5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
Additional Review and Applications
19 Organic Chemistry
SPECIAL NOTE

Although organic chemistry is not specifically tested on the AP Chemistry Exam, an overview of the subject will help you recognize and understand organic structures that appear in relation to other topics on the exam. In many cases, it will be important to be able to draw a Lewis structure for a molecule that happens to be organic. Organic molecules are good examples used to illustrate intermolecular forces. In addition, this material is taught by some high school and college teachers.
IN THIS CHAPTER
Summary: Organic chemistry is the study of the chemistry of carbon. Almost all the compounds containing carbon are classified as organic compounds. Only a few—for example, carbonates and cyanides—are classified as inorganic. It used to be thought that all organic compounds had to be produced by living organisms, but this idea was proven wrong in 1828 when German chemist Friedrich Wöhler produced the first organic compound from inorganic starting materials. Since that time, chemists have synthesized many organic compounds found in nature and have also made many never found naturally. It is carbon’s characteristic of bonding strongly to itself and to other elements in long, complex chains and rings that gives carbon the ability to form the many diverse and complex compounds needed to support life. It is important to remember that in organic compounds, carbon has four bonds.
Keywords and Equations

No keywords or equations specific to this chapter are listed on the AP Exam.
Hydrocarbons
Hydrocarbons are the simplest type of organic compound because they contain only the elements carbon and hydrogen. The electronegativities of carbon and hydrogen are similar, so these are nonpolar substances. As nonpolar substances, only London dispersion forces may be present.
There are four types of hydrocarbons; these are alkanes, alkenes, alkynes, and aromatics. Alkanes contain only single bonds. Alkenes contain at least one double bond between two carbon atoms. Alkynes contain at least one triple bond between two carbon atoms. Aromatics have ring structures with alternating single and double bonds between the carbon atoms. In all four categories, the carbon atoms have four bonds each and the hydrogen atoms have only one bond each.
The great variety of hydrocarbons is due to the ability of carbon atoms to form strong bonds to other carbon atoms. This is often seen as long chains of carbon atoms with hydrogen atoms off the side of the chain. The carbon atoms are bonded to each other with the hydrogen atoms off to the side. The number of hydrogen atoms depends on how many are needed to give each carbon atom four bonds. For example, if a carbon atom forms two single bonds to other carbon atoms, there will need to be two hydrogen atoms to bring the total to four bonds.
Hydrocarbons
Hydrocarbons are the simplest type of organic compound because they contain only the elements carbon and hydrogen. The electronegativities of carbon and hydrogen are similar, so these are nonpolar substances. As nonpolar substances, only London dispersion forces may be present.
There are four types of hydrocarbons; these are alkanes, alkenes, alkynes, and aromatics. Alkanes contain only single bonds. Alkenes contain at least one double bond between two carbon atoms. Alkynes contain at least one triple bond between two carbon atoms. Aromatics have ring structures with alternating single and double bonds between the carbon atoms. In all four categories, the carbon atoms have four bonds each and the hydrogen atoms have only one bond each.
The great variety of hydrocarbons is due to the ability of carbon atoms to form strong bonds to other carbon atoms. This is often seen as long chains of carbon atoms with hydrogen atoms off the side of the chain. The carbon atoms are bonded to each other with the hydrogen atoms off to the side. The number of hydrogen atoms depends on how many are needed to give each carbon atom four bonds. For example, if a carbon atom forms two single bonds to other carbon atoms, there will need to be two hydrogen atoms to bring the total to four bonds.
Structural Isomerism
An other reason why there are some many hydrocarbons is that it is possible to arrange the carbon atoms in different ways. These different arrangements are known as isomers. Compounds with the same molecular formulas but different structural formulas are called isomers. With hydrocarbons, this applies to a different arrangement of the carbon atoms. Isomers such as these are called structural isomers. Figure 19.1 shows the structural isomers of C5H12. Note that there are the same number of carbons and hydrogens in each structure. Only the way the carbons are bonded is different.
