5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
Additional Review and Applications
20 Experimental Investigations
IN THIS CHAPTER
Summary: The free-response portion of the AP Exam will contain a question concerning an experiment, and there may also be a few multiple-choice questions on one or more of these experiments. This chapter reviews the basic experiments that the AP Exam Committee believes to be important. You should look over all the experiments in this chapter and pay particular attention to any experiments you did not perform. In some cases, you may find, after reading the description, that you did a similar experiment. Not every AP class does every experiment, but any of these experiments may appear on the AP Exam.
The free-response questions on recent exams have been concerned with the equipment, measurements, and calculations required. In some cases, sources of error are considered. To answer the question completely, you will need an understanding of the chemical concepts involved.
To discuss an experiment, you must be familiar with the equipment needed. In the keywords section at the beginning of this chapter is a complete list of equipment for the experiments (see also Figure 20.1). Make sure you are familiar with each item. You may know an item by a different name, or you may need to talk to your teacher to get additional information concerning an item.
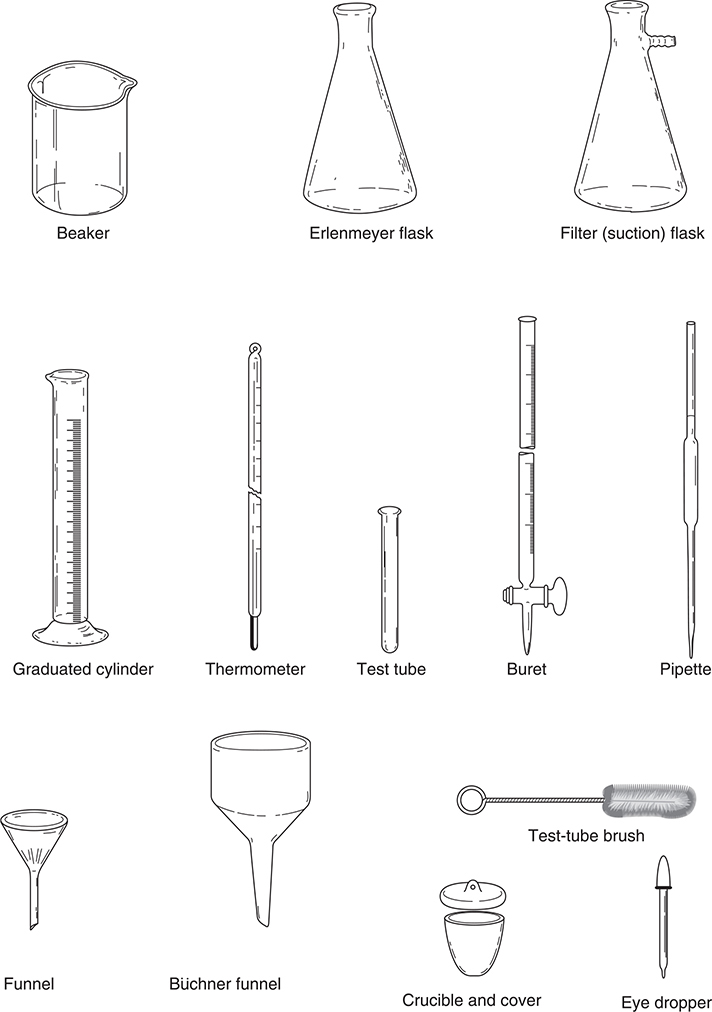
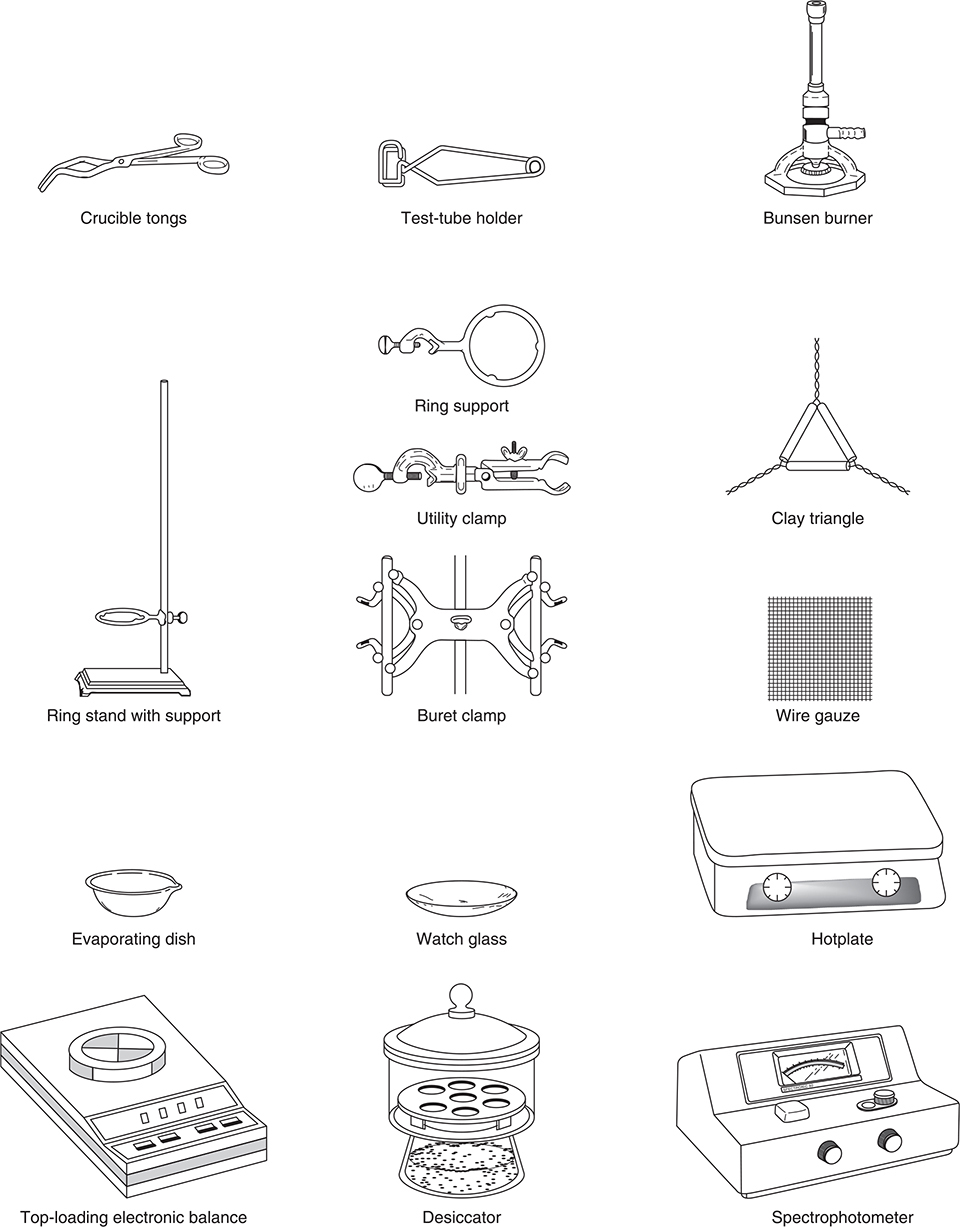
Figure 20.1 Common laboratory equipment.
