5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 4 Review the Knowledge You Need to Score High
17 Electrochemistry
IN THIS CHAPTER
In this chapter, the following AP topics are covered:
9.7 Galvanic (Voltaic) and Electrolytic Cells
9.8 Cell Potential and Free Energy
9.9 Cell Potential Under Nonstandard Conditions
9.10 Electrolysis and Faraday’s Law
Summary: Electrochemistry is the study of the chemical reactions that produce electricity and chemical reactions that take place because electricity is supplied. Electrochemical reactions may be of many types. Electroplating is an electrochemical process. So are the electrolysis of water, the production of aluminum metal, and the production and storage of electricity in batteries. All these processes involve the transfer of electrons and redox reactions.
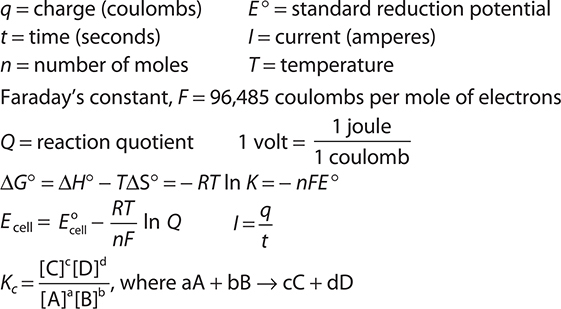
Keywords and Equations

A table of half-reactions is given in the exam booklet and in the back of this book. Gas constant, R = 8.314 J mol—1 K—1 = 0.08206 L atm mol—1 K—1
Redox Reactions
Electrochemical reactions involve redox reactions. In Chapter 12, Reactions and Periodicity, we discussed redox reactions, but here is a brief review: Redox is a term that stands for reduction and oxidation. Reduction is the gain of electrons, and oxidation is the loss of electrons. For example, suppose a piece of zinc metal is placed in a solution containing blue Cu2+ ions. Very quickly, a reddish solid forms on the surface of the zinc metal. That substance is copper metal. At the molecular level, the zinc metal atoms are losing electrons to form Zn2+ and Cu2+ ions are gaining electrons to form copper metal. These two processes can be shown as:
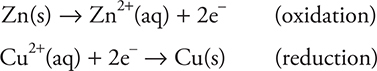
The electrons that are being lost by the zinc metal are the same electrons that are being gained by the cupric ion. The zinc metal is being oxidized, and the copper(II) cation is being reduced.
Something must cause the oxidation (taking of the electrons), and that substance is called the oxidizing agent (the reactant being reduced). In the example above, the oxidizing agent is Cu2+. The reactant undergoing oxidation is called the reducing agent because it is furnishing the electrons used in the reduction half-reaction. Zinc metal is the reducing agent above. The two half-reactions, oxidation and reduction, can be added together to give you the overall redox reaction. The electrons must cancel—that is, there must be the same number of electrons lost as electrons gained. Adding the two reactions above (oxidation and reduction) gives:
![]()
The next step is to cancel the electrons, which leaves:
![]()
In these redox reactions, like the electrochemical reactions we will show you, there is a simultaneous loss and gain of electrons. In the oxidation reaction (commonly called a half-reaction) electrons are being lost, but in the reduction half-reaction those very same electrons are being gained. So, in redox reactions electrons are being exchanged as reactants are being converted into products. This electron exchange may be direct, as when copper metal plates out on a piece of zinc, or it may be indirect, as in an electrochemical cell (battery). In this chapter, we will show you both processes and the calculations associated with each.
Knowing how to balance redox equations can be useful on the AP Exam, so we have included the half-reaction method of balancing redox reactions in the appendixes just in case you are having trouble with the technique in your chemistry class.
The definitions for oxidation and reduction given above are the most common and the most useful ones. A couple of others might also be useful: Oxidation is the gain of oxygen or loss of hydrogen and involves an increase in oxidation number. Reduction is the gain of hydrogen or loss of oxygen and involves a decrease in oxidation number.
Electrochemical Cells
In the example above, the electron transfer was direct; that is, the electrons were exchanged directly from the zinc metal to the copper(II) ions. But such a direct electron transfer doesn’t allow for any useful work to be done by the electrons. Therefore, in order to use these electrons, indirect electron transfer must be done. The two half-reactions are physically separated and connected by a wire. The electrons that are lost in the oxidation half-reaction flow through the wire to get to the reduction half-reaction. While those electrons are flowing through the wire, they can do useful work, like powering a calculator or a pacemaker. Electrochemical cells use indirect electron transfer to produce electricity by a redox reaction, or they use electricity to produce a desired redox reaction.
Galvanic (Voltaic) Cells
Galvanic (voltaic) cells produce electricity by using a redox reaction. Let’s take that zinc/copper redox reaction that we studied before (the direct electron-transfer reaction) and make it a galvanic cell by separating the oxidation and reduction half-reactions. (See Figure 17.1.) The reaction is a thermodynamically favorable reaction. The cell potential for a galvanic cell is positive.
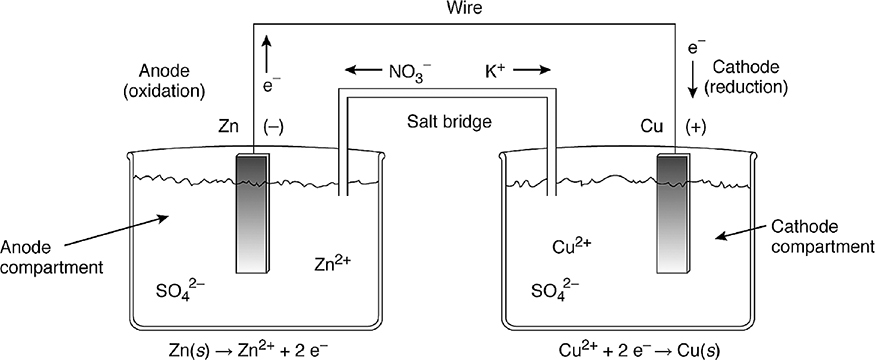
Figure 17.1 A galvanic cell.
