5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
Additional Review and Applications
18 Nuclear Chemistry
IN THIS CHAPTER
Summary: Radioactivity, the spontaneous decay of an unstable isotope to a more stable one, was first discovered by Henri Becquerel in 1896. Marie Curie and her husband expanded on his work and developed most of the concepts that are used today.
Nuclear chemistry is not a specific AP Chemistry topic; however, you should review parts of this chapter for two reasons. First, some of you need a review of atomic structure, a pre-AP topic that is prior knowledge, which will help you to understand the background for some AP Chemistry questions. Second, you need more practice with kinetics, and radioactivity contains the best examples of first-order kinetics.
Throughout this book, you have been studying traditional chemistry and chemical reactions. This has involved the transfer or sharing of electrons from the electron clouds, especially the valence electrons. Before you decided to take AP Chemistry, you learned information concerning atomic structure; it has been a while since you covered this material, so now is the time to make sure you remember the material.
Keywords and Equations

There are no specific nuclear equations provided; however, you will need to be able to use the first-order equations repeated below from Chapter 13, Kinetics.
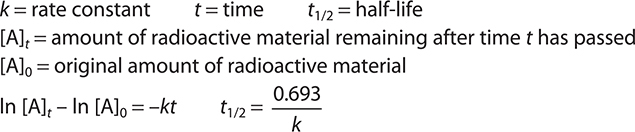
Nuclear Reactions
Balancing Nuclear Reactions
Prior to taking AP Chemistry, you learned that the nucleus of an atom contained protons and neutrons. In addition, you learned that the number of protons present is the atomic number. Also, you learned that the sum of the protons and neutrons is the mass number. Nuclear reactions involve changes in one or more atomic nuclei. During these changes, the total mass number and the total atomic number remains unchanged. Balancing nuclear equations involves balancing the atomic numbers and mass numbers.
Previously, you learned that the atomic number and mass numbers of an atom could be represented as:
![]()
In this notation, X is the symbol for the particle (normally the chemical symbol from the periodic table), A is the mass number for the particle (rarely from the periodic table), and Z is the atomic number for the particle (normally from the periodic table). (Hopefully, you still remember this.) The values of both A and Z are always integers. Many people make an error by using the atomic mass (atomic weight) in place of the mass number. Do not forget, both A and Z are important when balancing nuclear reactions. In a balanced nuclear reaction, the sum of all A values to the left of the reaction arrow must equal the sum of all A values to the right of the arrow. Similarly, the sum of all the Z values to the left of the reaction arrow must equal the sum of all the Z values to the right of the reaction arrow.
As an example of how to balance a nuclear equation, we will investigate the reaction of a chlorine-35 nucleus with a neutron to form hydrogen-1 and another nucleus. This process is known as a transmutation. In a transmutation, an element is converted into one or more different elements. This is entirely unlike “normal” chemical equations where balancing requires the atoms to remain unchanged. The first step in balancing this equation is to write the symbols of the known species on either side of the reaction arrow. You should already know how to write the symbols of chlorine-35 and hydrogen-1; however, you may not remember how to write the symbol of a neutron. We will use an X to indicate the unknown element. This gives the following:
![]()
In this equation, the mass numbers on the left sum to 36 = (35 + 1) and on the right they sum to 1 + A. This leads to 36 = 1 + A, which means A = 35. In an analogous fashion, sum of the atomic numbers on the left is 17 = (17 + 0), and 1 + Z on the right, which means 17 = 1 + Z, which means Z = 16. Checking the periodic table, we see the element with Z = 16 is sulfur. We can now include this material into the nuclear equation to produce a balanced equation:
![]()
Natural Radioactive Decay Modes

Three common types of radioactive decay are observed in nature, and two others are occasionally observed. While you do not need to know any specifics about these, it is useful to recognize their names and for you to get more practice in recognizing atomic symbols.
Alpha Emission
An alpha particle is a helium nucleus with two protons and two neutrons. It is represented as or ![]() or
or ![]() . As this particle is expelled from the nucleus of the radioisotope that is undergoing decay, it has no electrons and thus has a 2+ charge. However, it quickly acquires two electrons from its surroundings to form the neutral atom. Most commonly, the alpha particle is shown as the neutral particle and not the cation.