In some cases, the exam question will request a list of the equipment needed, while in other cases you will get a list from which to choose the items you need. Certain items appear in many experiments. These include the analytical balance, beakers, support stands, pipets, test tubes, and Erlenmeyer flasks. Burets, graduated cylinders, clamps, desiccators, drying ovens, pH meters, volumetric flasks, and thermometers are also commonly used. If you are not sure what equipment to choose, these serve as good guesses. Most of the remaining equipment appears in three or fewer experiments.

You will need to know the basic measurements required for the experiment. For example, you may need to measure the initial and final temperatures. Do not make the mistake of saying you measure the change in temperature. You calculate the change in temperature from your measured initial and final temperatures. You do not need to give a lot of detail when listing the required measurements, but you need to be very specific in what you measure. Many students have lost exam points for not clearly distinguishing between measured and calculated values.
The basic calculations fall into two categories. Simple calculations, such as the change in temperature or the change in volume, are the easiest to forget. Simple calculations may also include mass-to-mole conversions. The other calculations normally involve entering values into one of the equations given at the beginning of the previous chapters of this book.
Keywords and Equations

Pay special attention to the specific keywords and equations in the chapters associated with the individual experiments. This is especially true for experiments you did not do or if it is a name that is not familiar to you.
A = εbc (A = absorbance; ε = molar absorptivity; b = path length; c = concentration)
q = mcΔT (q = heat; m = mass; c = specific heat capacity; ΔT = change in temperature)

Experiment 1: Spectroscopy
Synopsis
Specific experiments of this type are often an introduction to the field of spectroscopy. Such experiments are designed to demonstrate the relationship between the amount of light absorbed by some solutions and their concentrations. Light of a specific wavelength is passed through both the solvent and a sample, or several wavelengths are used to determine the maximum absorbance. The amount of light transmitted by the solvent is subtracted from the amount of light transmitted by the sample. If you made a number of measurements at different concentrations, you could create a graphical relationship between the amount of light absorbed and the concentration of the solution. By using this relationship, you could determine the concentration of an unknown solution.
Equipment
Spectrophotometer
Cuvettes (sample tubes for the spectrophotometer)
Stock solutions (of known concentrations) of the solute (commonly some dye)
One or more solutions of unknown concentration (may be a household substance)
Assorted glassware, including volumetric glassware
Measurements
The student will make several dilutions of the stock solution and will calculate the concentration of each dilution (using MconVcon = MdilVdil or Mbefore Vbefore = Mafter Vafter). The transmittance (%T) will be measured for each solution (remember to subtract the transmittance of the solvent—this may be done by adjusting the spectrophotometer to 100% T and then measuring the transmittance of the solution). You measure the volumes and calculate one or both concentrations.
Calculations
To determine the relationship between the concentration of the solution and the transmittance, plot the molarity of the different solutions versus the transmittance (expressed as a decimal). The absorbance (Abs) of the solution (how much light is absorbed) is calculated by the formula Abs = —log (T), where T is the transmittance of the solution (not the percent transmittance or temperature). On most spectrophotometers you can read absorbance directly. You may need to use Beer’s law in the form of the following equation: A = εbc (A = absorbance; ε = molar absorptivity; b = path length; c = concentration).
Comments
If you are asked for the mass of the solute in the unknown, you first determine its molar concentration (c, in the Beer’s law equation) using your spectroscopy data. Then, using the molar concentration, the volume of the solution, and the molar mass of the solute, you can calculate the grams of solute present in the sample. Experiments of this type involve colored solutions and a spectrophotometer.
Experiment 2: Spectrophotometry
Synopsis
Specific experiments that are performed in this investigation use the concepts and techniques developed in Experiment 1: Spectroscopy to determine the mass percentage of a substance in a solid sample. For example, the determination of the amount of copper in a brass sample is a common experiment that is used in this category as well as the amount of iron in a vitamin pill. First, the “best” wavelength to be used is determined. The “best” wavelength is the one that gives the maximum absorbance of the chemical species being determined. Next, solutions of the solute being determined are prepared and their absorbance is measured using a spectrophotometer. A plot of absorbance versus concentration (Beer’s law) is prepared. The solid sample is dissolved and diluted to a certain volume. The absorbance of a portion of this sample is measured and its concentration is determined using the graph. From this information the mass of the substance can be found. Using this mass information and the mass of the sample allows you to calculate the mass percentage of the substance in the sample.
