5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 4 Review the Knowledge You Need to Score High
16 Acids and Bases
IN THIS CHAPTER
8.1 Introduction to Acids and Bases
8.2 pH and pOH of Strong Acids and Bases
8.3 Weak Acid and Base Equilibria
8.4 Acid—Base Reactions and Buffers
8.5 Acid—Base Titrations
8.6 Molecular Structure of Acids and Bases
8.7 pH and pKa
8.8 Properties of Buffers
8.9 Henderson—Hasselbalch Equation
8.10 Buffer Capacity
Summary: In Chapter 12, Reactions and Periodicity, we introduced the concept of acids and bases. Recall that acids are proton (H+) donors and bases are proton acceptors. Also, recall that acids and bases may be strong or weak. Strong acids completely dissociate in water (strong electrolytes); weak acids only partially dissociate (weak electrolytes). There are generally only two strong bases (strong electrolytes) to consider: the hydroxide and the oxide ion (OH- and O2-, respectively). All other common bases are weak (weak electrolytes). In Chapter 12, our discussion focused on strong acids and bases and their reactions. In this chapter we will concentrate on weak acids and bases, their equilibria and reactions.
It is important to be able to recognize the strong acids and the strong bases. You should assume all other acids and bases are weak unless told otherwise. As a reminder, the strong acids are: HCl, HBr, HI, HNO3, H2SO4, HClO3, and HClO4. The strong bases are the hydroxides of Li, Na, K, Rb, Cs, Ca, Sr, and Ba.
In this chapter we will also cover pH and pOH, alternate ways of expressing the concentration of weak acids and bases. We will also cover buffers and how they react to control the pH of solutions. In addition we will present the topic of the acid—base properties of salts. Finally, we will address the topic of acid—base titrations involving weak acids and weak bases.
It is important to remember equilibria is equilibria—the general techniques that you learned to handle general equilibrium situations still apply with regards to acid—base equilibria.
Keywords and Equations
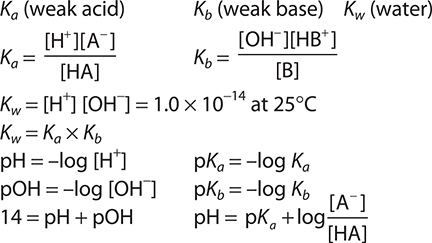
Acid—Base Equilibrium
Consider two acids: HCl (strong) and CH3COOH (weak). If each is added to water to form aqueous solutions, the following reactions take place:
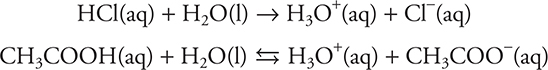
These reactions are sometimes simplified to:
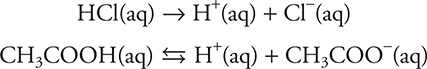
In these latter two equations, H+(aq) is equal to H3O+(aq). However, even though acids donate H+, using H+ in the equation for an aqueous solution is not correct. It should be noted that both H3O+(aq) and H+(aq) are simplifications, as the “true” formula of the hydrogen ion in solution is between H9O4+(aq) and H11O5+(aq).
The first reaction essentially goes to completion—there is no HCl left in solution. The second reaction is an equilibrium reaction—there are appreciable amounts of both reactants and products left in solution.
There are generally only two strong bases (strong electrolytes) to consider: the hydroxide and the oxide ion (OH- and O2-, respectively). All other common bases are weak (weak electrolytes). Weak bases, like weak acids, also establish an equilibrium system, as in aqueous solutions of ammonia:
![]()
In the Brønsted—Lowry acid—base theory, there is competition for an H+. Consider the acid—base reaction between acetic acid, a weak acid, and ammonia, a weak base:
![]()
Acetic acid donates a proton (H+) to ammonia in the forward (left-to-right) reaction of the equilibrium to form the acetate and ammonium ions. But in the reverse (right-to-left) reaction, the ammonium ion donates a proton to the acetate ion to form ammonia and acetic acid. The ammonium ion is acting as an acid and the acetate ion as a base. Under the Brønsted—Lowry system, acetic acid (CH3COOH) and the acetate ion (CH3COO-) are called a conjugate acid—base pair. Conjugate acid—base pairs differ by only a single H+. Ammonia (NH3) and the ammonium ion (NH4+) are also a conjugate acid—base pair. In this reaction, there is a competition for the H+ between the acetic acid and the ammonium ion. To predict on which side the equilibrium will lie, this general rule applies: the equilibrium will favor the side in which the weaker acid and base are present. Figure 16.1 shows the relative strengths of the conjugate acid—base pairs.
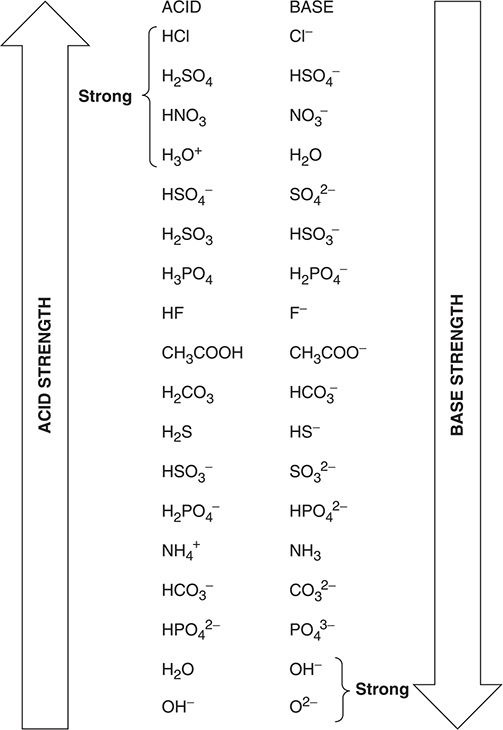
Figure 16.1 Conjugate acid—base pair strengths.
In Figure 16.1, you can see that acetic acid is a stronger acid than the ammonium ion and ammonia is a stronger base than the acetate ion. Therefore, the equilibrium will lie to the right.
The reasoning above allows us to find good qualitative answers, but to be able to do quantitative problems (how much is present, etc.), the extent of the dissociation of the weak acids and bases must be known. That is where a modification of the equilibrium constant is useful.
Ka—the Acid Dissociation Constant
Strong acids completely dissociate (ionize) in water. Weak acids partially dissociate and establish an equilibrium system. But as shown in Figure 16.1, there is a large range of weak acids based upon their ability to donate protons. Consider the general weak acid, HA, and its reaction when placed in water:
![]()
An equilibrium constant expression can be written for this system:
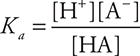
The [H2O] is assumed to be a constant and is incorporated into the Ka value. It is not shown in the equilibrium constant expression.
Since this is the equilibrium constant associated with a weak acid dissociation, this specific Kc is most commonly called the acid dissociation constant, Ka. The Ka expression is then:
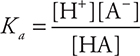
In some cases, the pKa is needed. The pKa is calculated using the following relationship:
![]()
In general, if the pH < pKa, there will be more of the acid form, HA, in solution than the base form, A-. Also if pH > pKa, then the base form will predominate. If pH = pKa the concentrations of the two forms will be equal.
Many times the weak acid dissociation reaction will be shown in a shortened notation, omitting the water:
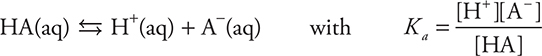
The greater the amount of dissociation is, the larger the value of Ka. Table 16.1 shows the Ka values of some common weak acids. Strong acids do not have a Ka.