. As this particle is expelled from the nucleus of the radioisotope that is undergoing decay, it has no electrons and thus has a 2+ charge. However, it quickly acquires two electrons from its surroundings to form the neutral atom. Most commonly, the alpha particle is shown as the neutral particle and not the cation.
Radon-222 undergoes alpha decay according to the following equation:
![]()
Notice that in going from Rn-222 to Po-218, the atomic number has decreased by 2 and the mass number by 4.
Beta Emission
A beta particle is an electron and can be represented as either ![]() or
or ![]() This electron comes from the nucleus, not the electron cloud, and results from the conversion of a neutron into a proton and an electron:
This electron comes from the nucleus, not the electron cloud, and results from the conversion of a neutron into a proton and an electron: ![]()
Nickel-63 will undergo beta decay according to the following equation:
![]()
Notice that the atomic number has increased by 1 in going from Ni-63 to Cu-63, but the mass number has remained unchanged.
Gamma Emission
Gamma emission is the giving off of high-energy, short-wavelength photons like X-rays. This radiation is commonly represented as γ. Gamma emission commonly accompanies most other types of radioactive decay but is often not shown in the balanced nuclear equation because it has neither appreciable mass nor charge.
Alpha, beta, and gamma emissions are the most common types of natural decay mode, but positron emission and electron capture are also observed occasionally.
Positron Emission
A positron is essentially an electron that has a positive charge instead of a negative one.
It is represented as ![]() or
or ![]() . Positron emission results from the conversion of a proton to a neutron and a positron:
. Positron emission results from the conversion of a proton to a neutron and a positron: ![]() . It is observed in the decay of some natural radioactive isotopes, such as
. It is observed in the decay of some natural radioactive isotopes, such as ![]()
Electron Capture
The four decay modes described above all involve the emission or giving off a particle; electron capture is the capturing of an electron from the energy level closest to the nucleus (1s) by a proton in the nucleus. This creates a neutron: ![]() . Electron capture leaves a vacancy in the 1s energy level, and an electron from a higher energy level drops down to fill this vacancy. A cascading effect occurs as the electrons shift downward and, as they do so, energy is released. This energy falls in the X-ray part of the electromagnetic spectrum. These X-rays give scientists a clue that electron capture has taken place.
. Electron capture leaves a vacancy in the 1s energy level, and an electron from a higher energy level drops down to fill this vacancy. A cascading effect occurs as the electrons shift downward and, as they do so, energy is released. This energy falls in the X-ray part of the electromagnetic spectrum. These X-rays give scientists a clue that electron capture has taken place.
Polonium-204 undergoes electron capture: ![]() Bi + X-rays. Notice that the atomic number has decreased by 1, but the mass number has remained the same. Remember that electron capture is the only decay mode that involves adding a particle to the left side of the reaction arrow.
Bi + X-rays. Notice that the atomic number has decreased by 1, but the mass number has remained the same. Remember that electron capture is the only decay mode that involves adding a particle to the left side of the reaction arrow.
Nuclear Stability

Predicting whether a specific isotope is stable and what type of decay mode it might undergo can be tricky. All isotopes containing 84 or more protons are unstable and will undergo nuclear decay. For these large, massive isotopes, alpha decay is observed most commonly. Alpha decay gets rid of four units of mass and two units of charge, thus helping relieve the repulsive stress found in these nuclei. For other isotopes, with atomic numbers less than 84, stability is best predicted determining the neutron-to-proton (n/p) ratio. Nuclei with a high ratio tend to undergo either positron emission or electron capture, while nuclei with a low ratio tend to undergo beta emission.
These predictions are only guidelines, and there are isotopes that utilize more than one decay mode. In addition, the isotope formed may not be stable and undergo further decay steps until a stable isotope results. For example, radioactive U-238 decays to stable Pb-206 in 14 steps, a majority of which are alpha emissions, as one might predict.