Equipment
Spectrophotometer
Cuvettes (sample tubes for the spectrophotometer)
Stock solution (known concentration) of the solute
Sample to be analyzed
Assorted glassware, including volumetric glassware
Measurements
The student will make several dilutions of the stock solution (solution of known concentration of the substance being determined) and will calculate the concentration of each dilution (using MconVcon = MdilVdil). (You measure the volumes and calculate one or both concentrations.) The absorbance of one of the stock solutions is measured at several wavelengths (generally 400—700 nm in 10- to 20-nanometer increments) using a spectrophotometer. The data of absorbance versus wavelength is plotted, and the wavelength that gives the maximum absorbance is chosen to be used for the rest of the experiment. The absorbance of each of the dilutions is measured. A plot of absorbance versus concentration (Beer’s law plot) is prepared either by hand or using a spreadsheet. The solid sample is dissolved (if it is copper, this will require the use of nitric acid) and diluted to a certain volume. The concentration of that solution is determined using the Beer’s law plot. Using the concentration of the solution and the solution’s volume, you can calculate the moles and then grams of the substance. Using the initial mass of the sample, you can finally calculate the mass percentage of the substance in the sample.
Calculations
You can determine the concentrations of the diluted stock solution by using the dilution equation (MconVcon = MdilVdil). The mass percentage is calculated by:
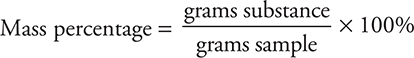
Comments
If you are doing a brass analysis for percentage of copper, you will dissolve the brass in concentrated nitric acid. Be extremely careful. The nitric acid is corrosive and the NO2 gas that is produced is toxic. On the AP Exam be sure to stress safety if you are describing this process. The preparation of other solutions may have other hazards you should be familiar with, and you should take appropriate precautions. Experiments of this type involve colored solutions and a spectrophotometer.
Experiment 3: Gravimetric Analysis
Synopsis
Specific experiments that are performed in this investigation use determination of the mass of a specific substance in a sample by precipitation, drying, and weighing. A common experiment done in this category is the determination of the hardness of a water sample. The hardness of a water sample is related to the amounts of calcium, magnesium, and iron ions in solution. These ions may be precipitated as the carbonate salts. For simplicity’s sake, hard-water samples are commonly prepared with only one of these ions, generally calcium. The carbonate salt is precipitated, separated from the solution by suction filtration, and dried in a drying oven. The mass of the dry salt is determined, and the water sample hardness is calculated as mg calcium carbonate per liter of water sample.
Equipment
Various salt solutions of known concentration
Analytical balance
Drying oven
Suction filtration apparatus
Büchner funnel
Filter paper
Aspirator
Ring stands
Assorted glassware, including volumetric glassware
Measurements
The student will make several measurements in gravimetric analysis, especially mass and volume determinations.
Calculations
If a water hardness analysis is being done, the grams of calcium carbonate per milliliter of water sample are initially calculated. This value is then converted to milligrams of calcium carbonate per liter of water sample (hardness) using appropriate conversions.
Comments
All measurements must be done accurately, especially the mass and volume measurements. The key to experiments of this type is mass measurements.
Experiment 4: Titration
Synopsis
In the titration procedure, the concentration of one solution is determined by adding small quantities at a time of another solution with known concentration until the point at which the moles of the first substance equal the moles of the other substance present (the endpoint). It is possible to do a titration by adding small amounts of a solution of a known concentration to determine the concentration of another solution. Many times this endpoint cannot be determined unaided, so an indicator or a pH meter is used. The point at which a color change happens with the indicator or an abrupt change in pH occurs with the pH meter is called the endpoint of the titration. Knowing the volume of the unknown, the concentration of the other reactant, and the number of milliliters it took to reach the endpoint, allows you to calculate the concentration of the unknown. Figure 20.2 shows a typical titrations setup.
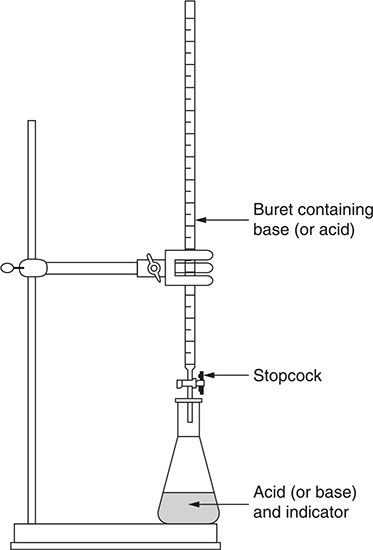
Figure 20.2 General acid−base titration setup.
Equipment
Burets
Erlenmeyer flasks
Pipets
Acid—base indicators or a pH meter
Acid or base solution of known concentration
Measurements
You will be placing a required volume of the unknown acid (or base) solution into the Erlenmeyer flask with a pipet. The buret will be filled with the other base (or acid) solution. As an alternative, a sample may be weighed directly into the flask and then dissolved in water. Be sure to record your initial volume. You will add small amounts of base drop by drop until the indicator changes color. Record the final volume. The final volume reading minus the initial volume reading is the volume of base added (this is a calculation, not a measurement).
Calculations
If the titration involves an acid—base reaction, the calculation of the concentration of the base is essentially a stoichiometry calculation. Most of the time you will be able to generalize the process using the equation:
H+(aq) + OH-(aq) → H2O(l)
From the molarity of the base and the volume used, you can calculate the moles of base (OH-). Because of the 1:1 stoichiometry that also will be the moles of acid. Dividing the moles by the liters of acid solution pipetted into the flask gives the acid’s molarity.
Titrations involving other reactions are similar. You still operate the equipment the same way and make the same measurements. You will need the specific chemical for the reaction used and to account for the stoichiometry.
Comments
If the titration involves an acid—base reaction, it may be performed with a pH meter without an indicator. The pH readings will be plotted against the volume. The endpoint is the point of inflection of the curve. A titration, either with an indicator or a pH meter, can be used to determine the acid content of household substances such as fruit juices or sodas. Nearly any type of reaction between two solutions may be utilized in a titration. Normally, one solution is added from a buret to another solution.