Table 16.1 The Formulas, Structures, and Ka Values for Some Acids. (Acids in Order of Decreasing Ka)
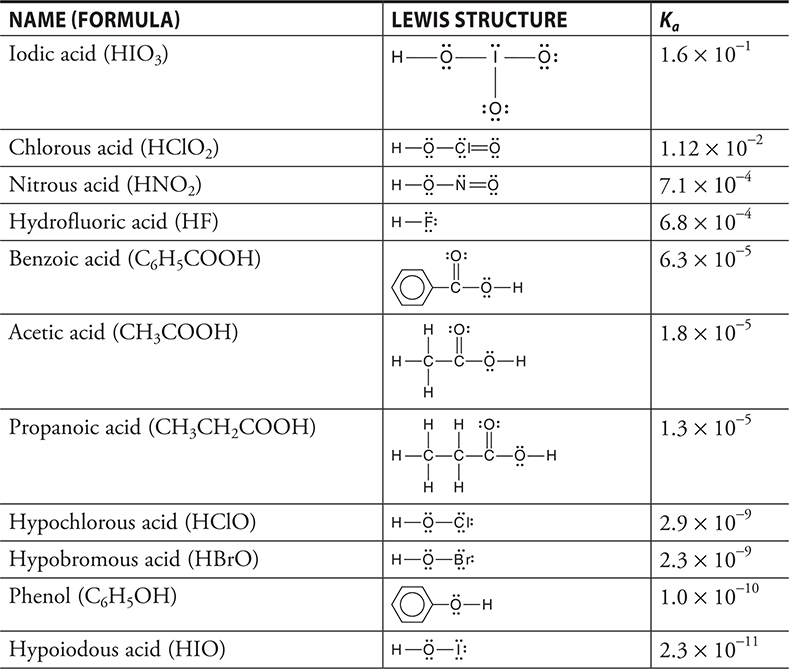
The [HA] is the equilibrium molar concentration of the undissociated weak acid, not its initial concentration. The exact expression would then be [HA] = Minitial — [H+], where Minitial is the initial concentration of the weak acid. This is true because for every H+ that is formed, an HA must have dissociated. However, many times if Ka is small, you can approximate the equilibrium concentration of the weak acid by its initial concentration, [HA] = Minitial.
If the initial molarity and Ka of the weak acid are known, the [H+] (or [A-]) can be calculated easily. And if the initial molarity and [H+] are known, Ka can be calculated.
For example, calculate the [H+] of a 0.250 M benzoic acid solution.
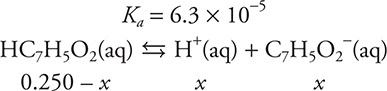
The quantity of acid that dissociated is x, which must be subtracted from the initial concentration (0.250 M) to give (0.250 — x)M. Each acid molecule that dissociates produces a hydrogen ion (H+) and a benzoate ion (C7H5O2-), so x dissociated molecules produces x H+ and x C7H5O2-. These equilibrium amounts are listed below the respective formulas in the equilibrium reaction and substituted for the formulas in the Ka expression.
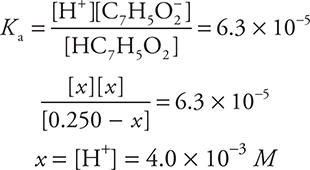
Strong acids are 100% dissociated, and weak acids are less than 100% ionized. In the problem just completed, we can calculate the percent dissociation of the acid by:
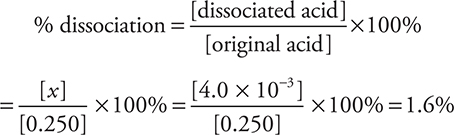
For polyprotic acids, acids that can donate more than one proton, the Ka for the first dissociation is much larger than the Ka for the second dissociation. If there is a third Ka, it is much smaller still. For most practical purposes, you can simply use the first Ka.
Remember: Ka expressions are for weak acids. Never ever use a Ka for a strong acid.
Kw—the Water Dissociation Constant
Before examining the equilibrium behavior of aqueous solutions of weak bases, let’s look at the behavior of water itself. In the initial discussion of acid—base equilibrium above, we showed water acting both as an acid (proton donor when put with a base) and a base (proton acceptor when put with an acid). Water is amphoteric; it will act as either an acid or a base, depending on whether the other species is a base or acid. But in pure water the same amphoteric nature is noted. In pure water, an exceedingly small amount of proton transfer is taking place:
![]()
This is commonly written as:
![]()
There is an equilibrium constant, called the water dissociation constant, Kw, which has the form:
![]()
Again, the concentration of water is a constant and is incorporated into Kw.
The numerical value of Kw of 1.0 × 10-14 is true for the product of the [H+] and [OH-] in pure water and for aqueous solutions of acids and bases. This equilibrium is always present any time H2O(l) is present.
In the discussion of weak acids, we indicated that the [H+] = [A-]. However, there are two sources of H+ in the system: the weak acid and water. The amount of H+ that is due to the water dissociation is exceedingly small and can be easily ignored.
Molecular Structure of Acids and Bases
The structures of some acids are shown in Table 16.1. The key to the strength of an acid is how easy it is for the acid to donate an H+. There are many factors influencing this, and while some of these are complimentary, others work against each other. One factor allowing an acid to donate an H+ in aqueous solution is the ability of H2O to hydrogen bond to an H atom before it is donated. The greater the strength of this hydrogen bond relative to the bond holding the H to the acid, the stronger the acid. The presence of very electronegative elements tends to stabilize the A- ions formed, and thus increase the strength of the acid. Multiple electronegative atoms are usually better than one.
If we compare the halogen acids HF(aq), HCl(aq), HBr(aq), and HI(aq), HF(aq) is weak and the other three are strong. The three strong acids are strong because the hydrogen—halogen bond is weak relative to the hydrogen bonding present. In the case of HF(aq), the acid is weak because fluorine forms a stronger hydrogen bond than oxygen.
Three of the acids in Table 16.1 are carboxylic acids. If you examine their structures, you will see a common feature of all carboxylic acids. The acidic hydrogen in these structures is the hydrogen to the far right of the structure. Carboxylic acids are a common category of weak acids. Even though these are organic acids, they may appear on the AP Exam in acid—base problems or Lewis structure problems. The organic chemistry of these compounds will not appear on the exam.
The conjugate bases of the carboxylic acids are the carboxylate ions, which are common weak bases. For example, the carboxylate ion derived from acetic acid, CH3COOH, is the acetate ion, CH3COO-. Other common weak bases are nitrogenous bases, the simplest of which is ammonia, NH3. Consider the following representation of the structure of the nitrogenous bases and their acceptance of an H+:
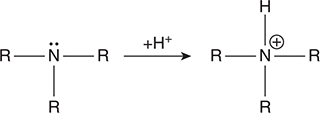
If all three R groups are hydrogen, the reaction is ammonia changing to the ammonium ion. If one or more of the R groups is an organic group, such a CH3—, the compound is an amine, which are the common organic bases. As with the carboxylic acids, the organic chemistry of amines is not an AP topic; however the acid—base chemistry of these compounds is an AP topic.
pH
Because the concentration of the hydronium ion, H3O+, can vary tremendously in solutions of acids and bases, a scale to easily represent the acidity of a solution was developed. It is called the pH scale and is related to the [H3O+]:
![]() using the shorthand notation
using the shorthand notation
Remember that in pure water ![]() . This value will change if the temperature is not 25°C; however, in most cases, the difference is not too large. This equilibrium is present whenever water is present, but in many cases, Kw is too small to make a difference. Since both the hydrogen ion and hydroxide ions are formed in equal amounts (both x), the Kw expression can be expressed as:
. This value will change if the temperature is not 25°C; however, in most cases, the difference is not too large. This equilibrium is present whenever water is present, but in many cases, Kw is too small to make a difference. Since both the hydrogen ion and hydroxide ions are formed in equal amounts (both x), the Kw expression can be expressed as:
![]()
Solving for [H+] gives us [H+] = 1.0 × 10-7. If you then calculate the pH of pure water:
![]()
The pH of pure water is 7.00 (at 25°C), slightly different at different temperatures. On the pH scale, this is called neutral. A solution whose [H+] is greater than in pure water will have a pH less than 7.00 and is called acidic. A solution whose [H+] is less than in pure water will have a pH greater than 7.00 and is called basic. Figure 16.2, on the next page, shows the pH scale and the pH values of some common substances. In this figure, all solutions below 7 contain an acid, and solutions above 7 contain a base.