Instead of one container, as before, two will be used. A piece of zinc metal will be placed in one container, and a piece of copper metal in another container. A solution of aqueous zinc sulfate (or some other water-soluble zinc compound) will be added to the container containing the zinc electrode and an aqueous solution of copper(II) sulfate [or some other water-soluble copper(II) compound] will be added to the container containing the copper metal. The zinc and copper metals will form the electrodes of the cell, the portion of the cell that conducts the electrons involved in the redox reaction. The solutions in which the electrodes are immersed are called the electrode compartments. The electrodes are connected by a wire and . . . nothing happens. If the redox reactions were to proceed, the container containing the zinc metal would build up a positive charge due to the zinc cations being produced in the oxidation half-reaction. The container containing the copper would build up a negative charge due to the loss of the copper(II) cations. The solutions (compartments) must maintain electrical neutrality. To accomplish this, a salt bridge will be used. A salt bridge is often an inverted U-tube that holds a gel containing a concentrated electrolyte solution, such as KNO3 in this example. Any electrolyte could be used if it does not interfere with the redox reaction. The anions in the salt bridge will migrate through the gel into the beaker containing the zinc metal (to balance the excess positive charge), and the salt-bridge cations will migrate in the opposite direction (to balance the excess negative charge). In this way, electrical neutrality is maintained. In electrical terms, the circuit has been completed and the redox reaction can occur. The zinc electrode is being oxidized in one container, and the copper(II) ions in the other container are being reduced to copper metal. The same redox reaction is happening in this indirect electron transfer as happened in the direct one:
![]()
The difference is that the electrons are now flowing through a wire from the oxidation half-reaction to the reduction half-reaction. And electrons flowing through a wire is electricity, which can do work. If a voltmeter was connected to the wire connecting the two electrodes, a current of 1.10 V would be measured. This galvanic cell shown in Figure 17.1 is commonly called a Daniell cell.
In the Daniell cell shown in Figure 17.1, note that the compartment with the oxidation half-reaction is on the left and the compartment undergoing reduction is on the right. The electrode at which oxidation is taking place is called the anode, and the electrolyte solution in which it is immersed is called the anode compartment. The electrode at which reduction takes place is called the cathode, and its solution is the cathode compartment. The anode is labeled with a negative sign (-), while the cathode has a positive sign (+). The electrons flow from the anode to the cathode, which is - to +, the thermodynamically favored direction.
Remember: oxidation is an anode process.

Sometimes the half-reaction(s) involved in the cell lack a conductive part to act as the electrode, so an inert (inactive) electrode, a solid conducting electrode that does not take part in the redox reaction, is used. Graphite and platinum are commonly used as inert electrodes.
Note: The electrode must be a conductor onto which a wire may be attached. It can never be an ion in solution.
Cell Notation
Cell notation is a shorthand notation representing a galvanic cell. To write the cell notation in Figure 17.1:
1. Write the chemical formula of the anode: Zn(s)
2. Draw a single vertical line to represent the phase boundary between the anode and the solution: Zn(s)|
3. Write the reactive part of the anode compartment with its initial concentration (if known) in parentheses (assume 1 M in this case): Zn(s) | Zn2+(1 M). (If unknown, the concentration may be left out.)
4. Draw a double vertical line to represent the salt bridge connecting the two electrode compartments: Zn(s) | Zn2+ (1 M ) ||
5. Write the reactive part of the cathode compartment with its initial concentration (if known) shown in parentheses: Zn(s) | Zn2+ (1 M) || Cu2+ (1 M). (If unknown, the concentration may be left out.)
6. Draw a single vertical line representing the phase boundary between the solution and the cathode: Zn(s) | Zn2+ (1 M) || Cu2+ (1 M) |
7. Finally, write the chemical formula of the cathode: Zn(s) | Zn2+ (1 M) || Cu2+ (1 M) | Cu(s)
If an inert electrode is used because one or both redox half-reactions do not have a suitable conducting electrode material associated with the reaction, the inert electrode is shown with its phase boundary. If the electrode components are in the same phase, they are separated by commas; if not, a vertical phase boundary line. For example, consider the following redox reaction:
![]()
The oxidation of the iron(II) ion to iron(III) doesn’t involve a suitable electrode material, so an inert electrode, such as platinum, would be used. The cell notation in this case would be:
![]()
Cell Potential
In the discussion of the Daniell cell, we indicated that this cell produces 1.10 volts. This voltage is really the difference in potential between the two half-cells. There are half-cell potentials associated with all half-cells. A list of all possible combinations of half-cells would be tremendously long. Therefore, a way of combining desired half-cells has been developed. The cell potential (really the half-cell potentials) depends on concentration and temperature, but initially we’ll simply look at the half-cell potentials at the standard temperature of 298 K (25°C) and all components in their standard states (1 M concentration of all solutions, 1 atmosphere pressure for any gases, and pure solid electrodes). All the half-cell potentials are tabulated as the reduction potentials, that is, the potentials associated with the reduction reaction. The hydrogen half-reaction has been defined as the standard and has been given a value of exactly 0.00 V. All the other half-reactions have been measured relative to it, some positive and some negative. The table of standard reduction potentials provided on the AP Exam is shown in Table 17.1.