Experiment 5: Chromatography
Synopsis
Many times the components (solutes) in a solution cannot be separated by simple physical means. This is especially true of polar solutes because of their interactions with the solvent or each other. One method that is commonly used is chromatography (Figure 20.3). A very small amount of the solution is spotted onto a strip of filter paper or chromatography paper and allowed to dry. The strip is placed vertically into a jar containing a small amount of solvent. As the solvent is drawn up the strip by capillary action, it dissolves the sample. The various solutes have different affinities to the paper and to the solvent and can thus be separated as the solvent moves up the strip. Choice of the solvent is critical and can be related to its polarity; however, the choice sometimes must be done by trial and error.
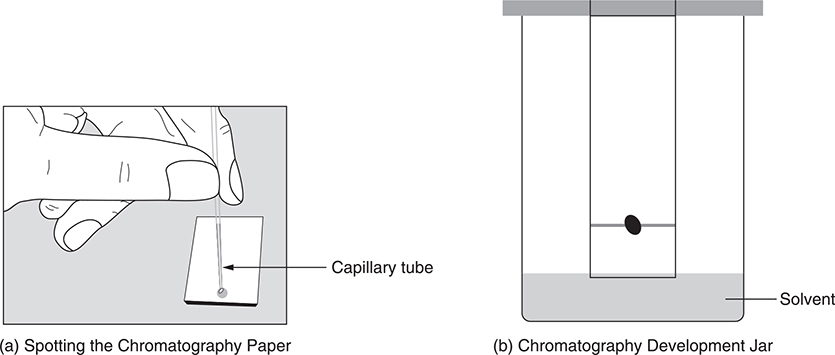
Figure 20.3 Basic procedure for a paper chromatography experiment.
Equipment
Filter paper or chromatography paper
Chromatography jar or large beaker
Various solvents
Metric ruler
Sample to be analyzed
Assorted glassware
Measurements
The student will make measurements of the distance that each component travels from the original spot and the distance that the solvent traveled.
Calculations
The calculations involve determining the Rf value for each component. The Rf value is the distance the component travels divided by the distance the solvent traveled. Substances that interact strongly with the paper do not travel very far (low Rf values), while those that interact strongly with the solvent travel much farther (high Rf values).
Comments
Chromatography is a very powerful separation technique.
Experiment 6: Determination of the Type of Bonding in Solid Samples
Synopsis
In this type of experiment, the student is given a set of bottles that contain solids with various types of bonding—ionic, covalent, or metallic. The student uses various physical and chemical tests to determine the bonding type. These tests might include melting point, conductivity, solubility, etc., along with observations of physical properties such as luster and hardness.
Equipment
Assorted solids—ionic, covalent, metals
Assorted solvents—polar and nonpolar
Conductivity tester
pH paper
Thermometer
Assorted glassware
Measurements
Many measurements and observations may be made. Some of these are:
Luster: metals tend to have a metallic luster; solid nonmetals often have a dull luster.
Melting point: ionic solids and metals have high melting points; covalent compounds have lower melting points.
Solubility: ionic compounds and polar covalent solids are generally soluble in water; metals and nonpolar covalent solids are generally insoluble or very slightly soluble in water.
Conductivity: aqueous solutions of ionic compounds are conductors; aqueous solutions of most polar covalent compounds are nonconductors.
Calculations
There are generally no calculations associated with this experiment.
Comments
Many other tests could be used: pH of the aqueous solutions, solubility on organic solvents, and so on.
Experiment 7: Stoichiometry
Synopsis
In this experiment, you are asked to verify the results of an experiment by checking both the stoichiometric calculations and the procedure. You will be asked to determine the percent by mass of substances such as sodium bicarbonate in a mixture. You will do this by making use of the unique properties of the components in this mixture.
Equipment
Bunsen burners and strikers
Digital balances
Ring stands and rings
Ceramic triangles
Crucibles and lids
Assorted glassware, including volumetric glassware
Measurements
A weighed sample mixture of sodium bicarbonate and sodium carbonate is heated to constant mass. The sodium bicarbonate decomposes to sodium carbonate, carbon dioxide (gas), and water vapor: 2 NaHCO3(s) → Na2CO3(s) + H2O(g) + CO2(g). The loss of mass is the loss in mass of CO2 + H2O. Examining the equation for the decomposition reaction, you can see that there is a 1:1 ratio of moles of water and carbon dioxide.
Calculations
If you let z = moles CO2 = moles H2O, then the total grams of mass lost can be shown as the sum of the moles of each (which will be the same) times the molar mass of each substance:
Mass lost (grams) = (z × 18.02 g H2O/mol) + (z × 44.01g CO2/mol)
You can then solve for z, the number of moles. As you can see from the balanced equation, the moles of NaHCO3 solid that decomposed is 2z. The mass of NaHCO3 that decomposed will be:
2z × 84.02g NaHCO3/mol
The percent of NaHCO3 in the mixture will be the mass of the sodium bicarbonate divided by the mass of the mixture sample times 100%:
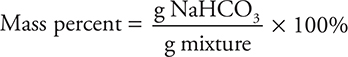
Comments
In order to increase the precision (and hopefully the accuracy) of the determination, several runs should be made, and an average taken.
This same procedure may be applied to many other reactions and mixtures. These samples could also be analyzed by a titration procedure.