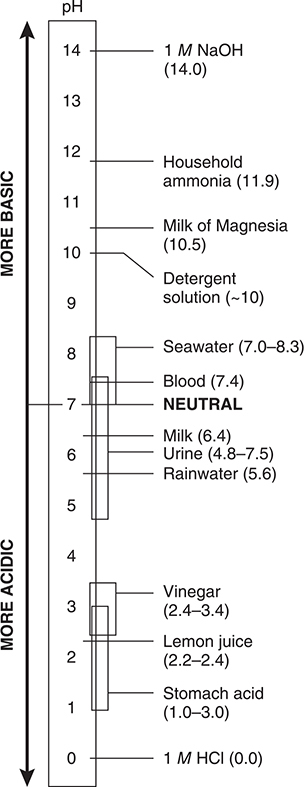
Figure 16.2 The pH scale.
The pOH of a solution can also be calculated. It is defined as pOH = —log[OH-]. The pH and the pOH are related:
pH + pOH = pKw = 14.00 at 25°C
In any of the problems above in which [H+] or [OH-] was calculated, you can now calculate the pH or pOH of the solution.
You can estimate the pH of a solution by looking at its [H+]. For example, if a solution has an [H+] = 1 × 10-5, its pH would be 5. This value was determined from the value of the exponent in the [H+]. This is why determining the pH of a solution of a strong acid or base is usually much simpler than calculations involving weak acid or bases. For example, the pH of a 0.015 M nitric acid solution is simply pH = — log (0.015) = 1.82, while the pH of a 0.015 M barium hydroxide solution may be found as pOH = — log (2 × 0.015) = 1.52 and pH = pKw — pH = 14.00 — 1.52 = 12.48 [the 2 is because each Ba(OH)2 has 2 hydroxide ions]. Care must be taken when using this approach. For example, what is the pH of a 1.0 × 10-8 M hydrochloric acid solution? If you get an answer of 8.0, and that does not immediately strike you as impossible, you have obviously missed something in the previous discussion, and you need to review the material again.
Kb—the Base Dissociation Constant
Weak bases (B), when placed into water, also establish an equilibrium system much like weak acids:
![]()
Ammonia is a common example of a weak base. The Kb reaction for ammonia is:
![]()
Many other weak bases substitute hydrocarbon (C + H) groups for one or more of the hydrogen atoms bonded to the nitrogen in ammonia.
The equilibrium constant expression is called the weak base dissociation constant, Kb, and has the form:
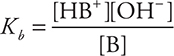
The same reasoning that was used in dealing with weak acids is also true here: [HB+] = [OH-]; [B] ≈ M initially. Calculations involving Kb are like those involving Ka.
As with Ka and pKa, the pKb is sometimes useful. The pKb is found by the following relationship:
pKb = —log Kb
For example, a 0.500 M solution of methylamine, CH3NH2, has a pH of 12.17. What is the Kb of methylamine? (Note that methylamine is an ammonia molecule with one H replaced by a CH3.)
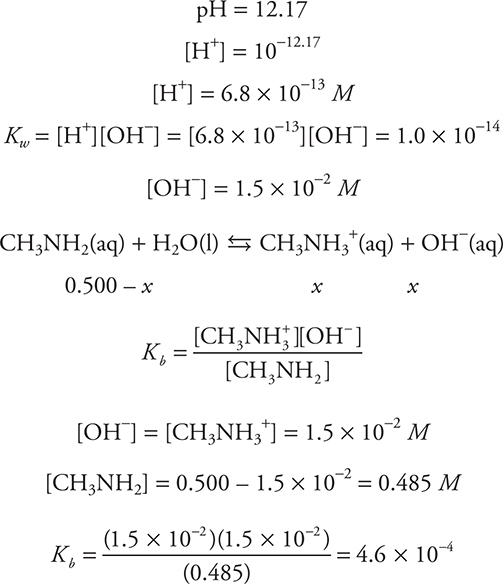
The percent ionization of a base may be calculated by a procedure similar to the calculation of the percent ionization of an acid. Strong bases, like strong acids, are 100% dissociated.
The Ka and Kb of conjugate acid—base pairs are related through the Kw expressions:
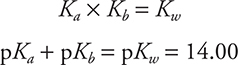
This equation shows an inverse relationship between Ka and Kb for any conjugate acid—base pair.
This relationship may be used in problems such as: Determine the pH of a solution made by adding 0.400 mol of strontium nitrite to enough water to produce 2.000 L of solution.
Solution:
The initial molarity is 0.400 mol/2.000 L = 0.200 M.
When a salt is added to water dissolution will occur:
![]()
The resultant solution, since calcium nitrite is soluble, has 0.200 M Ca2+ and 0.400 M NO2-.
Ions such as Cl- and Ca2+, which come from strong acids or strong bases, may be ignored in this type of problem. Ions such as NH4+ and NO2-, from weak acids or bases, will undergo hydrolysis. The nitrite ion is the conjugate base of nitrous acid (Ka = 4.5 × 10-4). Since nitrite is not a strong base, this will be a Kb problem, and OH- will be produced. The equilibrium is:
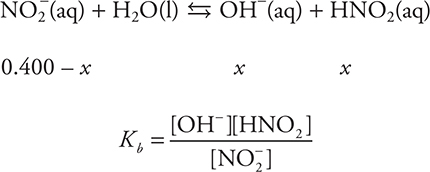
Determining Kb it from Ka (using ![]() ) gives:
) gives:
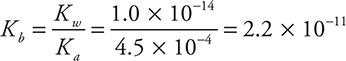
Entering the equilibrium (x) values into the Kb expression:
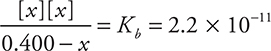
Solving for x gives ![]()
Using ![]() :
:
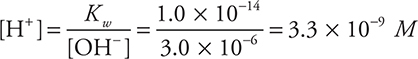
Finally:
![]()
Acidic/Basic Properties of Salts
The behavior of a salt will depend upon the acid—base properties of the ions present in the salt. The ions may lead to solutions of the salt being acidic, basic, or neutral. The pH of a solution depends on hydrolysis, a generic term for a variety of reactions with water. Some ions will undergo hydrolysis, and this changes the pH.