Table 17.1 Standard Reduction Potentials in Aqueous Solution at 25°C. All Ions Are in Aqueous Solution (aq)
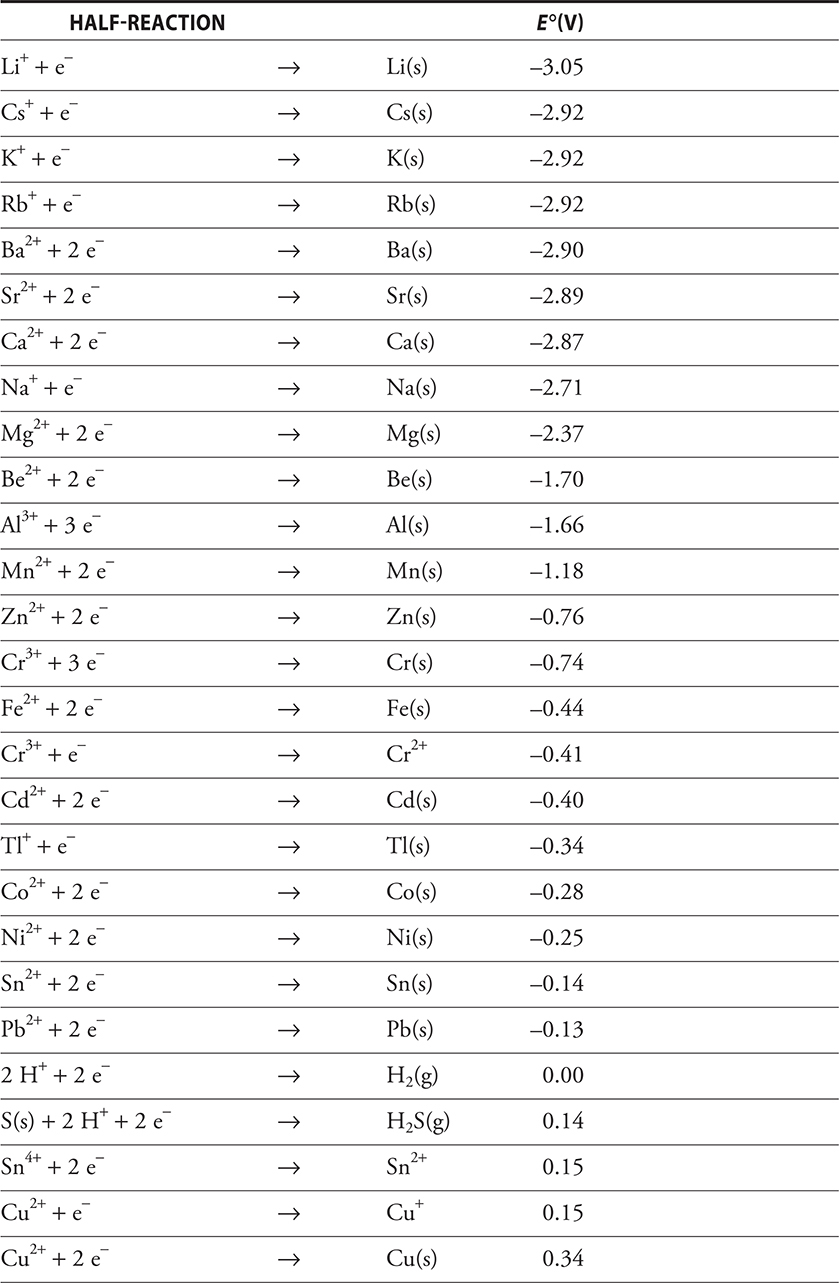
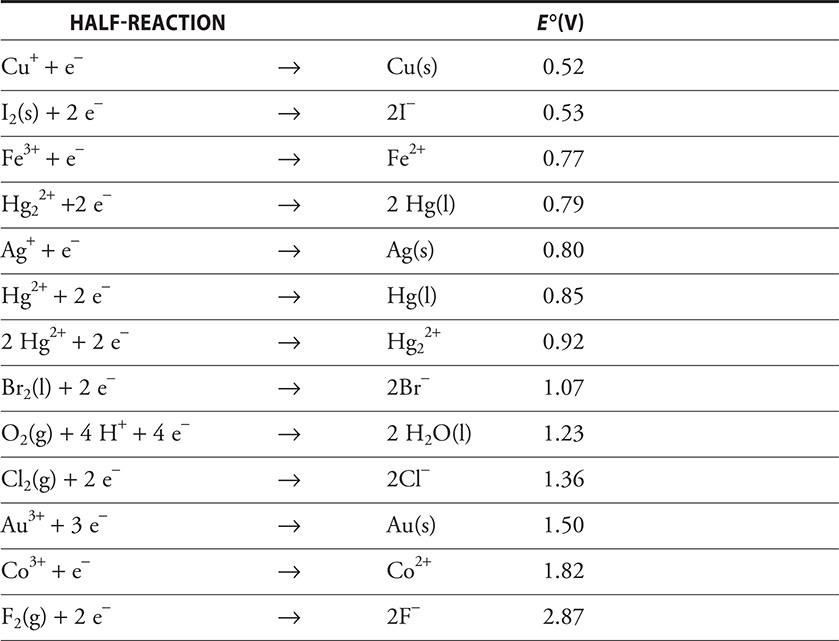
Here are some things to be aware of in looking at this table:
• All reactions are shown in terms of the reduction reaction relative to the standard hydrogen electrode.
• The more positive the value of the voltage associated with the half-reaction (E °), the more readily the reaction occurs.
• The strength of the oxidizing agent increases as the value becomes more positive, and the strength of the reducing agent increases as the value becomes more negative.
This table of standard reduction potentials can be used to write the overall cell reaction and to calculate the standard cell potential (E°), the potential (voltage) associated with the cell at standard conditions. There are a few things to remember when using these standard reduction potentials to generate the cell reaction and cell potential:
1. The standard cell potential for a galvanic cell must be a positive value, E ° > 0.
2. Because one half-reaction must involve oxidation, one of the half-reactions shown in the table of reduction potentials must be reversed to indicate the oxidation. If the half-reaction is reversed, the sign of the standard reduction potential must be reversed. However, this is not necessary to calculate the standard cell potential. Other than the sign, make no other change in the standard cell potential.
3. Because oxidation occurs at the anode and reduction at the cathode, the standard cell potential can be calculated from the standard reduction potentials of the two half-reactions involved in the overall reaction by using the equation:
![]()
But remember, both E ° cathode and E ° anode are shown as reduction potentials, used directly from the table without reversing.
Once the standard cell potential has been calculated, the reaction can be written by reversing the half-reaction associated with the anode and adding the half-reactions together, using appropriate multipliers if needed to ensure that the numbers of electrons lost and gained are equal.