Experiment 8: Redox Titration
Synopsis
The procedure here is basically the same as that in Experiment 4. In this experiment, the concentration of a substance will be determined by using a redox titration. The titrant will need to be standardized before it can be used in the titration. Commonly, the redox titration involves the titration of hydrogen peroxide (H2O2) with potassium permanganate (KMnO4), with the goal of analyzing the commercial hydrogen peroxide that can be found in a pharmacy. The KMnO4 solution can be standardized against a Fe(NH4)2(SO4)2 × 6H2O solution. You will prepare a standard (known concentration) solution of the Fe(NH4)2(SO4)2 × 6H2O, a sulfuric acid solution, and a solution of potassium permanganate. The redox half-reactions involved in the standardization are:
Fe2+(aq) → Fe3+(aq) + 1 e- and
MnO4-(aq) + 8 H+(aq) + 5 e- → Mn2+(aq) + 4 H2O(l)
giving an overall redox reaction of:
5 Fe2+(aq) + MnO4-(aq) + 8 H+(aq) → 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l)
The half-reactions involved in the titration of the hydrogen peroxide are:
H2O2(aq) → O2(g) + 2 H+(aq) + 2 e- and
MnO4-(aq) + 8 H+(aq) + 5 e- → Mn2+(aq) + 4 H2O(l)
giving the overall redox-reaction:
5 H2O2(aq) + 2 MnO4-(aq) + 6 H+(aq) → 2 Mn2+(aq) + 8 H2O(l) + 5 O2(g)
Equipment
Buret
Ring stand and clamps
Pipets of assorted volumes
Pipet bulbs
Assorted glassware, including volumetric glassware
Measurements
You will be making mass measurements of the Fe(NH4)2(SO4)2·6H2O and the KMnO4 and many volume measurements of the pipets, volumetric flasks, and the buret.
Calculations
For the standardization: from the number of grams of Fe(NH4)2(SO4)2·6H2O used, you can calculate the moles of Fe2+ used. Knowing this, you can determine the moles MnO4- used from the stoichiometry in the overall reaction (1 MnO4- : 5 Fe2+) and then its molarity.
For the peroxide titration: from the buret volume and the molarity of the KMnO4 solution, you can calculate the moles used in the titration, and by applying the overall reaction stoichiometry you can get the moles of hydrogen peroxide (5 H2O2 : 2 MnO4-). From the moles, you can get grams and finally mass percent (assuming the density of the peroxide solution is 1.00 g/mL).
A redox titration is not limited to the reactions shown here. There are many alternate combinations of oxidizing and reducing agents.
Comments
Be very careful in making your measurements. The same general procedure can be applied to other titration systems.
Experiment 9: Chemical and Physical Changes
Synopsis
Commonly this experiment involves separating the components of a mixture by using the chemical and physical properties of the mixture components. This is the basis of the analysis of commercially available samples such as over-the-counter acetaminophen- or aspirin-based pain relievers. The binder (many times sucrose), aspirin, and acetaminophen can be separated by the difference in their solubility in water and organic solvents, their acidity, and the difference in the way they react with hydrochloric acid and sodium bicarbonate solutions. Any chemical or physical change may be used.
Equipment
Büchner funnels
Vacuum filtration apparatus
Separatory funnel
Hot plate or drying oven
Assorted glassware
Measurements
You will be making mass measurements of the sample, and every time a component is separated as a solid, it is dried, and the mass determined.
Calculations
The overall percent recovery for the sample would be the sum of the masses of all the recovered components divided by the initial mass of the sample:
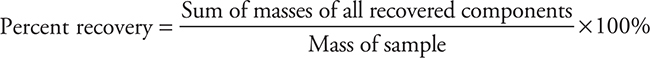
The percentage of each component can be calculated by dividing the mass of a component by the total mass of all recovered components:
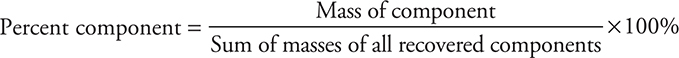
Comments
Be very careful in making your measurements. The same general procedure can be applied to numerous other systems.
Be especially careful when handling the acid solutions and organic solvents. Be sure to turn the separatory funnel upside down and open the stopcock (vent) to the funnel before opening it.
Experiment 10: Kinetics
Synopsis
In this experiment, some of the factors involved in the speed of a chemical reaction will be explored. Commonly this experiment focuses on the decomposition of calcium carbonate—limestone, CaCO3(s), and hydrochloric acid, HCl(aq). Pieces of calcium carbonate of different sizes (to test how the speed of reaction varies with surface area) and HCl solutions of different concentrations will be available. You can vary the temperature of the reaction mixture by using an ice bath or heating the mixture. In order to measure the speed of the reaction, the carbon dioxide gas product can be collected in a syringe, or a gas pressure probe can be used to monitor the production of the CO2(g) as a function of time. The mass of sample consumed (or the decrease in the total mass of the reaction flask) versus time can also be used as an indication of the speed of reaction.
Equipment
Balance
Hotplate
Syringes
Stopwatch
Assorted glassware
Magnetic stirrer and stir bar
Gas pressure probe and data collection device
Measurements
Measurements include the initial and final mass of the calcium carbonate sample, the volume of gas evolved, and time measurements.
Calculations
Calculations commonly involve determining the mass of sample consumed (lost) as a function of time. The results of the mass versus time measurements are commonly plotted.
Comments
When plotting the data, the time is commonly the horizontal axis, while the mass lost, or mL of gas produced, is the vertical axis.
Be especially careful when handling the hydrochloric acid.
Other reactions may be investigated. The only requirement is that the reaction be slow enough for you to be able to measure the time involved.
Experiment 11: Rate Laws
Synopsis
This is another type of kinetics experiment. In this experiment, you will determine the rate law for a specific chemical reaction. Commonly the reaction involved is the reaction of crystal violet (CV) with sodium hydroxide (NaOH). The progress of the reaction is followed with a spectrophotometer or colorimeter. You will initially create a Beer’s law calibration curve by measuring the absorbance of solutions of crystal violet of varying concentrations. Then you will use the same spectrophotometer to follow the change in concentration of crystal violet as it reacts with NaOH as a function of time:
CV+(aq) + OH-(aq) → CVOH(aq)
The rate expression for this reaction would be:
Rate = k [CV+]x [OH-]y
If we use a large stoichiometric excess of NaOH, then the rate equation becomes:
Rate = k* [CV+]x
since there is so much hydroxide ion present that its concentration essentially becomes constant.