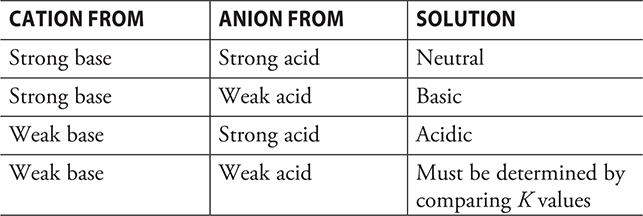
The reaction of an acid and a base will produce a salt. The salt will contain the cation from the base and the anion from the acid. In principle, the cation of the base is the conjugate acid of the base, and the anion from the acid is the conjugate base of the acid. Thus, the salt contains a conjugate acid and a conjugate base. This is always true in principle. In some cases, one or the other of these ions is not a true conjugate base or a conjugate acid. Just because the ion is not a true conjugate acid or base does not mean that we cannot use the ion as if it were. The conjugate base of any strong acid is so weak that it will not undergo any significant hydrolysis; the conjugate acid of any strong base is so weak that it, too, will not undergo any significant hydrolysis. Ions that do not undergo any significant hydrolysis will have no effect upon the pH of a solution and will leave the solution neutral. The presence of the following conjugate bases Cl-, Br-, I-, NO3-, ClO3-, and ClO4- will leave the solution neutral. The cations from the strong bases, Li+, Na+, K+, Rb+, Cs+, Ca2+, Sr2+, and Ba2+, while not true conjugate acids, will also leave the solution neutral. Salts containing only these cations and anions are neutral.
The conjugate base from any weak acid is a “strong” base and will undergo hydrolysis in aqueous solution to produce a basic solution. If the conjugate base (anion) of a weak acid is in a salt with the conjugate of a strong base (cation), the solution will be basic because only the anion will undergo any significant hydrolysis. Salts of this type are basic salts. All salts containing the cation of a strong base and the anion of a weak acid are basic salts.
The conjugate acid of a weak base is a “strong” acid, and it will undergo hydrolysis in an aqueous solution to make the solution acidic. If the conjugate acid (cation) of a weak base is in a salt with the conjugate base of a strong acid (anion), the solution will be acidic because only the cation will undergo any significant hydrolysis. Salts of this type are acidic salts. All salts containing the cation of a weak base and the anion of a strong acid are acidic salts.
There is a fourth category that consists of salts that contain the cation of a weak base with the anion of a weak acid. Prediction of the acid—base character of these salts is less obvious because both ions undergo hydrolysis. The two equilibria not only alter the pH of the solution but also interfere with each other. Predictions require a comparison of the K values for the two ions. The larger K value predominates. If the larger value is Ka, the solution is acidic. If the larger value is Kb, the solution is basic. In the rare case where the two values are equal, the solution would be neutral.
The following table summarizes this information:
For example, suppose you are asked to determine if a solution of sodium carbonate, Na2CO3, is acidic, basic, or neutral. Sodium carbonate is the salt of a strong base (NaOH) and a weak acid (HCO3-). Salts of strong bases and weak acids are basic salts. As a basic salt, we know the final answer must be basic (pH above 7).
Buffers
Buffers are solutions that resist a change in pH when an acid or base is added to them. The most common types of buffer are a mixture of a weak acid and its conjugate base or a mixture of a weak base and its conjugate acid. The weak acid will neutralize any base added, and the weak base of the buffer will neutralize any acid added to the solution. Thus, the presence of the conjugate acid—base pair will stabilize the pH of the solution. The hydronium ion concentration of a buffer can be calculated using an equation derived by rearranging the Ka expression:
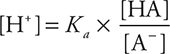
Taking the negative log of both sides yields the Henderson—Hasselbalch equation, which can be used to calculate the pH of a buffer:
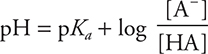
The weak base Kb expression can also be used, giving:
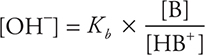 and
and 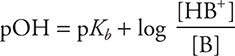
These equations allow us to calculate the pH or pOH of the buffer solution knowing K of the weak acid or base and the concentrations of the conjugate weak acid and its conjugate base. Also, if the desired pH is known, along with K, the ratio of base to acid can be calculated. The more concentrated these species are, the more acid or base can be neutralized and the less the change in buffer pH. This is a measure of the buffer capacity, the ability to resist a change in pH. When the buffer capacity of the buffer is exceeded by the addition of too much acid or base, the solution will cease to be a buffer.
Let’s calculate the pH of a buffer. What is the pH of a solution containing 2.50 mol of ammonia and 2.50 mol of ammonium chloride in a volume of 1.00 L?
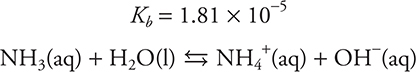
There are two ways to solve this problem.
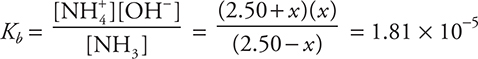
Assume x is small:
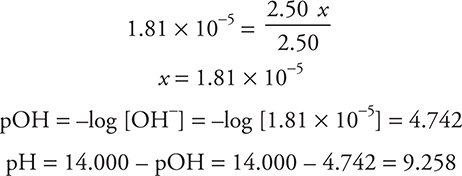
Alternative (shorter) solution:
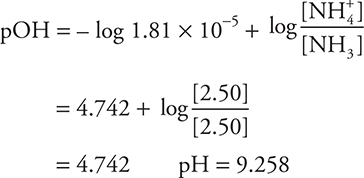
This example also illustrates that when the concentrations of the conjugates are the same, pH = pKa.
Acid—Base Reactions and Buffers
Acid—base reactions were first introduced in Chapter 12. At this time it would be helpful to review the Chapter 12 information in light of the new information that appears in this chapter.
The net ionic equation for the reaction of any strong acid with any strong base is:
![]()
Since this reaction occurs in every strong acid—strong base reaction, problems dealing with this type of reactions are relatively simple. For example, the pH at the equivalence point of such a titration is always 7. At points other than the equivalence point, either the acid or the base is the limiting reactant, and the concentration of the excess reactant controls the pH.
The net ionic equation for the reaction of any weak acid with any strong base may be written as:
![]()
As long as the excess reactant is the weak acid (if the base is being added to the acid, this is before the equivalence point), the resultant solution is a buffer, and it is possible to use the Henderson—Hasselbalch equation to determine the pH of the solution. (Note at half-way to the equivalence the pH = pKa of the acid.) When the strong base is in excess (if the base is being added to the acid, this is after the equivalence point), the pH is determined from the concentration of the excess hydroxide ion. At the equivalence point, the pH of the solution will be greater than 7. The pH at the equivalence point is determined by the Kb equilibrium for the conjugate base of the weak acid. This equilibrium is:
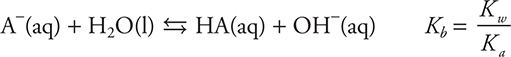
The net ionic equation for the reaction of any weak base with any strong acid may be written as:
![]()
As long as the excess reactant is the weak base (if the acid is being added to the base, this is before the equivalence point), the resultant solution is a buffer, and it is possible to use the Henderson—Hasselbalch equation to determine the pH of the solution. (Note at halfway to the equivalence the pOH = pKb of the acid.) When the strong acid is in excess (if the acid is being added to the base, this is after the equivalence point), the pH is determined from the concentration of the excess hydrogen ion. At the equivalence point, the pH of the solution will be less than 7. The pH at the equivalence point is determined by the Ka equilibrium for the conjugate acid of the weak base. This equilibrium is:
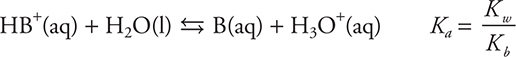
The net ionic equation for the reaction of any weak acid and any weak base may be written as:
![]()
The presence of two conjugate acid—base pairs significantly complicates the calculation, and such calculations are normally avoided.
Titration Equilibria
An acid—base titration is a common laboratory procedure used to determine the concentration of an unknown solution. A base solution of known concentration is added to an acid solution of unknown concentration (or vice versa) until an acid—base indicator visually signals that the endpoint of the titration has been reached. (A pH meter may also be used to determine the endpoint.) The equivalence point is the point at which a stoichiometric amount of the base has been added to the acid. Both chemists and chemistry students hope that the equivalence point (theoretical) and the endpoint (experimental) are close together. If there is an acid—base indicator, there is an additional equilibrium involving the indicator. Note: the members of the conjugate acid—base pair for the indicator are different colors.