Suppose a galvanic cell was to be constructed utilizing the following two half-reactions taken from Table 17.1:
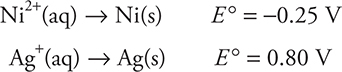
First, the cell voltage can be calculated using:
![]()
Since the cell potential must be positive (a galvanic cell), there is only one arrangement of -0.25 and 0.80 volts than can result in a positive value (a useful check):
![]()

This means that the Ni electrode is the anode and must be involved in oxidation, so its reduction half-reaction must be reversed, changing the sign of the standard half-cell potential, and added to the silver half-reaction. Note that the silver half-reaction must be multiplied by 2 to equalize electron loss and gain, but the half-cell potential remains the same:
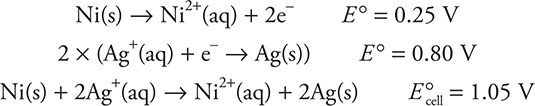
Note that the same cell potential is obtained as using: ![]()
If, for example, you are given the cell notation, you could use this method to determine the cell potential. In this case, the cell notation would be: ![]()
Electrolytic Cells
Electrolytic cells use electricity from an external source to produce a desired redox reaction. The reaction is a thermodynamically unfavorable reaction. Electroplating and the recharging of an automobile battery are examples of electrolytic cells. Like galvanic cells, oxidation occurs at the anode and reduction occurs at the cathode. The electrons flow from the + electrode to the - electrode, which is the thermodynamically unfavored direction. The cell potential for an electrolytic cell is negative.
Figure 17.2 shows a comparison of a galvanic cell and an electrolytic cell for the Sn/Cu system. On the left-hand side of Figure 17.2, the galvanic cell is shown for this system. Note that this reaction produces 0.48 V. But what if we wanted the reverse reaction to occur, the nonspontaneous reaction? This can be accomplished by applying a voltage in excess of 0.48 V from an external electrical source. This is shown on the right-hand side of Figure 17.2. In this electrolytic cell, electricity is being used to produce the nonspontaneous redox reaction.
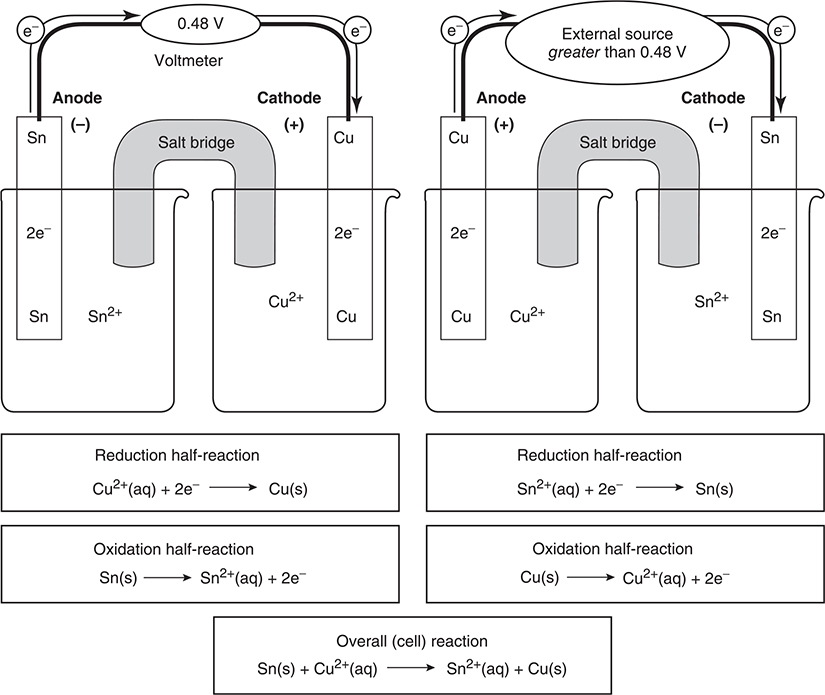
Figure 17.2 Comparison of a galvanic cell (left) and an electrolytic cell (right).
Quantitative Aspects of Electrochemistry
One of the most widely used applications of electrolytic cells is in electrolysis, the decomposition of a compound with electricity. Water may be decomposed into hydrogen and oxygen. Aluminum oxide may be electrolyzed to produce aluminum metal. In these situations, several questions may be asked: How long will it take; how much can be produced; what current must be used? Given any two of these quantities, the third may be calculated.
To answer these questions, the balanced half-reaction must be known. Then the following relationships can be applied:

Knowing the amperage and how long it is being applied (seconds), the coulombs can be calculated. Then the coulombs can be converted into moles of electrons, and the moles of electrons can be related to the moles (and then grams) of material being electrolyzed through the balanced half-reaction.
For example, while not the best ion for the purpose, gold plating may be done using a solution containing ![]() as the source of the gold. If a solution of sodium tetracyanoaurate(III),
as the source of the gold. If a solution of sodium tetracyanoaurate(III), ![]() , is electrolyzed by a current of 1.500 amperes for 3.000 h, how many grams of gold will be produced?
, is electrolyzed by a current of 1.500 amperes for 3.000 h, how many grams of gold will be produced?
Answer:
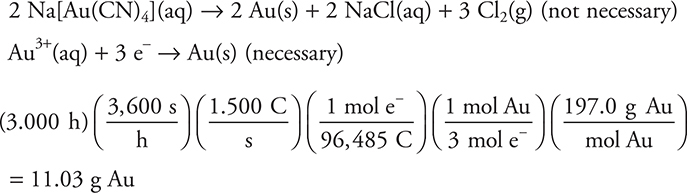
Note the change from 1.500 amperes to 1.500 coulombs per second using the definition above.
Calculation of E°cell also allows for the calculation of two other useful quantities—the Gibbs free energy (ΔG°) and the equilibrium constant (K). The equations (given on the exam) are:
![]()
The Gibbs free energy is the best single thermodynamic indicator of whether a reaction will be spontaneous (review Chapter 14, Thermodynamics). The Gibbs free energy for a reaction can be calculated from the E ° of the reaction using the following equation:
![]()
where F is Faraday’s constant of 96,485 C/mol e— = 96,485 J/V mol, and n is the number of electrons transferred.
If the redox reaction is at equilibrium, the equilibrium constant may be calculated by using the given equations:
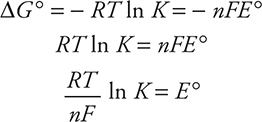
While it is perfectly acceptable to use this equation, traditionally, the values of the constants (R, T, and F ) are combined and the logarithm base is changed from base e to base 10, which leaves these two forms:
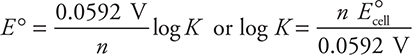
Let’s apply these relationships. Determine ΔG ° and K for the following reaction:
![]()
Answer:
For this reaction, two electrons are transferred from the Zn to the Ag. Thus, n is 2 for this reaction. The value of F (96,485 J/V mol) is given on the exam, so you will not need to memorize it.