Equipment
Spectrophotometer
Cuvettes (sample tubes for the spectrophotometer)
Pipettes and bulbs
Assorted glassware, including volumetric glassware
Measurements
You will be making measurements of absorbance and time. Be sure to use a blank containing only water and NaOH but no crystal violet.
Calculations
You will be making different concentrations of the stock crystal violet solution by dilution, so that you will use the dilution equation MconVcon = MdilVdil. You will be making three graphs:
(1) concentration versus time (straight line indicates zero order with respect to CV [x = 0 in rate expression]); (2) ln (concentration) versus time (straight line indicates first order with respect to CV [x = 1 in rate expression]); and (3) 1/concentration versus time (straight line indicates second order with respect to CV [x = 2 in rate expression]).
Comments
Be especially careful when handling the sodium hydroxide solution.
Experiment 12: Calorimetry
Synopsis
In this experiment, you will be measuring the heat produced (or absorbed) during the dissolving of various ionic substances in water with the goal of determining which of the salts is most efficient (with respect to cost) in generating or absorbing heat. Substances to test might include anhydrous calcium chloride (CaCl2), anhydrous sodium carbonate (Na2CO3), anhydrous ammonium nitrate (NH4NO3), anhydrous sodium acetate (NaC2H3O2), and similar salts. You will calculate the change in enthalpy of dissolution in kJ/mol (ΔHsoln) by using a coffee-cup calorimeter (see Figure 14.1 in Chapter 14, Thermodynamics). You may be using a magnetic stirrer instead of the stirring wire shown in the figure.
Equipment
Thermometers or temperature probes
Polystyrene cups
Magnetic stirrers and stir bars
Assorted glassware
Measurements
You will be making measurements of the initial and final temperatures of the solutions formed by adding a certain mass of solute to a measured amount of water. The ΔT is the final temperature minus the initial temperature. The value of ΔT is a calculated number and not a measured number.
Calculations
You may be given or may have to calculate the calorimeter constant, C, for your calorimeter—the heat absorbed by the calorimeter per degree of temperature change. The energy of solution formation (qrxn) is calculated by multiplying the mass times the specific heat of the solution (given) times the change in temperature (qrxn = mcΔT), and the energy of solution (qsoln) is calculated by qsoln = —(qrxn + CΔT). The enthalpy of dissolution (ΔHsoln) is calculated by dividing the qsoln (in kJ) by the number of moles of salt used.
Comments
Be especially careful with the ammonium nitrate—it is a strong oxidizer.
The thermometer is part of the surroundings, not the system.
Experiment 13: Chemical Equilibrium—Le Châtelier’s Principle
Synopsis
Experiments that fall into this category examine systems that are at equilibrium and what happens when that equilibrium is disturbed. Many times this involves having a small tray of reagents and testing an equilibrium system by mixing selected reagents and making observations. You may change concentrations (adding more reagent) or change the temperature of the solutions. This may involve an acid—base equilibrium or complex-ion equilibriums. Reactions will be given, and you should be able to describe the stress that you imposed and how the system reacted to that stress.
Equipment
Test tubes
Stirring rods
Spatula
Assorted glassware
Measurements
This experiment normally involves no measurements, only estimations of volumes and masses.
Calculations
This experiment usually involves no calculations. If calculations are involved, at a minimum, you will need to know the concentrations of any solutions used.
Comments
Be very careful when working with concentrated ammonia and hydrochloric acid. Always wear goggles, gloves, and an apron, and keep these reagents in the hood.
Experiment 14: Acid—Base Titrations
Synopsis
The procedure here is basically the same as that in Experiment 4. Experiments that fall into this category are acid—base titrations involving weak acids or weak bases versus a strong acid or strong base. Many times the course of the titration is followed by a pH meter and the equivalence point is determined graphically. This allows you to determine not only the concentration of the weak acid or base but also its pKa or pKb. Both monoprotic and polyprotic acids may be examined. From an examination of the specific reaction involving a weak acid or base, you should be able to determine whether the solution at the equivalence point will be acidic or basic.
Equipment
Stirring rods
pH meters or pH probes
Buret
Assorted glassware
Measurements
You will be making pH measurements and plotting them against volume of titrant added. In many cases, you will titrate various acids (strong and weak) with a NaOH solution of known concentration. The endpoint for such a titration is the point at which a dramatic increase in pH occurs; this is called the point of inflection of the curve. The pH at the volume corresponding to half the endpoint volume is the pKa of the acid. The same is true of bases, except the pH will be decreasing during the titration. A polyprotic acid or base will give you two or more points of inflection, and two or more pKs and Ks may be calculated.
Calculations
The Ka of the acid can be calculated by the equation Ka = 10-pKa. If the Kb of a weak base is to be determined, use Kb = 10-pKb.
Comments
Be extremely careful when working with the acids and bases and wear personal protective equipment, especially your goggles. When making dilutions, always add the acid (or base) to water, NOT water to acid.
Experiment 15: Buffer pH
Synopsis
A buffer is a substance that resists a change in pH when an acid or base is added to it. It is normally a mixture of a weak acid and its conjugate base, but it can be a weak base and its conjugate acid. Experiments in this category involve examining the properties of buffers and household substances that are buffers. This will involve titrating a substance with an acid or base while following the course of the titration with a pH meter, plotting the pH versus mL of titrant added, and determining the endpoint graphically. At any point before the endpoint, you have a buffer present. Common household substances may be tested for their buffer ability. The curve of pH versus mL of a substance that has some buffering ability rises sharply initially and then levels off much more than a titration of a substance that is not a buffer. You can use this to determine whether an unknown solution exhibits any buffering capability.