Nuclear Decay Calculations
A radioactive isotope may be unstable, but it is impossible to predict when a certain atom will decay. However, if a statistically large enough sample is examined, some trends become obvious. The radioactive decay follows first-order kinetics (see Chapter 13, Kinetics, for a more in-depth discussion of first-order reactions and equations). If the number of radioactive atoms in a sample is monitored, it can be determined that it takes a certain amount of time for half the sample to decay; it takes the same amount of time for half the remaining sample to decay; and so on. The amount of time it takes for half the sample to decay is called the half-life of the isotope and is given the symbol t1/2. The table below shows the percentage of radioactive isotope remaining versus half-life.
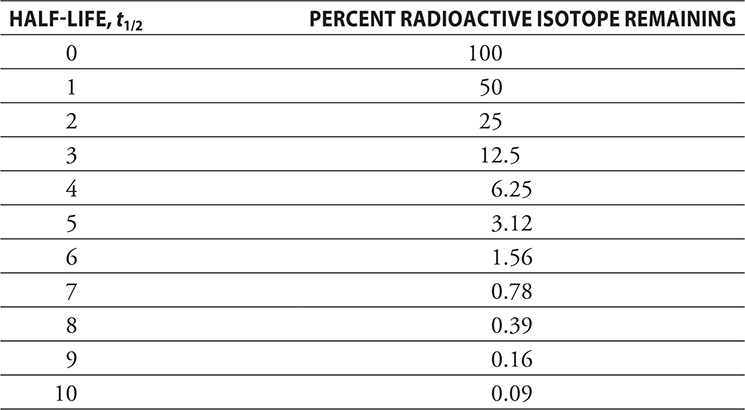
Half-lives may be very short, 4.2 × 10—6 seconds for Po-213, or very long, 4.5 × 109 years for U-238. The long half-lives of some waste products is a major problem with nuclear fission reactors.
If only multiples of half-lives are considered, the calculations are very straightforward. For example, I-131 is used in the treatment of thyroid cancer and has a t1/2 of 8 days. How long would it take to decay to 25% of its original amount? Looking at the chart, you see that 25% decay would occur at two half-lives, or 16 days. However, radioactive decay is not a linear process; you cannot use the chart to predict how much would still be radioactive at the end of 12 days or at some time (or amount) that is not associated with a multiple of a half-life. To solve these types of problems, one must use the mathematical relationships associated with first-order kinetics that were presented in the Kinetics chapter. In general, two equations are used:
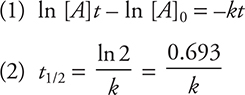
In these equations, the ln is the natural logarithm; [A]t is the amount of isotope radioactive remaining after some time t; [A]0 is the amount initially radioactive; and k is the rate constant for the decay. If you know initial and final amounts and are looking for the half-life, you will use equation (1) to solve for the rate constant and then use equation (2) to solve for t1/2.
For example: What is the half-life of a radioisotope that takes 45 min to decay to 80% of its original activity?
First use equation (1) to determine k; then use the value of k in equation (2) to determine the half-life:
Using equation (1): 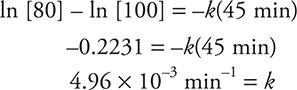
Using equation (2): 
If one knows the half-life and amount remaining radioactive, equation (2) can be used to calculate the rate constant k and equation (1) can then be used to solve for the time. This is the basis of carbon-14 dating, which is used to determine the age of objects that were once alive.
For example, suppose a wooden tool is discovered and its carbon-14 activity is determined to have decreased to 73% of the original. How old is the object?
The half-life of carbon-14 is 5,730 yr. Substituting this into equation (2):
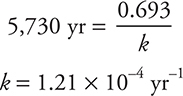
Substituting this rate constant into equation (1):
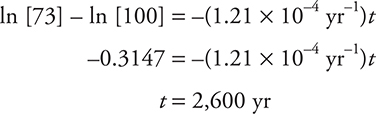
Mass—Energy Relationships
Like “normal” chemical reactions, nuclear reactions involve energy. In general, nuclear reactions involve much more energy than do “normal” chemical reactions. In a nuclear process, the energy results from the conversion of a very small amount of matter into energy. (Remember that in nuclear reactions there is no conservation of matter, as in ordinary chemical reactions.) The amount of energy that is produced can be calculated by using Einstein’s equation E = mc2, where E is the energy produced, m is the mass converted into energy (the mass defect), and c is the speed of light. The amount of matter that is converted into energy is normally very small, but when it is multiplied by the speed of light (a very large number) squared, the amount of energy produced is very large.