The first answer is:
![]()
The second answer is:
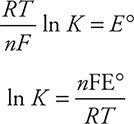
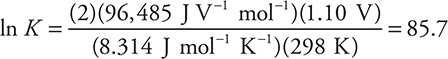
or, using the other form of the equation:
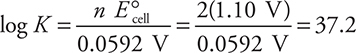
In either case, the calculation gives a K of about 1037 (actually K = 1.5 × 1037). In many cases, the approximate value will be all you need for the AP Exam.
Nernst Equation
So far, all the calculations have used the standard cell potential or standard half-cell potentials, which means the reaction is under standard conditions (defined previously). However, what if the conditions are not standard? When the conditions are not standard, an adjustment is necessary. The most common nonstandard condition is where the molarity is no longer 1 M. The nonstandard cell potential, E cell, (no degree symbol) is calculated using the Nernst equation, which appears on the AP Exam as:
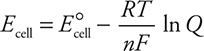
where R is the ideal gas constant (8.314 J mol—1 K—1), T is the Kelvin temperature, n is the number of electrons transferred, F is Faraday’s constant (96,485 C/mol e— = 96,485 J/V mol), and Q is the reaction quotient discussed Chapter 15, Equilibrium. Note: at equilibrium Q = K, and Ecell = 0, which leads to:
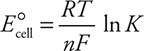
As seen previously, it is possible to enter the values of the constants and convert to base 10 logarithms to give:
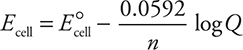
The second form, involving log Q, is the more useful form in that it is no longer necessary to enter all the constants every time. If one knows the cell reaction or half-reaction, the concentrations of ions, and E °, then the actual cell potential can be calculated. Another useful application of the Nernst equation is in calculating the concentration of one of the reactants from cell-potential measurements. Knowing the actual cell potential and E °cell allows calculation of Q, the reaction quotient. Knowing Q and all but one of the concentrations allows the calculation of the unknown concentration.
Another application of the Nernst equation is in concentration cells. A concentration cell is an electrochemical cell in which the same chemical species is used in both cell compartments; however, the concentrations differ. Because the half-reactions are the same, E °cell = 0.00 V. Simply substituting the appropriate concentrations into the reaction quotient allows calculation of the actual cell potential.
When using the Nernst equation on a cell reaction in which the overall reaction is not supplied, only the half-reactions and concentrations, there are two equivalent methods to work the problem. The first way is to write the overall redox reaction based upon E ° values, and then apply the Nernst equation. The second method is to treat each half-reaction independently and then combine the two results to produce the overall potential. Let’s practice the second method. Calculate the potential of a half-cell containing 0.20 M K2Cr2O7(aq), 0.10 M Cr3+(aq), and 1.0 × 10—3 M H+(aq).
Answer:
The following half-reaction is given on the AP Exam:
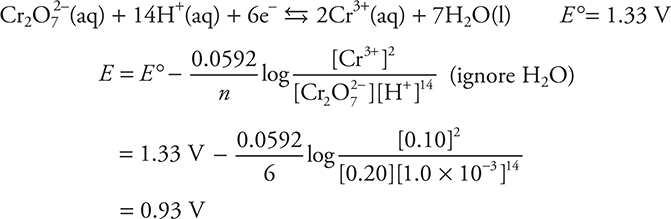
Electrolysis and Faraday’s Law
It is possible to use Faraday’s law for reactions occurring in electrochemical cells. The equation (from the AP Exam) is ![]() , where I is the current, q is the charge transferred, and t is the elapsed time.
, where I is the current, q is the charge transferred, and t is the elapsed time.
In general, this equation is not often used directly. You will see in the section Quantitative Aspects of Electrochemistry how this equation is applied indirectly. Schematically this process may be represented as:

This process will work for both galvanic and electrolytic cells. For galvanic cells, the key is the electricity produced. For electrolytic cells, the key is the electricity required.
Experiments
Electrochemical experiments are normally concerned with standard cell voltages. Measurements of the cell potential are essential and require a voltmeter (potentiometer). These measurements may be taken from different combinations of half-cells, or from measurements before and after changes of some aspect of the cell were made.
Using measurements of different half-cell combinations, a set of “standard” reduction potentials may be constructed. This set will be similar to a table of standard reduction potentials. The solutions used in the half-cells must be of known concentration. These solutions are produced by weighing reagents and diluting to volume. The measurements will require a balance and a volumetric flask. It is also possible to produce known concentrations by diluting solutions. This method requires a pipette and a volumetric flask. Review Chapter 11, Solutions, for solution techniques.
Common Mistakes to Avoid

1. Be sure your units cancel to give the unit wanted in your final answer.
2. Be sure to round your answer off to the correct number of significant figures.
3. Remember that oxidation is the loss of electrons and reduction the gain, and that in redox reactions the same number of electrons is lost and gained.
4. When diagramming an electrochemical cell, be sure the electrons go from anode to cathode. (It will help if you follow the convention of placing the reduction [cathode] on the right.)
5. Be sure that for a galvanic cell, the cell potential is greater than 0.
6. In cell notation, be sure to write anode, anode compartment, salt bridge, cathode compartment, cathode in this specific order. (The cathode [reduction] is on the right.)
7. When using a multiplier to equalize electron loss and gain in reduction half-cell potentials, do not use the multiplier on the voltage of the half-cell.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. First are 20 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. Some of these questions are designed to review prior knowledge. Following those is a long free-response question like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may use the periodic table and the equation sheet at the back of this book.
Choose one of the following for Questions 1 and 2.
(A) There is no change in the voltage.
(B) The voltage becomes zero.
(C) The voltage increases.
(D) The voltage decreases but stays positive.
The following reaction takes place in a voltaic cell:
![]()
The cell has a voltage that is measured and found to be +1.10 V.
1. What happens to the cell voltage when the copper electrode is made smaller?
2. What happens to the cell voltage when the salt bridge is filled with deionized water instead of 1 M KNO3?
3. 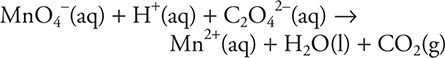
What is the coefficient of H+ when the reaction is balanced?
(A) 16
(B) 2
(C) 8
(D) 5
4. ![]()
After the above half-reaction is balanced, which of the following are the respective coefficients of OH— and SO42—?