If the acid being titrated is a weak acid, then there are equilibria that will be established and must be accounted for in the calculations. Typically, a plot of pH of the weak acid solution being titrated versus the volume of the strong base added (the titrant) starts at a low pH and gradually rises until close to the equivalence point, where the curve rises dramatically. After the equivalence point region, the curve returns to a gradual increase. This is shown in Figure 16.3. The titration of a polyprotic acid will contain the same type of plot with a break as indicated in the plot for each of the acidic hydrogen atoms present in the acid.
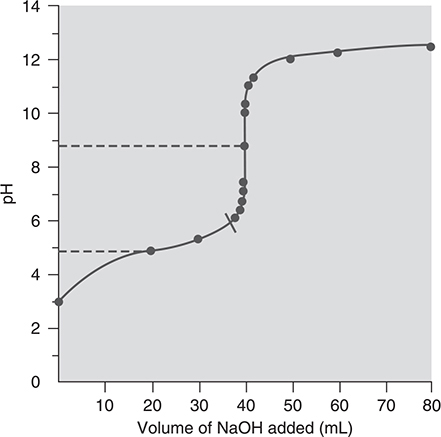
Figure 16.3 The titration of a weak acid with a strong base.
In many cases, one may know the initial concentration of the weak acid but may be interested in the pH changes during the titration. To study the changes, one can divide the titration curve into four distinctive areas in which the pH is calculated:
1. Calculating the initial pH of the weak acid solution is accomplished by treating it as a simple weak acid solution of known concentration and using its Ka.
2. As base is added, a mixture of the remaining weak acid and its conjugate base is formed. This is a buffer solution and can be treated as one in the calculations. Determine the moles of acid consumed from the moles of titrant added—that will be the moles of conjugate base formed. Then calculate the molar concentration of weak acid and conjugate base, taking into consideration the volume of titrant added. Finally, apply your buffer equations.
3. At the equivalence point, all the weak acid has been converted to its conjugate base. The conjugate base will react with water, so treat it as a weak base solution and calculate the [OH-] using Kb of the conjugate base of the weak acid 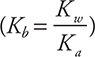 . Finally, calculate the pH of the solution.
. Finally, calculate the pH of the solution.
4. After the equivalence point, you have primarily the excess strong base that will determine the pH.
All these steps are reversed if acid is being added to base.
Let’s consider a typical titration problem. A 50.00-mL sample of 0.150 M formic acid, HCHO2, (pKa = 3.74) was titrated with 0.300 M sodium hydroxide. Determine the pH of the solution after the following quantities of base have been added to the acid solution:
a. 0.00 mL
b. 12.50 mL
c. 24.50 mL
d. 25.00 mL
e. 27.50 mL
f. 37.50 mL
Note that throughout this problem we will express the molarity as 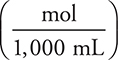 instead of
instead of 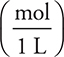 , which will simplify the calculations.
, which will simplify the calculations.
a. 0.00 mL. Since no base has been added, only HCHO2 is present. HCHO2 is a weak acid, so this can only be a Ka problem.
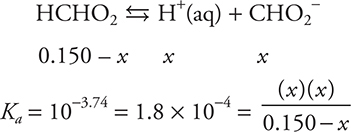
Quadratic needed: 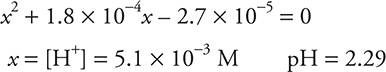
b. 12.50 mL. Since both an acid and a base are present (and they are not conjugate to each other), this must be a stoichiometry problem. Stoichiometry requires a balanced chemical equation and moles.
![]()
 could be written as NaCHO2, but the separated ions are more useful.
could be written as NaCHO2, but the separated ions are more useful.
Initial moles acid: 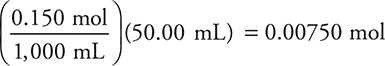
(This number will be used in all remaining steps. It does not change because no more acid is added.)
Added moles base: 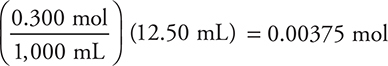
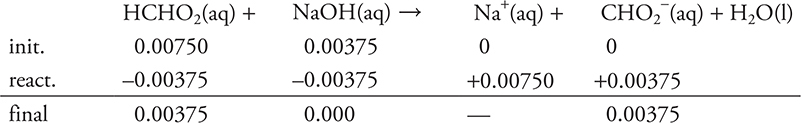
The stoichiometry portion of part (b) is finished.
The solution is no longer HCHO2 and NaOH, but HCHO2 and CHO2- (a conjugate acid—base pair).
Since a CA/CB pair is present, this is now a buffer problem, and the Henderson—Hasselbalch equation may be used.
![]()
Note the simplification in the CB/CA concentrations. Both moles are divided by exactly the same volume (since they are in the same solution), so the identical volumes cancel.

Another useful fact to remember occurs here. At the halfway point in the titration of a weak acid or a weak base, the concentration of both members of the conjugate acid—base pair are present in equal amounts. This leads to pH = pKa or pOH = pKb at this point in the titration.
c. 24.50 mL. Since both an acid and a base are present (and they are not conjugate to each other), this must be a stoichiometry problem again. Stoichiometry requires a balanced chemical equation and moles.
![]()
Base: 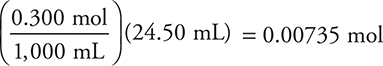
Use the previously calculated moles of acid (0.00750 mole).
Based on the stoichiometry of the problem, and on the moles of acid and base, NaOH is the limiting reagent.
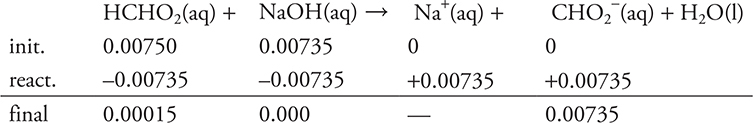
The stoichiometry portion of part (c) is finished.
The solution is no longer HCHO2 and NaOH, but HCHO2 and CHO2- (a conjugate acid—base pair).
Since a CA/CB pair is present, this is now a buffer problem, and the Henderson—Hasselbalch equation may be used.
![]()
d. 25.00 mL. Since both an acid and a base are present (and they are not conjugate to each other), this must be a stoichiometry problem. Stoichiometry requires a balanced chemical equation and moles.
![]()
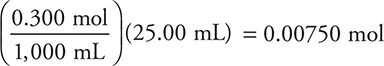
Use the previously calculated moles of acid (0.00750 moles).
Based on the stoichiometry of the problem, and on the moles of acid and base, both are limiting reagents.
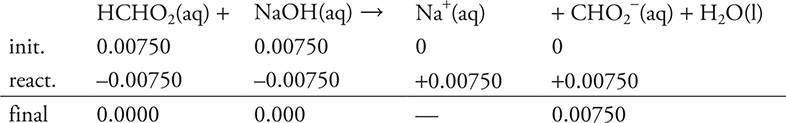
![]()
The stoichiometry portion of part (d) is finished.
The solution is no longer HCHO2 and NaOH, but an CHO2- solution (a conjugate base of a weak acid).
Since the CB of a weak acid is present, this is a Kb problem.
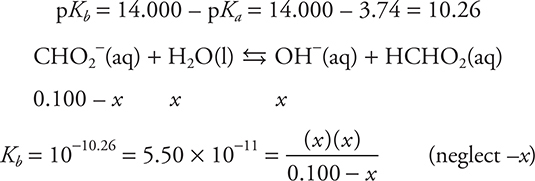
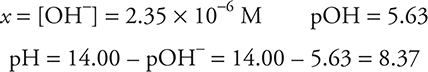
e. 27.50 mL. Since both an acid and a base are present (and they are not conjugate to each other), this must be a stoichiometry problem. Stoichiometry requires a balanced chemical equation and moles.