Common Mistakes to Avoid

1. Make sure your answer is reasonable. Don’t just write down the answer from your calculator.
2. Make sure your units cancel in your calculations, leaving the unit you want.
3. In half-life problems, don’t omit the minus sign. Watch your units. Half-lives must be in time units.
4. In half-life problems, be sure to use the amount of isotope still radioactive as [A]t and not the amount decayed.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. First are 10 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. Following those is a long free-response question like the ones in Section II of the exam. These questions are primarily designed to review prior knowledge and kinetics. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 15 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. When ![]() decays, it emits 2 α particles, then a β particle, followed by another α. The resulting nucleus is:
decays, it emits 2 α particles, then a β particle, followed by another α. The resulting nucleus is:
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
2. The formation of ![]() from
from ![]() occurs by:
occurs by:
(A) electron capture
(B) α decay
(C) β decay
(D) positron decay
3. Which of the following lists the types of radiation in the correct order of increasing penetrating power?
(A) α, γ, β
(B) β, α, γ
(C) α, β, γ
(D) β, γ, α
4. Choose the correct statement from the following that is applicable to β particles.
(A) Beta particles are electrons and have a mass number of zero and a charge of —1.
(B) Beta particles have a mass number of zero, a charge of —1, and are less penetrating than α particles.
(C) Beta particles are electrons with a charge of +1 and are less penetrating than α particles.
(D) Beta particles have a mass number of zero and a charge of +1.
5. An atom of ![]() undergoes radioactive decay by α emission. What is the product nuclide?
undergoes radioactive decay by α emission. What is the product nuclide?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
6. If 75% of a sample of pure ![]() decays in 24.6 years, what is the half-life of
decays in 24.6 years, what is the half-life of ![]() ?
?
(A) 24.6 yr
(B) 18.4 yr
(C) 12.3 yr
(D) 6.15 yr
7. Which of the following will have the smallest mass defect?
(A) 238U
(B) 12C
(C) 56Fe
(D) 1H
8. Several types of radioactive decay were mentioned in this chapter. How does electron capture differ from the other types mentioned?
(A) Electron capture is the only radioactive decay process that involves an electron.
(B) Electron capture is the only radioactive decay process in which something enters the nucleus.
(C) Electron capture is the only radioactive decay process that does not change the mass defect.
(D) Electron capture is the only radioactive decay process that involves no change in atomic number.
9. The age of Earth is over 4.4 billion years. This was determined by radioactive dating. Which of the following radioactive isotopes could NOT be used to determine the age of the Earth?
(A) 87Rb
(B) 238U
(C) 40K
(D) 14C
10. Uranium-236 undergoes spontaneous fission as indicated by the following partial equation: ![]() . What is the missing product?
. What is the missing product?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
![]() Answers and Explanations
Answers and Explanations
1. D—The mass of the starting nucleus is 226 and its atomic number is 88 (prior knowledge). Each α particle reduces the mass by 4 and each β reduces the mass by 0. The final mass should be 226 — (4 + 4 + 0 + 4) = 214. Each α particle reduces the atomic number by 2 and each β reduces the atomic number by —1. The final atomic number should be 88 — (2 + 2 — 1 + 2) = 83.
2. B—The calculations are as follows: mass difference = 234 — 230 = 4; atomic number difference = 92 — 90 = 2. These correspond to an α particle.
3. C—Alpha particles are the least penetrating, and gamma rays are the most penetrating (prior knowledge).
4. A—The complete symbol for a β particle is ![]() (prior knowledge). As indicated by the symbol, the mass number is zero. Beta particles are electrons, which means they have a —1 charge.
(prior knowledge). As indicated by the symbol, the mass number is zero. Beta particles are electrons, which means they have a —1 charge.