(A) 8 and 3
(B) 6 and 2
(C) 10 and 2
(D) 5 and 2
5. How many moles of Pt may be deposited on the cathode when 0.80 F of electricity is passed through a 1.0-M solution of Pt4+?
(A) 1.0 mole
(B) 0.60 mole
(C) 0.20 mole
(D) 0.80 mole
6. A chemistry student obtains the following answer when balancing a redox equation:
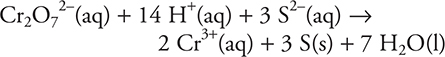
Which of the following is a true statement concerning the balanced equation?
(A) The Cr2O72— reduces the S2—.
(B) The oxidation number of chromium changes from +7 to +3.
(C) The oxidation number of the sulfur remains -2.
(D) The Cr2O72— oxidizes the S2—.
7. ![]()
What is the coefficient for water when the above half-reaction is balanced?
(A) 3
(B) 4
(C) 2
(D) 1
8.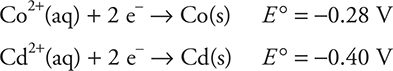
Given the above standard reduction potentials, estimate the approximate value of the equilibrium constant for the following reaction: ![]()
![]()
(A) e—4.60
(B) e—9.35
(C) e9.35
(D) e36.84
9. A sample of silver is to be purified by electro-refining. This will separate the silver from an impurity of gold. The impure silver is made into an electrode. Which of the following is the best way to set up the electrolytic cell?
(A) an impure silver cathode and an inert anode
(B) an impure silver cathode and a pure gold anode
(C) a pure silver cathode with an impure silver anode
(D) a pure gold cathode with an impure silver anode
10. ![]()
The reaction shown above was used in an electrolytic cell. The voltage measured for the cell was not equal to the calculated E ° for the cell. Which of the following could cause this discrepancy?
(A) The anion in the anode compartment was chloride instead of nitrate, as in the cathode compartment.
(B) One or more of the ion concentrations was not 1 M.
(C) Both solutions were at 25°C instead of 0°C.
(D) The solution in the salt bridge was Na2SO4 instead of KNO3.
11. How many grams of metallic mercury could be produced by electrolyzing a 1.0 M Hg(NO3)2 solution with a current of 2.00 A for 3.00 h?
(A) 22.4 g
(B) 201 g
(C) 11.2 g
(D) 44.8 g
12. An electrolysis cell was constructed with two platinum electrodes in a 1.00 M aqueous solution of KCl. An odorless gas evolves from one electrode, and a gas with a distinctive odor evolves from the other electrode. Choose the correct statement from the following list.
(A) The gas with the distinctive odor was evolved at the anode.
(B) The odorless gas was oxygen.
(C) The gas with the distinctive odor was evolved at the cathode.
(D) The odorless gas was evolved at the anode.
13. 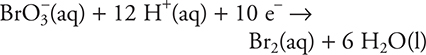
Which of the following statements is correct for the above reaction?
(A) The ![]() undergoes oxidation at the anode.
undergoes oxidation at the anode.
(B) Br goes from a -1 oxidation to a 0-oxidation.
(C) Br2 is oxidized at the anode.
(D) The ![]() undergoes reduction at the cathode.
undergoes reduction at the cathode.
14. 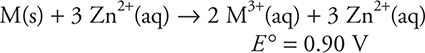
![]()
Using the above information, determine the standard reduction potential for the following reaction: M3+(aq) + 3 e— → M(s).
(A) 0.90 V
(B) -1.66 V
(C) 0.00 V
(D) -0.62 V
15. 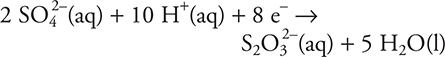
A chemistry student constructs a voltaic cell. The above half-reaction occurs in one of the cell compartments. Which of the following statements is true concerning this half-reaction?
(A) The sulfur undergoes oxidation.
(B) This half-reaction occurs at the cathode.
(C) The oxidation state of sulfur does not change.
(D) The H+ serves as a catalyst.
Use the information on standard reduction potentials in the following table to answer questions 16-18.
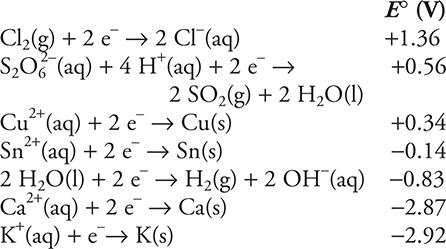
16. A chemist constructs an electrolysis cell with two platinum electrodes in a 1.00 M aqueous solution of sodium chloride, NaCl. When she connects the cell to a power source, an odorless gas forms at one electrode, and a gas with a distinctive odor forms at the other electrode. Choose the correct statement from the following list.
(A) The odorless gas was oxygen.
(B) The odorless gas was the result of oxidation.
(C) The gas with the distinctive odor was the result of oxidation.
(D) The odorless gas was evolved at the positive electrode.
17. A student mixed an acidic 1.0 M sodium dithionate, Na2S2O6, solution with a 1.0 M tin(II) bromide, SnBr2, solution containing some tin metal, Sn, and observed a gas forming. Which of the following is the gas?
(A) H2(g)
(B) SO2(g)
(C) Br2(g)
(D) Sn(g)
18. A chemist constructs a galvanic cell with a tin, Sn, electrode in a compartment containing a 1.0 M tin(II) perchlorate, Sn(ClO4)2, solution and a platinum, Pt, electrode in a compartment containing a 1.0 M copper(II) chloride, CuCl2, solution. The salt bridge connecting the two compartments contains a 1.0 M potassium sulfate, K2SO4. Which of the following is the net ionic equation for the cell?
(A) ![]()
(B) ![]()
(C) ![]()
(D) 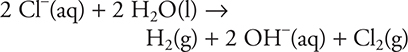
19. 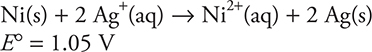
A student builds a galvanic cell utilizing the above reaction. At 25°C, what could the student do for the cell to generate a potential greater than that of the original standard cell.
(A) Increase the Ag+(aq) concentration
(B) Increase the size of the Ni(s) electrode
(C) Decrease the size of the Ag(s) electrode
(D) Increase the pressure
20. 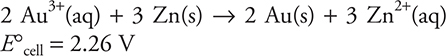
The above standard cell is constructed and allowed to operate. At what point will ΔGcell = 0?