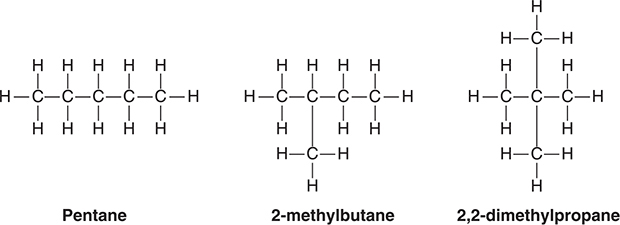
Figure 19.1 Structural isomers of C5H12.
In writing structural isomers, or any other organic compounds, remember that carbon forms four bonds. This should be apparent to you when inspecting Figure 19.1. One of the most common mistakes that a chemistry student makes is writing an organic structure with a carbon atom having fewer or more than four bonds.
There are additional types of isomers. One of the additional types of isomerism is optical isomerism. Optical isomers are extremely important in biological systems. If you enter a college program leading to a medical career, you will see many examples of optical isomers.
Common Functional Groups
Organic molecules may include functional groups. A functional group is anything in the molecule other than carbon—carbon single bonds or carbon—hydrogen bonds. The double and triple carbon—carbon bonds in alkenes and alkynes are functional groups. The presence of any atom other than carbon or hydrogen is a functional group.
The addition of other elements to hydrocarbons results in polar molecules. The intermolecular forces in polar molecules are either dipole—dipole forces or hydrogen bonding. The presence of polar molecules results in molecules that have higher melting and boiling points than hydrocarbons. (Note that if you are going to compare the melting or boiling points of molecules, the molecules you are comparing should be about the same size.)
Any of the hydrogen atoms in an organic compound may be replaced by a halogen (F, Cl, Br, or I).
The other carbon compounds that may appear on the AP Exam contain oxygen and/or nitrogen. Both oxygen and nitrogen are significantly more electronegative than either carbon or hydrogen. This significantly higher electronegativity means that oxygen and nitrogen containing organic compounds are polar. In addition, if a hydrogen atom is attached to the oxygen or nitrogen, hydrogen bonding is possible.
Oxygen containing organic compounds, with only single bonds, include ethers and alcohols. In an ether, an oxygen atom is attached to two carbon atoms, leaving a polar molecule with dipole—dipole forces. In an alcohol, an oxygen atom is attached to a carbon atom and a hydrogen atom, which leads to hydrogen bonding being the key intermolecular force.
Nitrogen containing organic compounds, with only single bonds, are the amines. In an amine, the nitrogen atom forms a single bond to one, two, or three carbon atoms. Amines are ammonia molecules with one, two, or three of the hydrogen atoms replaced by carbon-containing groups. If there are one or two hydrogen atoms attached to the nitrogen, there will be hydrogen bonding; otherwise, dipole—dipole forces are important. The key property of amines is that they are bases. As bases, they react with acids. The reaction with an acid is very much like the reaction of ammonia with an acid. Acid—base reactions of amines should be treated like reactions of ammonia with acids.
It is also possible for an oxygen atom to form a double bond to a carbon atom. Compounds containing a carbon—oxygen double bond include aldehydes, ketones, carboxylic acids, and esters. The present of oxygen makes these compounds polar with intermolecular dipole—dipole forces; in addition, carboxylic acids have hydrogen bonding. Besides recognizing the intermolecular forces, it will be important for you to deal with carboxylic acids.
Carboxylic acids are the most common organic acids. You have already dealt with one carboxylic acid—acetic acid. All other carboxylic acids behave like acetic acid, so you should not be distracted by their different formulas.
