
(C) —485 kJ
(D) 464 kJ
14. Which of the following reactions is accompanied by the greatest increase in entropy?
(A) 2 H2(g) + O2(g) → 2 H2O(g)
(B) 2 Mn2O7(l) → 4 MnO2(s) + 3 O2(g)
(C) 2 C(s) + O2(g) → 2 CO(g)
(D) 2 Mg(s) + O2(g) → 2 MgO(s)
15. Hydrogen gas burns in oxygen gas according to the following reaction:
2 H2(g) + O2(g) → 2 H2O(l) ΔH = —572 kJ
What is the energy change when 2.00 moles of water decompose to the elements at constant pressure?
(A) —286 kJ
(B) —572 kJ
(C) +572 kJ
(D) +286 kJ
16. A solution is prepared by dissolving solid ammonium nitrate, NH4NO3, in water. The initial temperature of the water was 25°C, but after the solid had dissolved, the temperature fell to 20°C. What conclusions may be made about ΔH and ΔS?
(A) ΔH < 0 and ΔS > 0
(B) ΔH > 0 and ΔS > 0
(C) ΔH > 0 and ΔS < 0
(D) ΔH < 0 and ΔS < 0
17. The temperature increases when lithium sulfate, Li2SO4, dissolves in water. Which of the following conclusions may be related to this?
(A) Lithium sulfate is less soluble in hot water.
(B) The hydration energies of lithium ions and sulfate ions are very low.
(C) The heat of solution for lithium sulfate is endothermic.
(D) Lithium sulfate solutions are not ideal solutions.
18. Calcium carbonate, CaCO3, decomposes when heated according to the following reaction:
CaCO3(s) ⇆ CaO(s) + CO2(g)
Increasing the temperature shifts the equilibrium to the right. Which of the following is applicable to this reaction?
(A) The reaction is faster at higher temperatures.
(B) The process is enthalpy and entropy driven.
(C) The process is enthalpy driven.
(D) The process is entropy driven.
19. Which of the following combinations is true when ethanol, C2H5OH, boils?
(A) ΔH > 0 and ΔS > 0
(B) ΔH = 0 and ΔS > 0
(C) ΔH > 0 and ΔS < 0
(D) ΔH < 0 and ΔS < 0
Use the following information to answer questions 20—23:
![]()
The above reaction is the standard heat of formation reaction for xenon tetrafluoride.
20. For this reaction, what can be said about the value of ΔS°?
(A) ΔS° is near zero.
(B) ΔS° is positive.
(C) ΔS° is negative.
(D) The reaction does not involve ΔS°.
21. This reaction is spontaneous under standard conditions. What will happen to the value of ΔG for this reaction when the temperature increases if you assume ΔS is negative?
(A) ΔG will decrease.
(B) ΔG will increase.
(C) ΔG will remain the same.
(D) Only ΔHf° is important; ΔG does not matter.
22. What will happen to the value of K for this reaction as the temperature increases? Assume that ΔG for this reaction is increasing.
(A) K will decrease.
(B) K will increase.
(C) K will approach 1.
(D) K is a constant and will not change
23. If you have a table of standard thermodynamic values for the substances involved in the reaction, how might the temperature at which the reaction changes from spontaneous to nonspontaneous be predicted?
(A) It is impossible to predict the temperature.
(B) The change will occur at 298 K (25°C).
(C) ΔG cannot change from spontaneous to nonspontaneous.
(D) At this point, ΔG = 0, which leads to T = ΔH/ΔS.
24. Both carbon monoxide, CO, and nitrogen, N2, have about the same molar mass (28 g mol—1). Both molecules have a triple bond between the two atoms. At absolute zero, the entropy of CO(s) is greater than 4 J mol—1 K—1, and that of N2(s) is 0 J mol—1 K—1. Why is the standard entropy of CO(g) greater than that of N2(g)?
(A) CO is more disordered than N2.
(B) The presence of carbon leads to greater entropy.
(C) Oxygen is more electronegative than nitrogen.
(D) The triple bond in CO is polar, whereas the triple bond in N2 is nonpolar.
![]() Answers and Explanations
Answers and Explanations
1. D—You may wish to review the Kinetics chapter if you have forgotten what the activation energy is.
2. A—The free energy is the minimum energy required for a nonspontaneous reaction to occur and the maximum energy available from a spontaneous reaction.
3. B—The Law of Conservation of Energy (First Law of Thermodynamics) says the amount of heat lost must be equal to the heat gained. For each metal, the heat (q) involved is determined from q = mcΔT. The hot metal will lose heat (—q), and the cold metal will gain heat (+q).
4. B—This process is the reverse of the lattice energy definition.
5. D—This is a Hess’s law problem. Double the first equation given (and ΔH). Use the second equation as is. Reverse the third equation (changing the sign of ΔH). Cancel equal amounts of each substance that appears on each side of the reaction arrow and add the results. If the sum exactly matches the desired equation, the sum of the ΔH values is the answer. If you do not get an exact match, you made an error.