5. B—An α particle reduces the mass by 4 and reduces the atomic number by 2. The calculations are as follows: mass number = 238 — 4 = 234; atomic number = 92 — 2 = 90.
6. C—After one half-life, 50% would remain. After another half-life, this amount would be reduced by one-half to 25%. The total amount decayed is 75%. Thus, 24.6 years must be two half-lives of 12.3 years each. This is reviewed in Chapter 13, Kinetics.
7. D—1H has no mass defect because there is nothing to bind to the single proton in the nucleus (prior knowledge).
8. B—During electron capture, an electron is absorbed into the nucleus, which will alter the mass defect and change the atomic number. Beta decay also involves electrons.
9. D—The half-life of carbon-14 (5,730 years) is much too short to determine an age in the billions of years.
10. C—It is necessary to determine both the mass number and the atomic number of the nucleus formed. The mass number difference depends on the superscripts. The total on each side of the reaction arrow must be identical. Mass difference = 236 — 4(1) — 136 = 96. The atomic number difference depends on the subscripts. The total on each side of the reaction arrow must be identical. Atomic number difference = 92 — 4(0) — 53 = 39. The mass numbers and atomic number values should all be prior knowledge, covered in your pre-AP chemistry course when you learned about the structures of atoms.
![]() Free-Response Question
Free-Response Question
You have 10 minutes to answer the following question. You may use a calculator and the tables in the back of the book.
Question
It is possible to determine the age of a sample from a meteorite by determining the amounts of thorium-232 and lead-208 present. Thorium-232 decays to lead-208 with a half-life of 1.40 × 1010 years. A mineral sample contains 40.2 mg of thorium-232 and 18.0 mg of lead-208. Answer the following two questions concerning this sample.
(a) How many milligrams of thorium-232 were originally in the sample?
(b) What is the age of the sample?
![]() Answer and Explanation
Answer and Explanation
Note that, while nuclear chemistry is not an AP topic, all the materials in these questions depend on basic AP Chemistry knowledge, which is why this chapter can be valuable. It is especially important to remember that nuclear decay processes follow first-order kinetics; for this reason, this question is reviewed in Chapter 13, Kinetics.
(a) This is a stoichiometry calculation. The calculation of the original mass is the mass at present plus the mass of the thorium-232 that decayed. To simplify the mole calculation, it is useful to remember that numerically, g/mole is the same as mg/mmole.

The molar mass of 208Pb is 208 g/mole and not 207.2 g/mole (from the periodic table) because only one isotope is present. Using the molar mass from the periodic table gives an incorrect answer of 60.4 mg 232Th. If you did not convert the mass of lead-208 to mass of thorium-232, you got an incorrect answer of 58.2 mg 232Th.
The set up earns 1 point even if you incorrectly used 207.2 g/mole and 0 points if you did not convert lead to thorium. The correct answer (60.3 mg 232Th) earns a second point.
(b) The equation given in the exam booklet relating the quantities of material in a first-order reaction to time (integrated rate law) is ln [A]t — ln [A]0 = —kt. Another equation, also given in the exam booklet, is t1/2 = ln 2/0.693 = 0.693/k. It is possible to rearrange and combine these equations. Rearrangement gives:
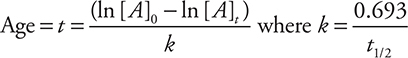
Entering the values ![]() years gives:
years gives:

Both [A]t and [A]0 must be in the same units, which, in this case, are mg 232Th. Using the wrong initial mass (60.4 mg) gives 8.22 × 109 yr (an error of 30 million years). Using the mass of lead-208 instead of converting to thorium-232 gives 7.48 × 109 yr.
Give yourself 1 point for the correct setup. You still get the point if you use a wrong answer from part (a). The correct answer (2.16 × 109 yr) earns a second point.
Total your points. There are 4 points possible. Subtract 1 point if your answers do not have the correct number of significant figures.
![]() Rapid Review
Rapid Review
• Know how to incorporate the mass number and the atomic number into a chemical symbol.
• Nuclear decay processes follow first-order kinetics. Use the appropriate equations.