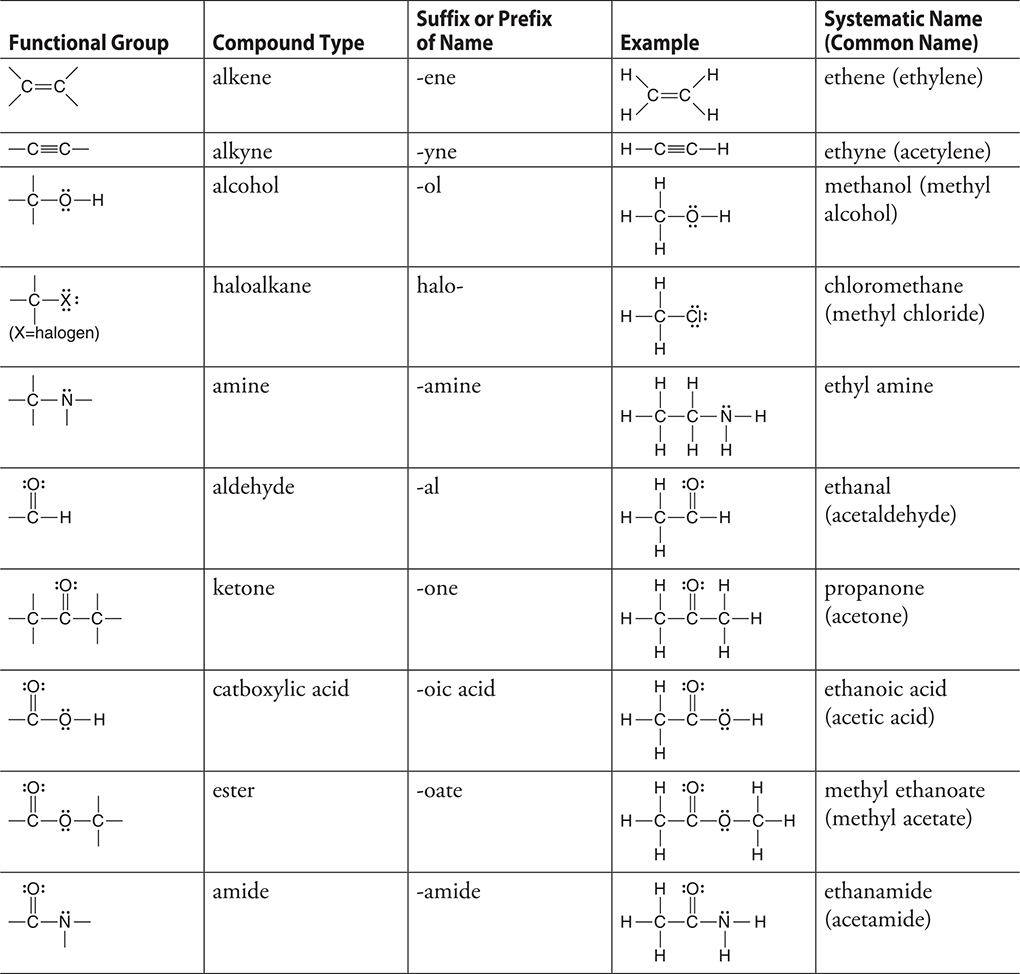
The introduction of other atoms (N, O, Cl, etc.) to organic compounds gives rise to many other functional groups. The major functional groups are shown in Table 19.1 on the next page.
Table 19.1 Common Functional Groups
Acid—Base Chemistry
While organic chemistry is not an AP topic, acid—base chemistry is a topic. These compounds do illustrate many of the AP topics. Organic acids (carboxylic acids) and bases (amines) are weak acids or weak bases. For this reason, they will have a Ka or a Kb (amino acids are organic compounds that are acids and bases). While the structures are different, the acid—base equilibria are identical to the acid—base equilibria presented in Chapter 15 (Unit 8).
A typical weak-acid equilibrium is:
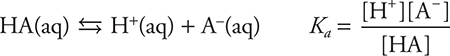
You probably have seen the acetic acid, a common weak acid, where A = C2H3O2. Substituting for A in the above equilibrium gives:
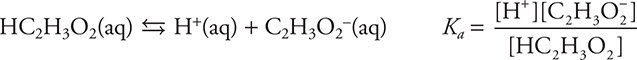
It is possible to write the formula of any carboxylic acid as:
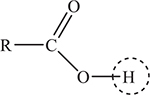
The circled hydrogen atom is the acid hydrogen. R may represent hydrogen or nearly any organic group. R = H— in formic acid, R = CH3— in acetic acid, and so on. The structural formula is the reason why acetic acid is sometimes written as CH3COOH instead of HC2H3O2. Do not let the identity of R distract you; as far as solving Ka problems, R makes no difference in the procedure.