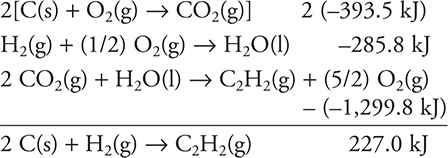
Simple rounding to the nearest 100 kJ gives 200 kJ.
6. D—The system is insulated, and no work can be done on or by the system (rigid container); thus, the energy is constant. At the melting point, some of the mercury will spontaneously melt; changing from a solid to a liquid increases the entropy.
7. A—The process is endothermic (the ammonium chloride is absorbing heat to cool the water). Endothermic processes are “helped” by higher temperatures. C and possibly D would give an increase in temperature. There is insufficient information about B. Do not forget, the thermometer measuring the temperature is part of the surroundings and not part of the system.
8. B—The reaction showing the greatest increase in the number of moles of gas will show the greatest entropy increase. If no gases are present, then the greatest increase in the number of moles of liquid would yield the greatest increase.
9. D—The reaction occurs readily; therefore, it must be a spontaneous reaction. If the reaction is spontaneous, then ΔG° < 0. If the free energy is negative free energy, then K must be large (> 1). (The calculation would be ΔG = —RT ln Keq.)
10. A—Heat is required to melt something (ΔH > 0). A transformation from a solid to a liquid gives an increase in entropy (ΔS > 0).
11. D—This equation has an overall decrease in the amount of gas present (decreases entropy). The other answers produce more gas (increasing entropy).
12. C—Nonspontaneous means that ΔG > 0. Since the reaction becomes spontaneous, the sign must change. Recall: ΔG = ΔH — TΔS (given in the exam booklet). The sign change at higher temperature means that the entropy term (with ΔS > 0) must become more negative than the enthalpy term (ΔH > 0).
13. C—The calculation is [2(436 kJ) + 499 kJ] — {2[2(464 kJ)]} = —485 kJ. (Using bond energies ΔH = Σ bonds broken — Σ bonds formed.)
14. B—This is the reaction that has the greatest increase in the number of moles of gas.
15. C—The decomposition of water is the reverse of the reaction shown; therefore, the enthalpy change is reversed (positive instead of negative). The amount of water decomposing is 2.00 moles, which is the same amount of water in the reaction.
16. B—Dissolving almost always has ΔS > 0. A decrease in temperature means the process has ΔH > 0 (the system is absorbing energy from the surroundings). The thermometer measuring the temperature is part of the surroundings.
17. A—The increase in temperature of the solution indicates that the process is exothermic. An exothermic process will shift toward the starting materials (solid lithium sulfate and water) when heated.
18. D—This is a thermodynamic problem, so a kinetics answer (A) is not applicable. For the reaction to occur, ΔG must be negative. It is possible to determine ΔG from ΔG = ΔH — TΔS. Since heating causes the equilibrium to shift to the right, ΔH must be positive. A positive ΔH will not lead to a negative ΔG; therefore, enthalpy cannot be the driving force (eliminating B and C). Thus, the reaction must be entropy driven because ΔS is positive owing to the release of the carbon dioxide gas.
19. A—Heat is required to boil a substance; therefore, ΔH is greater than 0. A transformation from a liquid to a gas gives an increase in entropy (ΔS > 0).
20. C—The value is negative because there is a decrease in the number of moles of gas during the reaction.
21. B—The key relationship is ΔG = ΔH — TΔS. From this relationship, it is apparent that the value of ΔG will increase (become less negative) as the temperature increases. In general, both ΔH and ΔS are relatively constant with respect to small temperature changes. As the temperature increases, the value of the entropy term, TΔS, becomes more negative. The negative sign in front of this term leads to a positive contribution. The value of ΔG will first become less negative (more positive) and eventually become positive (no longer spontaneous).
22. A—Recall that ΔG = —RT ln K. As the value of ΔG increases, the value of K will decrease.
23. D—Recalling ΔG = ΔH — TΔS, it is possible to determine ΔG, ΔH, and ΔS from the standard thermodynamic values. The change from spontaneous to nonspontaneous occurs when ΔG = 0. Rearranging this equation and setting ΔG = 0 gives T = ΔH/ΔS, which will allow the temperature to be estimated.
24. A—Carbon monoxide exhibits two molecular orientations, CO and OC, while the orientations of N2 both appear the same. For this reason, there are more states (disorder and higher entropy) present in solid CO.
![]() Free-Response Question
Free-Response Question
You have 10 minutes to answer the following question. You may use a calculator and the tables in the back of the book.
Question
![]()
Under standard conditions, the enthalpy change for the above reaction going from left to right (forward reaction) is ΔH ° = —294 kJ.
(a) In the above reaction, is the value of ΔS° positive or negative? Justify your conclusion.
(b) The above reaction is spontaneous under standard conditions. Predict what will happen to ΔG for this reaction as the temperature is increased. Justify your prediction.