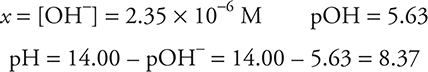
Use the previously calculated moles of acid (0.00750 moles).
Based on the stoichiometry of the problem, and on the moles of acid and base, the acid is now the limiting reagent.
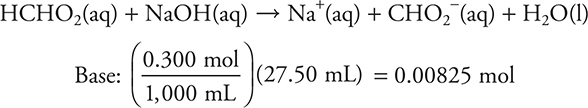
The strong base will control the pH.
![]()
The stoichiometry portion of part (e) is finished.
Since this is now a solution of a strong base, it is now a simple pOH/pH problem.
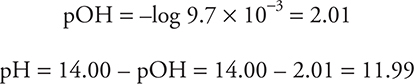
f. 37.50 mL. Since both an acid and a base are present (and they are not conjugate to each other), this must be a stoichiometry problem. Stoichiometry requires a balanced chemical equation and moles.
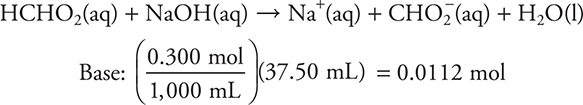
Use the previously calculated moles of acid (0.00750 moles).
Based on the stoichiometry of the problem, and on the moles of acid and base, the acid is again the limiting reagent.
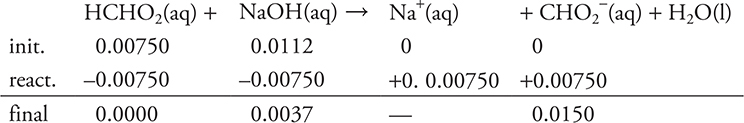
The strong base will control the pH.
![]()
The stoichiometry portion of part (f ) is finished.
Since this is now a solution of a strong base, it is now a simple pOH/pH problem.
Experiments

Equilibrium experiments such as 10, 11, and 13 in Chapter 20, Experimental Investigations, directly or indirectly involve filling a table like the following:

The initial amounts—concentrations or pressures—are normally zero for the products, and a measured or calculated value for the reactants. Once equilibrium has been established, the amount of at least one of the substances is determined. Based on the change in this one substance and the stoichiometry, the amounts of the other materials may be calculated (not measured).
Measurements may include the pressure, the mass (to be converted to moles), the volume (to be used in calculations), and the pH (to be converted into either the hydrogen ion or hydroxide ion concentration). Some experiments measure the color intensity (with a spectrophotometer), which may be converted to a concentration.
Do not make the mistake of “measuring” a change. Changes are never measured; they are always calculated.
Common Mistakes to Avoid

1. Be sure to check the units and significant figures of your final answer.
2. Be sure, when working weak-base problems, to use Kb and not Ka.
3. In titration problems, make sure you compensate for dilution when mixing two solutions together.
4. A Ka expression must have [H+] in the numerator, and a Kb expression must have [OH-] in the numerator. There are NO exceptions.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. First are 20 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. These questions also include ones designed to help you review prior knowledge. Following those is a long free-response question like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. A 0.1 molar solution of acetic acid, CH3COOH, has a pH of about:
(A) 1
(B) 3
(C) 7
(D) 10
2. 
Using the given information, choose the best answer for preparing a buffer with a pH of 8.
(A) K2HPO4 + KH2PO4
(B) H3PO4
(C) K2HPO4 + K3PO4
(D) K3PO4
Use the following information for Questions 3 and 4.

3. Which of the following is a solution with an initial KCOOH concentration of 1 M and an initial K2HPO2 concentration of 1 M ?
(A) a solution with a pH > 7, which is a buffer
(B) a solution with a pH < 7, which is not a buffer
(C) a solution with a pH < 7, which is a buffer
(D) a solution with a pH > 7, which is not a buffer
4. Which of the following is a solution with an initial H3PO2 concentration of 1 M and an initial KH2PO2 concentration of 1 M ?
(A) a solution with a pH > 7, which is a buffer
(B) a solution with a pH < 7, which is not a buffer
(C) a solution with a pH < 7, which is a buffer
(D) a solution with a pH > 7, which is not a buffer
5. A solution of a weak base is titrated by adding a solution of a standard strong acid. The progress of the titration is followed with a pH meter. Which of the following observations would occur?
(A) The pH of the solution gradually decreases throughout the experiment.
(B) Initially the pH of the solution drops slowly, and then it drops much more rapidly.
(C) At the endpoint, the pH is 7.
(D) After the equivalence point, the pH becomes constant because this is the buffer region.
6. What is the ionization constant, Ka, for a weak monoprotic acid with a 0.10-molar solution having a pH of 3.0?
(A) 1 × 10-5
(B) 1 × 10-2
(C) 1 × 10-6
(D) 1 × 10-4
7. Phenol, C6H5OH, has Ka = 1.0 × 10-10. What is the pH of a 0.010-M solution of phenol?
(A) between 3 and 7
(B) 10
(C) 2
(D) between 7 and 10
8. You are given equimolar solutions of each of the following. Which has the lowest pH?
(A) NH4Cl
(B) NaCl
(C) K3PO4
(D) Na2CO3
9. When sodium nitrite dissolves in water:
(A) The solution is acidic because of the hydrolysis of the sodium ion.
(B) The solution is basic because of the hydrolysis of the NO2- ion.
(C) The solution is basic because of the hydrolysis of the sodium ion.
(D) The solution is acidic because of the hydrolysis of the NO2- ion.
10. Which of the following solutions has a pH nearest 7?
(A) 1 M H2C2O4 (oxalic acid) and 1 M KHC2O4 (potassium hydrogen oxalate)
(B) 1 M KNO3 (potassium nitrate) and 1 M HNO3 (nitric acid)
(C) 1 M NH3 (ammonia) and 1 M NH4NO3 (ammonium nitrate)
(D) 1 M CH3NH2 (methylamine) and 1 M HC2H3O2 (acetic acid)
11. Determine the OH-(aq) concentration in a 1.0 M aniline (C6H5NH2) solution. The Kb for aniline is 4.0 × 10-10.
(A) 2.0 × 10-5 M
(B) 4.0 × 10-10 M
(C) 3.0 × 10-6 M
(D) 5.0 × 10-7 M
12. ZnS(s) + 2 H+(aq) ⇆ Zn2+(aq) + H2S(aq)
What is the equilibrium constant for the above reaction? The successive acid dissociation constants for H2S are 9.5 × 10-8 (Ka1) and 1 × 10-19 (Ka2). The Ksp, the solubility product constant, for ZnS equals 1.6 × 10-24.
(A) 1.7 × 10-17
(B) 6.3 × 10-56
(C) 1.7 × 102
(D) 5.9 × 1016
13. The addition of nitric acid increases the solubility of which of the following compounds?
(A) KCl(s)
(B) Pb(CN)2(s)
(C) Cu(NO3)2(s)
(D) NH4NO3(s)
14. Which of the following is the strongest Brønsted—Lowry acid?
(A) HBrO
(B) HBrO3
(C) HBrO2
(D) HBrO4
15. 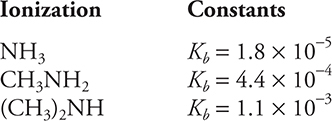
How would a solution with an initial NH4Cl (NH4+ + Cl-) concentration of 1 M and an initial concentration of CH3NH3Cl (CH3NH3+ + Cl-) concentration of 1 M be classified?