(A) At no point
(B) When the cell reaches equilibrium
(C) When [Au3+] = 0
(D) When no more zinc remains
![]() Answers and Explanations
Answers and Explanations
1. A—The size of the electrode is not important when determining the cell voltage.
2. B—The salt bridge serves as an ion source to maintain charge neutrality. Deionized water would not be an ion source, so the cell could not operate (cell voltage = 0 V).
3. A—The balanced equation is:
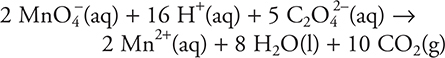
4. C—The balanced equation is:
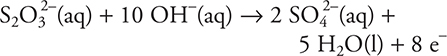
5. C—It takes 4 moles of electrons (4F ) to change the platinum ions to platinum metal. The calculation would be 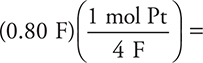
![]()
6. D—The dichromate ion oxidizes the sulfide ion to elemental sulfur as the sulfide ion reduces the dichromate ion to the chromium(III) ion. Chromium goes from +6 to +3, while sulfur goes from -2 to 0. The hydrogen remains at +1 and the oxygen remains -2, so neither hydrogen nor oxygen is oxidized nor reduced.
7. C—The balanced chemical equation is:
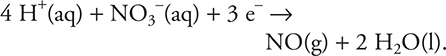
8. C—It is necessary to reverse the Cd half-reaction to ![]()
The standard cell potential is (0.40 — 0.28)V = 0.12 V
It is necessary to use two equations from the list provided on the exam:
ΔG° = -RT ln K and ΔG° = -nFE°
These may be used separately or combined. It is faster to use the combined equation, which comes from setting the two ΔG° equations equal to each other:
-RT ln K = -nFE°
and rearranging:
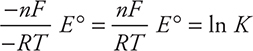
Entering values and substituting V = J/coul:
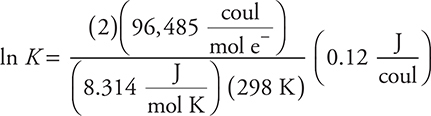
To save time, you can do some rounding; however, you should recall that calculations involving logarithms of any type are extremely sensitive to rounding.
Rounding gives:
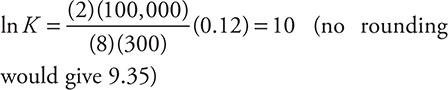
This leads to K = e10 (actually e9.35).
The closest answer in the problem is e9.35.
The actual value is K = 1.1 × 104 (if the rounded value were used, the K would be double the actual K or 2.2 × 104, showing the sensitivity of logarithms to rounding).
9. C—The impure silver must be oxidized so it will go into solution. Oxidation occurs at the anode. Reduction is required to convert the silver ions to pure silver. Reduction occurs at the cathode. The cathode must be pure silver; otherwise, it could be contaminated with the cathode material.
10. B—If the voltage was not equal to E°, then the cell was not standard. Standard cells have 1 M concentrations and operate at 25°C with a partial pressure of each gas equal to 1 atm. No gases are involved in this reaction, so the cell must be operating at a different temperature or a different concentration (or both).
11. A—The half-reaction is ![]()
![]() .
.
The calculation based on this half-reaction is:
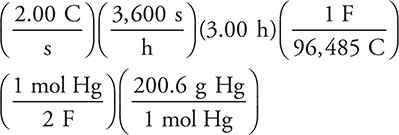
You can estimate the answer by replacing 96,485 with 100,000; 3,600 × 3 with 10,000; and 200.6 with 200, which gives:
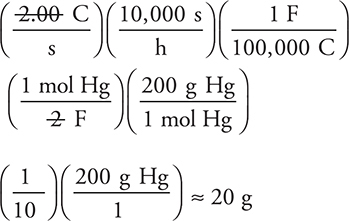
(which is slightly low owing to the rounding).
12. A—The possible gases are H2, O2, and Cl2. The only one of these three gases with a distinctive odor is Cl2. The half-reaction producing ![]() which is an oxidation (at the anode). The cathode reaction must produce H2 (producing O2 requires an oxidation).
which is an oxidation (at the anode). The cathode reaction must produce H2 (producing O2 requires an oxidation).
13. D—The bromate ion, BrO3—, is gaining electrons, so it is being reduced. Reduction always occurs at the cathode.
14. B—The half-reactions giving the overall reaction must be as follows:
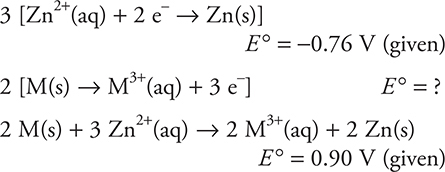
Thus, -0.76 + ? = 0.90, giving ? = 1.66 V. The half-reaction under consideration is the reverse of the one used in this combination, so the sign of the calculated voltage must be reversed. Do not make the mistake of multiplying the voltages when the half-reactions were multiplied to equalize the electrons.
15. B—This is a reduction half-reaction (electrons are being gained). Reductions take place at the cathode.
16. C—The only gases in the table are hydrogen, sulfur dioxide, and chlorine. Of the three gases, the only odorless gas is hydrogen. The formation of hydrogen is through reduction; therefore, it is necessary to determine the oxidation half-reaction. Sulfur dioxide forms through reduction, so it cannot be the gas. The gas with the distinctive odor must be chlorine, which is produced through oxidation. (There will be a longer table given on the AP Exam.)
17. B—Based on the reduction potentials in the table, tin is a sufficiently strong reducing agent to reduce the dithionate ion to sulfur dioxide, a gas with a distinctive odor. Tin cannot reduce water to produce hydrogen, and tin cannot oxidize chloride ion to produce chlorine. None of the other substances is a gas.
18. C—Using the cell potentials, the copper(II) ions will undergo reduction (use the half-reaction from the table), and the tin metal will undergo oxidation (reverse the half-reaction from the table). Combine the resultant half-reactions and cancel electrons to get this answer. A is not a net ionic equation. B is the reverse reaction. D is non-spontaneous (according to the cell potentials).