(c) Will the value of K remain the same, increase, or decrease as the temperature increases? Justify your prediction.
(d) Show how the temperature at which the reaction changes from spontaneous to nonspontaneous can be predicted. What additional information is necessary?
(e) Draw the Lewis structure of XeF6.
![]() Answer and Explanation
Answer and Explanation
(a) The value is negative. The decrease in the number of moles of gas (from 4 to 1) during the reaction means there is a decrease in entropy.
Give yourself 1 point if you predicted negative. Give yourself 1 point for discussing the decreasing number of moles of gas. You may get this point even if you did not get the first point.
(b) Recall that ΔG = ΔH — TΔS. (This equation is given on the equation page of the AP Exam.) The value of ΔG will increase (become less negative) as the temperature is increased.
Give yourself 1 point for this answer if it is obvious that increasing means less negative.
In general, both ΔH and ΔS are relatively constant with respect to small temperature changes. As the temperature increases, the value of the entropy term, TΔS, becomes more negative. The negative sign in front of this term leads to a positive contribution. The value of ΔG will first become less negative (more positive) and eventually become positive (no longer spontaneous).
Give yourself 1 point for the ΔG = ΔH — TΔS argument even if you did not get the first point.
(c) Recall that ΔG = —RT ln K. (This equation is given on the equation page of the AP Exam.) The value of K will decrease as the temperature increases (due to the negative sign).
You get 1 point for this answer.
As the value of ΔG increases [see part (b)], the value of K will decrease. You get 1 point for using ΔG = —RT ln K in your discussion.
If you got the justification for part (b) wrong, and you used the same argument here, you will not be penalized twice. You still get your point here.
(d) The reaction changes from spontaneous to nonspontaneous when ΔG = 0.
Recall that ΔG = ΔH — TΔS (given on exam).
Rearranging this equation to T = — ΔH/ΔS allows the temperature to be estimated.
This rearranged equation is worth 1 point. To do the calculation, the value of ΔS is the additional information needed. Give yourself 1 point for this.
(e) The Lewis structure is
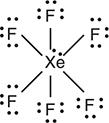
The complete Lewis structure, with all fluorine atoms attached to the xenon atom, is worth 2 points. If all the fluorine atoms do not have an octet of electrons, the structure is worth only 1 point. Even if you have not worked a Lewis structure of this type, you should know that fluorine will follow the octet rule. This illustrates the diversity of the free-response questions on the AP Exam.
![]() Rapid Review
Rapid Review
• Thermodynamics is the study of heat and its transformations.
• Kinetic energy is energy of motion, while potential energy is stored energy.
• The common units of energy are the joule, J, and the calorie, cal.
• A calorimeter is used to measure the heat released or absorbed during a chemical or physical change. Know how a calorimeter works.
• The specific heat capacity is the amount of heat needed to change the temperature of 1 g of a substance by 1 K, while the molar heat capacity is the heat capacity per mole.
• The heat lost by the system in calorimetry is equal to the heat gained by the surroundings.
• The specific heat (c) of a solid can be calculated by: —(csolid × msolid × ΔTsolid) = cwater × mwater × ΔTwater or by q = cmΔT.
• The First Law of Thermodynamics states that the total energy of the universe is constant. (Energy is neither created nor destroyed.)
• The Second Law of Thermodynamics states that all spontaneous processes move in a way that increases the entropy (disorder) of the universe.
• The enthalpy change, ΔH, is equal to the heat lost or gained by the system under constant-pressure conditions.
• ΔH values are associated with a specific reaction. If that reaction is reversed, the sign of ΔH changes. If one must use a multiplier on the reaction, the multiplier must also be applied to the ΔH value.
• The standard enthalpy of formation of a compound, ΔHf°, is the enthalpy change when 1 mole of the substance is formed from its elements and all substances are in their standard states.
• The standard enthalpy of formation of an element in its standard state is zero.
• ΔH°rxn = Σ ΔHf° products — Σ ΔHf° reactants. Know how to apply this equation.
• ΔH°rxn is usually negative for a spontaneous reaction.
• ΔS° = Σ S° products — Σ S° reactants. Know how to apply this equation.
• ΔS° is usually positive for a spontaneous reaction.
• The Gibbs free energy is a thermodynamic quantity that relates the enthalpy, entropy, and temperature and is the best indicator if a reaction is spontaneous, nonspontaneous, or at equilibrium.
• If ΔG° > 0, the reaction is not spontaneous; if ΔG° < 0, the reaction is spontaneous; and if ΔG° = 0, the reaction is at equilibrium.
• ΔG° = Σ ΔGf° products — Σ ΔGf° reactants. Know how to apply this equation.
• ΔG° = ΔH°rxn — T ΔS°rxn. Know how to apply this equation.
• For a system at equilibrium: ΔG ° = —RT ln K = —2.303 RT log K. Know how to apply this equation to calculate equilibrium constants.