Equipment
pH meter
Burets and clamps
Magnetic stirrer
Assorted glassware
Measurements
You will be making measurements of volume and pH for a wide variety of substances. The point in a titration involving a buffer that corresponds to halfway to the endpoint is called the point of maximum buffering.
Calculations
The Ka of the acid can be calculated by the equation Ka = 10-pKa. If the Kb of a weak base is to be determined, then use Kb = 10-pKb.
Comments
Be careful in handling the acid and base solutions.
Experiment 16: The Capacity of a Buffer
Synopsis
Experiments in this category are designed to explore the capacity of a buffer, which is the amount of acid or base that can be neutralized by the buffer. You can determine this by using different amounts of the conjugate acid and base components or by changing the concentration of each by the same amount. Normally, the higher the concentration of the conjugate acid and base in the buffer, the more moles of added base or acid can be neutralized and thus the higher the buffer capacity. You will also be asked to create a buffer of a specific pH.
Equipment
Balance
Burets and clamps
Assorted glassware
Measurements
You will be making measurements of volume and pH for a wide variety of substances. You will be making graphs of pH versus mL of titrant added.
Calculations
You can calculate the initial pH of a conjugate acid/base buffer by using the following equations:
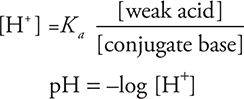
If you want a buffer of a certain pH, then put in the Ka of the weak acid you want to use and the [H+] desired and solve for the ratio of acid to base. If you have a choice of several acid/base systems, then choose the one whose pKa is closest to the desired pH. It is also possible to use the Kb of a weak base in your calculations.
Comments
Be extremely careful when working with the acids and bases and wear personal protective equipment, especially your goggles. When making dilutions, always add the acid (or base) to water, NOT water to acid. The equations used may be in a different form.
Common Mistakes to Avoid
1. You measure initial and final values but calculate the change.
2. You use an analytical balance to weigh the mass (grams) but not the moles.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. First are 20 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. Following those is a long free-response question and four short free-response questions like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. You have an aqueous solution of sugar. The simplest method to separate the sugar from the solution is to:
(A) evaporate the solution to dryness
(B) centrifuge the solution
(C) filter the solution
(D) electrolyze the solution
2. A chemistry student adds 25.0 g of sodium hydroxide, NaOH, to 500.0 g of water. Which of the following procedures should he employ to determine the molarity of the solution?
(A) He should convert the grams of NaOH to moles and measure the volume of the solution.
(B) He should titrate the solution with standard potassium hydroxide solution.
(C) He should determine the freezing point of the solution.
(D) He should determine the vapor pressure of the solution at room temperature.
3. A chemistry student prepares a solution of an unknown solid with a molar mass of 78.3 g/mol. She prepares the solution by dissolving 2.50 g of the unknown substance in 100.0 g of water. Which of the following procedures could she use to determine whether the unknown substance is an electrolyte?
(A) She could measure the specific heat of the solution.
(B) She could measure the volume of the solution.
(C) She could measure the freezing point of the solution.
(D) She could determine the specific heat of the solution.
4. A chemist makes a solution by dissolving 10 g of urea in 100 g of water. What additional information does he need to calculate the molarity of this solution?
(A) He needs the density of the solution and the molar mass of urea.
(B) He needs the density of urea and the molar mass of urea.
(C) He needs the density of water and the density of the solution.
(D) He needs the molar mass of urea and the density of water.
5. A certain reaction follows the rate law Rate = k[Br-][BrO-][H+]2. What are the units of the rate constant, k?
(A) s-1M -3
(B) M s-1
(C) M 3 s
(D) M s
Use the following diagram for questions 6 and 7.
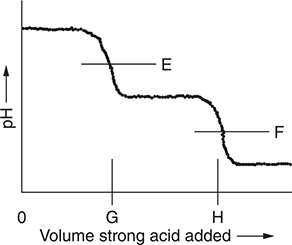
The diagram above represents the idealized titration curve for the reaction of sodium oxalate (Na2C2O4) with a strong acid like hydrochloric acid (HCl). E and F represent the pH at the endpoints. G and H will depend on the composition of the sample with the possibility that one may not be present.
6. A trial run used a sample of pure sodium oxalate. How does the volume of acid necessary to reach G compare to the volume of acid necessary to get from G to H?
(A) The volumes required will be the same.
(B) A larger volume is necessary to reach G.
(C) A larger volume is necessary to get from G to H.
(D) It is impossible to determine.
7. In addition to water, what are the predominant species in solution at E?
(A) Na2C2O4 and HCl
(B) Na+, Cl-, and HC2O4-
(C) C2O42- and H+
(D) Na+, H+, and C2O42-
8. A student is attempting to prepare an electrochemical cell, which ideally should produce 1.23 V. Unfortunately, he was unable to make a salt bridge. He reasoned that a copper wire would conduct electricity like a salt bridge, so he used a copper wire in place of the salt bridge. How will the voltage of the cell with the copper wire compare to that of the ideal cell?
(A) The voltage is ideal.
(B) The voltage becomes zero.
(C) The voltage is greater than ideal.
(D) The voltage less than ideal but greater than zero.
9. A chemist constructs an electrochemical cell with an iron anode and a copper cathode. She measures the cell voltage. Next, she replaces the copper electrode with a larger copper electrode. What does she find when she measures the cell voltage of the cell with the larger copper electrode?
(A) There is no change in the voltage.
(B) The voltage becomes zero.
(C) The voltage increases.
(D) The voltage decreases but stays positive.
10. While investigating a radioactive decay process, a chemist constructs a linear graph. What information did she plot?
(A) She plotted the concentration remaining versus time.
(B) She plotted the natural logarithm of the concentration remaining versus time.
(C) She plotted the reciprocal of the concentration remaining versus time.
(D) She plotted the natural logarithm of the concentration remaining versus the inverse of the time.