19. A—For this cell, the Nernst equation is:
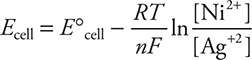
For this cell ![]() is a constant at 25°C and therefore changing any of these is not an option. There are no gases involved, so a pressure change is unlikely to make a difference. Solids do not appear in the Q expression, and as always, the amount of solid does not alter the value, which means changing the size of an electrode will not make a difference. Increasing the Ag+(aq) concentration will make Q smaller number is subtracted from the E°cell.
is a constant at 25°C and therefore changing any of these is not an option. There are no gases involved, so a pressure change is unlikely to make a difference. Solids do not appear in the Q expression, and as always, the amount of solid does not alter the value, which means changing the size of an electrode will not make a difference. Increasing the Ag+(aq) concentration will make Q smaller number is subtracted from the E°cell.
20. B—The cell will cease to operate when [Au3+] = 0 or when no more zinc remains; however, ΔGcell may not equal zero under these circumstances. ΔG = 0 at equilibrium.
![]() Free-Response Question
Free-Response Question
You have 15 minutes to answer the following long question. You may use a calculator and the tables in the back of the book.
Question
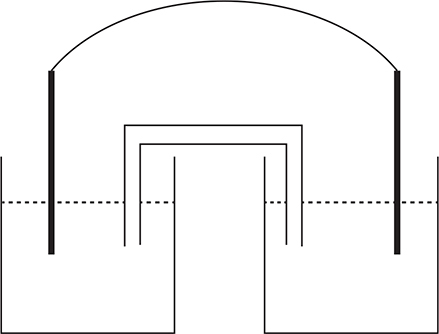
The galvanic cell pictured is constructed with a cobalt electrode in a 1.0 M Co(NO3)2 solution in the left compartment and a silver electrode in a 1.0 M AgNO3 solution in the right compartment. The salt bridge contains a KNO3 solution. The cell voltage is positive.
(a) What is the balanced net ionic equation for the reaction, and what is the cell potential?
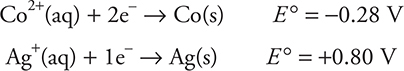
(b) Which electrode is the anode? Justify your answer.
(c) If some solid Co(NO3)2 is added to the cobalt compartment, what will happen to the voltage? Justify your answer.
(d) If the cell operates until equilibrium is established, what will the potential be? Justify your answer.
(e) Write the electron configurations for Co and Co2+.
![]() Answer and Explanation
Answer and Explanation
(a) The cell reaction is:
![]()
The calculation of the cell potential may be done in different ways. Here is one method:
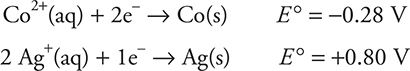
Based upon the half-reaction potentials, the cobalt half-reaction must be reversed to get an oxidation (-0.28 V changes to +0.28 V) and then the two equations added to get the correct answer.
![]()
Give yourself 1 point if you got the correct equation. The physical states are not necessary. Give yourself 1 point for the correct answer (1.08 V) regardless of the method used. The most common mistake is to multiply the silver voltage by 2. You do not get the point for an answer of 1 V.
(b) The cobalt is the anode. The reason Co is the anode is because the Co is oxidized.
You get 1 point for identifying the anode. You get 1 point for the explanation of why Co is the anode or if you state that the Co loses electrons (undergoes oxidation).
(c) The voltage would decrease. The excess Co2+, from the Co(NO3)2, would impede the reactions from proceeding as written.
An alternate explanation uses the Nernst equation (given on the AP Exam).
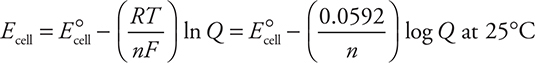
For this cell, the Nernst equation becomes:
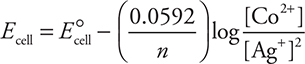
Increasing the cobalt ion concentration means a larger value will be subtracted from E°cell to decrease Ecell.
Give yourself 1 point for saying decrease. Give yourself 1 point for the explanation.
(d) At equilibrium, the cell voltage would be 0 V. At equilibrium no work is done, so the potential must be zero.
Give yourself 1 point for saying 0 V and give yourself 1 point for the equilibrium explanation.
(e) The electron configurations are as follows:
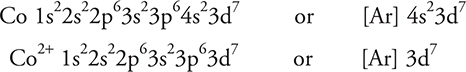
Give yourself 1 point for each correct electron configurations. A common error is to forget that the first electrons removed are the 4s electrons. The orbitals do not need to be in the “correct” order, but they must have the correct superscripts.
Total your points. There are 10 points possible.
![]() Rapid Review
Rapid Review
• In redox reactions, electrons are lost and gained. Oxidation is the loss of electrons, and reduction is the gain of electrons.
• The same number of electrons is lost and gained in the redox half-reactions.
• Galvanic (voltaic) cells produce electricity from a redox reaction.
• The anode is the electrode at which the oxidation half-reaction takes place. The anode compartment is the solution in which the anode is immersed.
• The cathode is the electrode at which reduction takes place, and the cathode compartment is the solution in which the cathode is immersed.
• A salt bridge is used in an electrochemical cell to maintain electrical neutrality in the cell compartments.
• Be able to diagram an electrochemical cell.
• The cell notation is a shorthand way of representing a cell. It has the form: anode | anode compartment || cathode compartment | cathode
• Standard reduction potentials are used to calculate the cell potential under standard conditions. All half-reactions are shown in the reduction form.
• For a galvanic cell, E°cell
• ![]() . Know how to use this equation to calculate E°cell.
. Know how to use this equation to calculate E°cell.
• Electrolytic cells use an external source of electricity to produce a desired redox reaction.
• Review how to diagram an electrolytic cell.
• The following relationships can be used to calculate quantitative changes that occur in an electrochemical cell, especially an electrolytic one: 1 F = 96,485 C per mole of electron (F = 96,485 C/mol e— = 96,485 J/V mol) and 1 amp = 1 C/s (A = C/s).
• The standard cell potential can be used to calculate the Gibbs free energy for the reaction: ΔG ° = -nF (given on the AP Exam). Know how to use this equation.
• The standard cell potential can also be used to calculate the equilibrium constant for a reaction (from the equation given on the exam): ΔG° = - RT ln K = - nFE °. Know how to use this equation.