The amines (organic bases) are related to ammonia, which means all amine Kb equilibria are related to the Kb for ammonia.
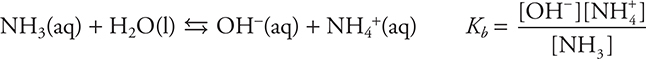
The formation of the ammonium ion may be represented as:
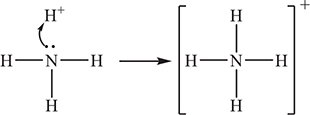
The formula of an amine may be represented as ![]() , where the different Rs may or may not be the same. For ammonia,
, where the different Rs may or may not be the same. For ammonia, ![]() . For a primary amine, one of the Rs ≠ H. For a secondary amine, two Rs ≠ H. In a tertiary amine, none of the Rs is an H. Based upon the general formula of an amine the above reaction becomes:
. For a primary amine, one of the Rs ≠ H. For a secondary amine, two Rs ≠ H. In a tertiary amine, none of the Rs is an H. Based upon the general formula of an amine the above reaction becomes:
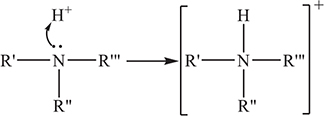
As with organic acids, do not be distracted by the identities of the Rs.
All organic acids have a hydrogen atom attached to an oxygen atom, and in all amines, except tertiary amines, there is at least one hydrogen atom attached to a nitrogen atom. This means that in all organic acids and in most amines, hydrogen bonding is present, which means that everything you learned about hydrogen bonding as an intermolecular interaction applies.
Experiments
Many experiments involving the concepts of organic chemistry are synthesis reactions. In addition, acid—base titrations involving organic materials are important.
Common Mistakes to Avoid
1. When writing organic formulas, make sure that every carbon has four bonds.
2. Hydrocarbons are nonpolar, and the intermolecular forces are London dispersion forces.
3. Adding other elements to a hydrocarbon may make the molecule polar. If it is polar, there are dipole—dipole forces and/or hydrogen bonding between the molecules.
4. Be sure that every carbon has four bonds!
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and improve your understanding of chemistry. Although organic chemistry won’t specifically be tested on the exam, this section contains questions of the same types as those found on the AP Exam. First are nine multiple-choice questions. Following those is a long free-response question. Time yourself following the instructions provided to practice pacing yourself.
Multiple-Choice Questions
Answer the following questions in 15 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. Cycloalkanes are hydrocarbons with the general formula CnH2n. If a 14-g sample of any alkene is combusted in excess oxygen, how many moles of water will form?