(A) a solution with a pH > 7, which is a buffer
(B) a solution with a pH < 7, which is not a buffer
(C) a solution with a pH < 7, which is a buffer
(D) a solution with a pH > 7, which is not a buffer
16. Which of the following species CANNOT serve as both a Brønsted base and a Brønsted acid?
(A) H2PO42-
(B) CO32-
(C) HSeO4-
(D) HCO3-
17. A buffer stock solution is made by mixing 2.0 moles of NH3 and 3.0 moles of NH4Cl and diluting to 1.0 L. What is the buffer capacity of 100.0 mL of this solution toward NaOH?
(A) 0.20 moles NaOH
(B) 0.30 moles NaOH
(C) 3.0 moles NaOH
(D) 2.0 moles NaOH
18. Acetic acid, HC2H3O2, is a commonly used weak acid, which is also an organic compound. This acid, and many other organic acids, own their acidic behavior to a specific structure of a particular group of atoms. What is the one way of writing the general formula of this particular group of atoms?
(A) —COOH
(B) HC2-
(C) HC-
(D) There is no general formula.
19. In the titration of a weak base solution with a strong acid solution where does the pOH = pKb of the weak base?
(A) At the equivalence point
(B) At the endpoint
(C) At no point in the titration
(D) Halfway to the equivalence point
20. Under what circumstances will it not be a problem to add the indicator after an acid—base titration has begun?
(A) Any time if the acid and base are both strong.
(B) Any time if either the acid or base is weak.
(C) As long as it is added before the endpoint.
(D) It is never acceptable to add the indicator late.
![]() Answers and Explanations
Answers and Explanations
1. B—Any acid will have a pH below 7; thus, C and D can be eliminated. A 0.1 molar solution of a strong acid would have a pH of 1. Acetic acid is not a strong acid, which eliminates A.
2. A—The K nearest 10-8 will give a pH near 8. The answer must involve the H2PO4- ion. The potassium ions are spectator ions and have no effect on the pH.
3. D—The two substances are not a conjugate acid—base pair, so this is not a buffer, which eliminates answers A and C. Both compounds are salts of a strong base and a weak acid; such salts are basic (pH > 7).
4. C—The two substances constitute a conjugate acid—base pair of a weak acid, so this is a buffer. The pH should be near —log Ka1. This is about 2 (acid).
5. B—Anytime an acid is added, the pH will drop. The reaction of the weak base with the acid produces the conjugate acid of the weak base. The combination of the weak base and its conjugate is a buffer, so the pH will not change very much until all the base is consumed. After all the base has reacted, the pH will drop much more rapidly. The equivalence point of a weak base—strong acid titration is always below 7 (only strong base—strong acid titrations will give a pH of 7 at the endpoint). The value of pOH is equal to pKb halfway to the equivalence point.
6. A—If pH = 3.0, then [H+] = 1 × 10-3 = [A-], and [HA] = 0.10 — 1 × 10-3 ≈ 0.10. The generic 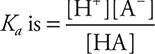 and when the values are entered into this equation,
and when the values are entered into this equation, 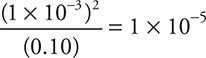 . Since you can estimate the answer, no actual calculations are necessary.
. Since you can estimate the answer, no actual calculations are necessary.
7. A—This is an acid dissociation constant; thus, the solution must be acidic (pH < 7). The pH of a 0.010 M strong acid would be 2.0. This is not a strong acid, so the pH must be above 2. Even though phenol has an OH in its formula, the fact that it has a Ka means that it must be an acid. Phenol is an organic compound, which is irrelevant since there is only one way to handle a Ka equilibrium.
8. A—A is the salt of a strong acid and a weak base; therefore, this salt is acidic. B is a salt of a strong acid and a strong base; such salts are neutral. C and D are salts of a weak acid and a strong base; such salts are basic. Since A is the only acidic salt, it is the solution with the lowest pH.
9. B—Sodium nitrite is a salt of a weak acid and a strong base. Ions from strong bases (Na+ in this case) do not undergo hydrolysis and do not affect the pH. Ions from weak acids (NO2- in this case) undergo hydrolysis to produce basic solutions.
10. D—The weak acid and the weak base partially cancel each other to give a nearly neutral solution. Methylamine is related to ammonia, and like ammonia it is a weak base.
11. A—The equilibrium constant expression is Kb = 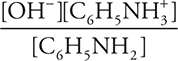 (not needed). This expression becomes
(not needed). This expression becomes 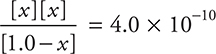 (needed), which simplifies to
(needed), which simplifies to 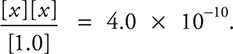 Taking the square root of each side gives x = 2.0 × 10-5 = [OH-]. Since you can estimate the answer, no calculator is necessary. The formula of the compound is irrelevant since there is only one way to do a Kb problem.
Taking the square root of each side gives x = 2.0 × 10-5 = [OH-]. Since you can estimate the answer, no calculator is necessary. The formula of the compound is irrelevant since there is only one way to do a Kb problem.
12. C—The equilibrium given is the sum of the following three equilibriums:
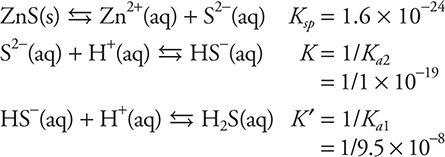
Summing these equations means you need to multiply the equilibrium constants: ![]()
 Estimate the answer by using the exponents to get the exponent of the correct answer (+3) and pick the closest.
Estimate the answer by using the exponents to get the exponent of the correct answer (+3) and pick the closest.
13. B—Nitric acid, being an acid, will react with a base. In addition to obvious bases containing OH-, the salts of weak acids are also bases. All the anions, except CN- are from strong acids and, as such, are not bases. The removal of the CN- ion shifts the Ksp equilibrium for Pb(CN)2 to the right (more soluble).
14. D—Perbromic acid, HBrO4, is expected to be the strongest acid in this group because the greater number of oxygen atoms pulls more electron density from the hydrogen atom, making it easier to be lost as a hydrogen ion.
15. B—The two substances (NH4Cl and CH3NH3Cl) do not contain a conjugate acid— base pair, so the mixture is not a buffer, which eliminates A and C. Both compounds are salts of a weak base (NH3 or CH3NH2) and a strong acid (HCl); such salts are acidic (pH < 7). Salts of a strong acid and a weak base are acidic salts. The Kb values shown in the table indicate that all three compounds are weak bases.
16. B—All the ions can serve as Brønsted bases (accept a hydrogen ion to form H3PO4, HCO3-, H2SeO4, and H2CO3). All but B can behave as Brønsted acids (donate a hydrogen ion to form HPO42—, SeO42—, and CO32—).
17. B—A 100.0 mL sample of the stock is one-tenth of the solution; therefore, it contains one-tenth of the solutes: 0.20 mole of NH3 and 0.30 mole NH4Cl. The added NaOH will react with the acid component of the buffer, NH4Cl. The reaction is:
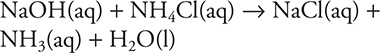
Since the reaction stoichiometry is 1:1, 0.30 moles of NH4Cl will react with 0.30 mole of NaOH.
18. A—Acetic acid is an example of a carboxylic acid. In some cases, the formula of acetic acid is written as CH3COOH to emphasize this. Two other carboxylic acids are formic acid, HCOOH, and propanoic acid, CH3CH2COOH. You may have seen or even used oxalic acid, H2C2O4, which is a combination of two carboxylic acid groups as seen when the formula is writer as HOOCCOOH or (COOH)2. Note, this question deals with the AP topic 8.6 The Molecular Structure of Acids and Bases. A similar question might deal with some of the organic bases, which have structures derived from ammonia, NH3.