(A) 14 moles
(B) 2.0 moles
(C) 1.0 mole
(D) 28 moles
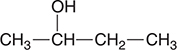
2. The organic compound shown above would be classified as:
(A) an organic base
(B) a hydrocarbon
(C) an alcohol
(D) a carboxylic acid
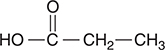
3. The above compound would be classified as:
(A) an organic base
(B) a hydrocarbon
(C) an alcohol
(D) a carboxylic acid
4. Which of the following compounds can form hydrogen bonds?
(A) CH3CH2CH2CH2OH
(B) CH3CH=CHCH2CH2
(C) CH3CH2CHClCH2CH3
(D) CH3CHClCH2CH2CH3
5. A carboxylic acid may be represented as:
(A) ROH
(B) RCHO
(C) R-O-R
(D) RCOOH
6. Which of the following compounds will give the highest pH when dissolved in water?
(A) CH3CHO
(B) CH3OH
(C) HCOOH
(D) CH3NH2
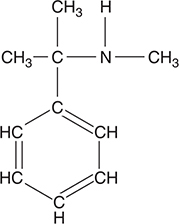
7. The organic compound shown above would be classified as:
(A) an organic base
(B) a hydrocarbon
(C) an alcohol
(D) a carboxylic acid
8. Which basic concept from Chapter 8, Bonding, best explains why carbon in stable organic compounds has four bonds?
(A) VSEPR
(B) octet rule
(C) Avogadro’s hypothesis
(D) electronegativity
9. Which of the following is the one reaction that all organic compounds will undergo?
(A) neutralization
(B) precipitation
(C) combustion
(D) polymerization
![]() Answers and Explanations
Answers and Explanations
1. C—Using the empirical formula gives the following combustion reaction:
![]()
The general formula CnH2n means that 1 mole of H2O will form per mole of empirical formula unit, regardless of the value of n. The moles of water formed are the mass of the alkene divided by the empirical formula mass:

2. C—Organic bases are, in general, amines (contain N). A hydrocarbon only contains C and H. A carboxylic acid (like acetic acid) has one oxygen double-bonded to a carbon and an —OH bonded to a carbon atom. Alcohols have an —OH bonded to a carbon atom.
3. D—Organic bases are, in general, amines (contain N). A hydrocarbon only contains C and H. A carboxylic acid (like acetic acid) has one oxygen double-bonded to a carbon and an —OH bonded to a carbon atom. Alcohols have an —OH bonded to a carbon atom.
4. A—Just because these are organic compounds does not alter the fact that hydrogen bonding requires a hydrogen atom bonded to one of the following: N, O, or F. Answer A has an H attached to an O.
5. D—A common example of a carboxylic acid is acetic acid (often written as CH3COOH), where R = CH3. A is an alcohol, B is an aldehyde, and C is an ether.
6. D—Only acids and bases change the pH. The acids are RCOOH, and the bases contain N. The highest pH is the result of dissolving a base in water. A is an aldehyde, which is neutral. B is an alcohol, which is neutral (or very slightly acidic). C is a carboxylic acid, which is an acid. D is an amine, which is a base. You should be able to recognize acids and bases.
7. A—Organic bases are, in general, amines (contain N). A hydrocarbon only contains C and H. Alcohols and carboxylic acids contain O. Do not be distracted by the “complicated” structure.
8. B—A carbon atom, with four bonds, is surrounded by 4 × 2 = 8 electrons (an octet).
9. C—All organic compounds burn.
![]() Free-Response Questions
Free-Response Questions
You have 20 minutes to answer the following questions. You may use a calculator and the tables in the back of the book.
Question 1
The alkane hexane, C6H14, has a molecular mass of 86.17 g mol—1.
(a) Like all hydrocarbons, hexane will burn. Write a balanced chemical equation for the complete combustion of hexane. This reaction produces gaseous carbon dioxide, CO2, and water vapor, H2O.
(b) The complete combustion of 10.0 g of hexane produces 487 kJ. What is the molar heat of combustion (ΔH) of hexane?
(c) Determine the pressure exerted by the carbon dioxide formed when 5.00 g of hexane is combusted. Assume the carbon dioxide is dry and stored in a 20.0 L container at 27°C. Show all work.
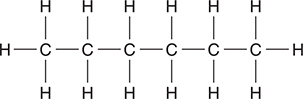
(d) Hexane, like most alkanes, may exist in different isomeric forms. The structural formula of one of these isomers is pictured below. Draw the structural formula of any two other isomers of hexane. Make sure all carbon atoms and hydrogen atoms are shown.
Question 2
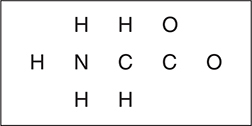
Amino acids are biological molecules with an organic acid group and an organic base group present. These two groups react with each other. The simplest natural amino acid is glycine, NH2CH2COOH, which, after its internal acid—base reaction, is better formulated as [N+H3CH2COO—].
(a) Complete the Lewis structure below for glycine in its ionic form. If resonance is possible, you only need to show one resonance form.
(b) Determine the formal charge for each nonhydrogen atom.
![]() Answers and Explanations
Answers and Explanations
Note that, while organic chemistry is not an AP topic, all the materials in these questions depend on basic AP Chemistry knowledge, which is why this chapter can be valuable.
Question 1
(a) 2 C6H14(g) + 19 O2(g) → 12 CO2(g) + 14 H2O(g)
Give yourself 2 points for the answer shown above or for the coefficients 1, 9/2, 6, and 7. Give yourself 1 point if you have one or more, but not all, of the elements balanced. This question reviews balancing equations.
(b) 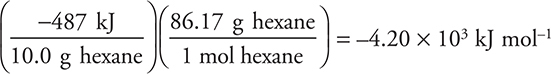
Give yourself 2 points for the above setup and correct answer (this requires a negative sign in the answer). If the set up is partially correct, give yourself 1 point. This question reviews thermochemistry.
(c) The ideal gas equation should be rearranged to the form P = nRT/V.
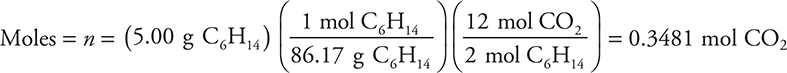
This answer has an extra significant figure. The mole ratio should match the one given in your balanced equation. You will not be penalized again for an incorrectly balanced equation.
R = 0.08206 L atm mol—1 K—1 (This value is in your test booklet.)
V = 20.0 L
T = 27°C + 273 = 300 K (In this case, there is no penalty if you forget to use the Kelvin temperature.)
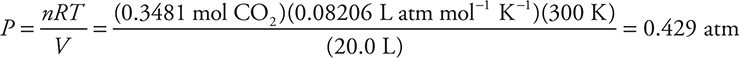
Give yourself 2 points for the correct setup and answer. Give yourself 1 point if you did everything correctly, except the mole ratio or the kelvin conversion. This question reviews gas stoichiometry.
(d) You may need to redraw one or more of your answers to match the answers shown below.
Give yourself 1 point for each correct answer, with a 4-point maximum. There are no bonus points for additional answers.
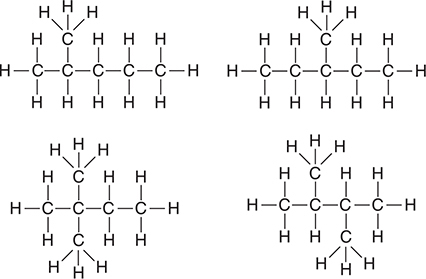
While it is unnecessary to name these compounds. The compounds are 2-methylpentane; 3-methylpentane; 2,2-dimethylbutane; and 2,3-dimethylbutane, respectively. These four, along with the original n-hexane, are the only isomers. If you think you have another isomer, you have simply redrawn one of these. Try naming your answer and see if it matches one of these names. This question reviews bonding concepts.
Total your points. There are 10 points possible. Subtract 1 point if your numerical answers do not have the correct number of significant figures.
Question 2
(a) As shown below, there are two different resonance forms. You only need to draw one of these.
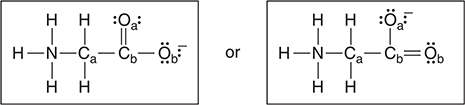
You get 1 point for a correct Lewis structure around the nitrogen and carbon atoms. You get 1 point for a correct Lewis structure around both oxygen atoms. This question reviews bonding. Since the atom placement was given to you it is irrelevant whether this is an organic compound or not.
(b) There are two acceptable resonance forms, differing only in the bonding to the oxygen atoms (and hence their formal charges). For an atom, the formal charge = valence electrons — nonbonding electrons — ½ (bonding electrons).
The formal charges for the left structure are in the following table. For the other structure, simply reverse the lines for Oa and Ob.

You get 1 point for a correct set of formal charges and 1 point if the total of all the formal charges is 0. This question helps review the bonding concepts.
Total your points. There are 4 points possible.
![]() Rapid Review
Rapid Review
• Organic chemistry is the chemistry of carbon and its compounds.
• Hydrocarbons are organic compounds of just carbon and hydrogen atoms.
• Alkanes are hydrocarbons in which there are only single bonds.
• Isomers are compounds that have the same molecular formulas but different structural formulas. Review the writing of the various structural isomers of alkanes. Make sure that each carbon atom has four bonds.
• A functional group is a group of atoms that is the reactive part of the molecule. Review the general functional groups. Especially review amines and carboxylic acids.