19. D—While it may seem simpler to simply memorize this fact for a multiple-choice question, free-response questions require explanations.
This may be demonstrated by using the pOH form of the Henderson—Hasselbalch equation, which may be derived from the acid form:
Acid form: 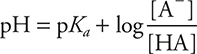 given on the exam
given on the exam
In this form, CA and CB being the members of a conjugate acid-base pair.
Alternate acid form: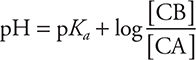
In this form, CA and CB being the members of a conjugate acid-base pair.
Analogous base form: 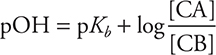
At halfway to the equivalence point, [CA] = [CB], which means 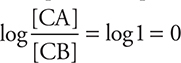
20. C—The purpose of the indicator is to determine the endpoint; therefore, as long as the indicator is present before the endpoint is reached, the titration should work.
Expect questions involving laboratory experiments throughout the exam.
![]() Free-Response Question
Free-Response Question
You have 10 minutes to answer the following short question. You may use a calculator and the tables in the back of the book.
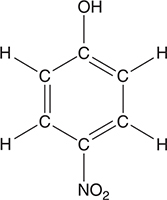
The compound p-nitrophenol (pictured above) may be used as an acid—base indicator. It is typically used as an indicator in the form of a 0.1% aqueous solution. In acid, this indicator is colorless, while in base the indicator is yellow. The maximum absorption for the colorless form is 313 nm and the maximum absorption for the yellow form is 402 nm. As an indicator, it reacts with hydroxide ions according to the following equilibrium:
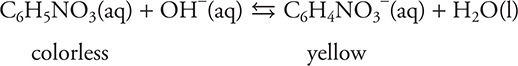
(a) Write the Keq for the above reaction.
(b) Like many acid—base indicators, p-nitrophenol is a weak acid. An indicator solution is made by adding 0.100 g of this compound to a 100-mL volumetric flask containing a little water. The sample is diluted to volume by the addition of more water. If the molar mass of this compound is 139.110 g mol-1, what is the molarity of this solution?
(c) Several samples of p-nitrophenol are prepared by diluting the indicator solution with pH = 10 buffer to assure conversion of all the C6H5NO3(aq) to C6H4NO3-(aq). These samples were used to create the calibration plot below at a wavelength of 402 nm.
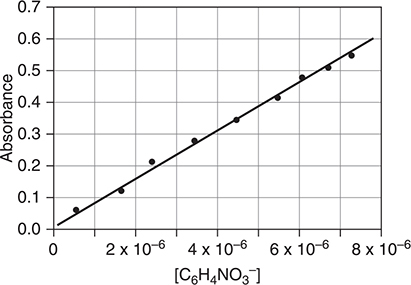
The experimental data points and the best-fit line is shown on the graph. What is the approximate absorbance of a solution with [C6H4NO3-] = 3 × 10-6 M?
(d) The color change of the indicator is in the 5.6—7.6 pH range. This indicator would work best for which of the following titrations: (i) Na2CO3 with standard HCl; (ii) HCl with standard NaOH; (iii) NH3 with standard HC2H3O2?
![]() Answer and Explanation
Answer and Explanation
(a) 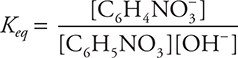
1 point for the correct equilibrium expression unless H2O is included in the expression.
(b) To determine the molarity, the moles of indicator must be divided by the liters of solution. The grams of p-nitrophenol need to be converted to moles and the volume of the solution needs to be converted to liters. These may be done as two separate calculations or as one combined calculation as shown below.
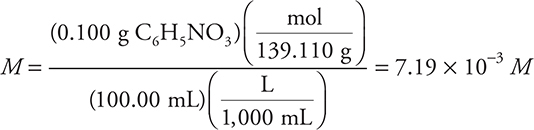
1 point for the correct calculation unless the answer has the wrong number of significant figures (3).
(c) 0.23
1 point for this answer. Significant figures are not important. Values from 0.21 through 0.25 are acceptable. Units are unnecessary; however, a.u. or absorbance (units) may be used.
(d) The best choice is (ii).
The midpoint of the pH range is 6.6; therefore, a titration with an equivalence point near this value would be best. The indicator works better when the pH is increasing (colorless → yellow), since it is easier to see the color appearing than disappearing (yellow → colorless). This is one reason why (ii) is a better choice than (i). The other reason why (ii) is better than (i) is that it is a strong acid—strong base titration, and as such the equivalence point is at pH = 7 (close to 6.6). (iii) is not a good choice regardless of the indicator since it is a weak base—weak acid titration.
1 point for choosing (ii) and giving either of the two reasons listed.
![]() Rapid Review
Rapid Review
• Strong acids completely dissociate in water, whereas weak acids only partially dissociate (have an equilibrium constant).
• Weak acids and bases establish an equilibrium system.
• Under the Brønsted—Lowry acid—base theory, acids are proton (H+) donors and bases are proton acceptors.
• Conjugate acid—base pairs differ only in a single H+; the one that has the extra H+ is the acid.
• The equilibrium for a weak acid is described by Ka, the acid dissociation constant. It has the form: 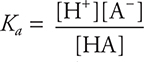 . Know how to apply this equation. If there is an exponent other than 1 anywhere in the equation, you have made an error.
. Know how to apply this equation. If there is an exponent other than 1 anywhere in the equation, you have made an error.
• Most times the equilibrium concentration of the weak acid, [HA], can be approximated by the initial molarity of the weak acid.
• Knowing Ka and the initial concentration of the weak acid allows the calculation of the [H+].
• Water is an amphoteric substance, acting either as an acid or a base.
• The product of the [H+] and [OH-] in a solution or in pure water is a constant, Kw, called the water dissociation constant, 1.0 × 10-14. Kw = [H+] [OH-] = 1.0 × 10-14 at 25°C. Know how to apply this equation.
• The pH is a measure of the acidity of a solution. pH = —log[H+]. Know how to apply this equation and estimate the pH from the [H+].
• On the pH scale 7 is neutral; pH > 7 is basic; and pH < 7 is acidic.
• pH + pOH = pKw = 14.00. Know how to apply this equation.
• Kb is the ionization constant for a weak base. 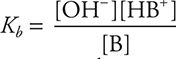 . Know how to apply this equation. If there is an exponent other than 1 anywhere in the equation, you have made an error.
. Know how to apply this equation. If there is an exponent other than 1 anywhere in the equation, you have made an error.
• Ka × Kb = Kw for conjugate acid—base pairs. Know how to apply this equation.
• Buffers are solutions that resist a change in pH by neutralizing either an added acid or an added base.
• The Henderson—Hasselbalch equation allows the calculation of the pH of a buffer solution: 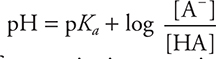 . Know how to apply this equation.
. Know how to apply this equation.
• The buffer capacity is a quantitative measure of the ability of a buffer to resist a change in pH. The more concentrated the acid—base components of the buffer, the higher its buffer capacity.
• A titration is a laboratory technique to determine the concentration of an acid or base solution.
• An acid—base indicator is used in a titration and changes color in the presence of an acid or base.
• The equivalence point or endpoint of a titration is the point at which an equivalent amount of acid or base has been added to the base or acid being neutralized.
• Know how to determine the pH at any point of an acid—base titration.
• If you have a solution of an acid, the pH will be below 7.
• If you have a solution of a base, the pH will be above 7.