11. The teacher gave a student a 0.10 M solution of an unknown indicator solution. The student was to prepare a calibration graph of the absorbance versus indicator concentration for this unknown indicator. The 0.10 M solution was sufficiently dilute to be used directly in a spectrophotometer. What should be the student’s first step?
(A) Determine the wavelength of maximum absorbance.
(B) Make a series of dilutions to produce a set of solutions of varying concentrations.
(C) Determine the molar mass of the unknown indicator.
(D) Measure the mass and volume of the solution to determine its density.
12. The teacher gave another student a 0.10 M solution of an unknown indicator solution. The wavelength of maximum absorbance is 640 nm, the molar mass of the unknown indicator was 347 g mol-1, and the density of the solution was 1.00 g mL-1. To facilitate the construction of the calibration curve, the student used a standard cuvette as the reference in the spectrophotometer and placed the various dilutions in a series of test tubes. The resulting calibration curve seemed very erratic. What was the student’s error?
(A) There were no precautions taken to keep the temperature constant.
(B) Test tubes should never be used as a substitute for a standard cuvette.
(C) The unknown was one of the few substances that do not produce a linear calibration curve.
(D) The concentrations of the diluted solutions were all too high.
13. A student is given the task of preparing a 0.10 M solution of H2SO4 (molar mass = 98 g mol-1) to be used by the class in an experiment. The class needs 1.00 L of this solution. The student reasons that they will need to weigh 9.8 g of sulfuric acid and add this to a 1 L volumetric flask, add about 500 mL distilled water and swirl the flask to mix. After the solution is mixed, additional distilled water is added to bring the level to the line in the volumetric flask. Finally the flask is stoppered and inverted several times to assure the solution is well mixed. The student presents this procedure to the teacher, who rejects it because of a mistake. What was the student’s mistake?
(A) The student does not allow tie for the solution to cool.
(B) The student used the wrong mass of H2SO4.
(C) The student suggests adding water to acid.
(D) The solution will not have the correct molarity.
14. Two students, A and B, are conducting an experiment in gravimetric analysis. They are attempting to determine the quantity of silver in a sample by precipitating the silver as silver acetate, AgC2H3O2, which has Ksp = 4 × 10-3. Both students precipitate the silver from solution by adding an excess of 0.10 M sodium acetate, NaC2H3O2 solution. Once all the silver has precipitated, student A washes the precipitate several times with sodium acetate solution, and finally once with a little distilled water while student B washes the solution several times with distilled water. Once washed, both students dry and weigh the precipitate. Based upon the masses of the dried precipitates, each student calculates the percent silver in the sample. How do the results of the student results compare to the correct percent of silver in the sample?
(A) The values are too low with student B being closer than student A.
(B) The values are too high with student B being closer than student A.
(C) The values are too high with student A being closer than student B.
(D) The values are too low with student A being closer than student B.
15. 2 KMnO4(aq) + 5 Na2C2O4(aq) + 8 H2SO4(aq) →2 MnSO4(aq) + 10 CO2(g) + K2SO4(aq) + 5 Na2SO4(aq) + 8 H2O(l)
A student is planning on doing a redox titration involving the above chemical reaction. The first step the student must perform is to standardize the KMnO4 solution (approximately 0.1 M ) with primary standard sodium oxalate (molar mass 134.0 g mol-1). The student carefully weighs 5.360 g of Na2C2O4 into a flask and adds about 25 mL of distilled water to dissolve the sample and 50.00 mL of 1.000 M H2SO4 to the flask. The Na2C2O4 solution is then titrated with the KMnO4. This titration requires 40.00 mL of the KMnO4 solution. From this information, the student calculates the molarity. The student carefully repeats this procedure three more times and averages the consistent results of the four titrations. However, when the standardized KMnO4 solution is used to determine the amount of Na2C2O4 in an unknown, the results are inaccurate. What did the student do wrong?
(A) The student did not add sufficient H2SO4 to the samples.
(B) The student incorrectly weighed the Na2C2O4 for one of the solutions used for standardization.
(C) The student incorrectly recorded the volume at the endpoint of one of the samples used for standardization.
(D) The student titrated the solution in one titration too fast and missed getting and accurate endpoint.
16. A student adds 100.0 g of water to a “coffee-cup” calorimeter. The initial temperature of the water was 20.00°C. The student add 4.000 g of solid NaOH, and the temperature increases to 35.00°C. The heat of solution for sodium hydroxide is —44.50 kJ mol-1. Determine the heat capacity of the solution in J g-1 °C-1.
(A) -2.833 J g-1 °C -1
(B) 2.833 J g-1 °C -1
(C) 73.67 J g-1 °C -1
(D) -73.67 J g-1 °C -1
17. A student wishes to determine the Ka of an unknown weak acid, HA, with a molar mass of 140 g mol-1. The student first accurately weighs 1.400 g of the unknown acid. The HA is dissolved in a little water, transferred to a 100 mL volumetric flask and distilled water added until the line on flask is reached. A pipette is used to transfer 50.00 mL of this solution to an Erlenmeyer flask. The solution in the Erlenmeyer flask is titrated to the endpoint with standard NaOH solution. The untitrated and titrated samples are mixed in a beaker and the pH of the mixture is determined with a pH meter. What must the student now due to determine the Ka of the solution?
(A) Determine the moles of acid present in the titrated sample.
(B) Determine the value of 10-pH.
(C) Determine the final volume of the mixed solution.
(D) Determine the moles of untitrated acid present.
18. A student is preparing to do an experiment to determine the mass percent of copper in brass. Unfortunately, while collecting the materials for the experiment, the student learns that there is no nitric acid. The student rationalizes that if should be possible to substitute hydrochloric acid for the nitric acid since they are both strong acids. So the student sets up the experiment using hydrochloric acid. After completing the experiment, the student obtains results that are:
(A) the same as with nitric acid
(B) no results
(C) lower than with nitric acid
(D) higher than with nitric acid
Use the following graph for Question 19.
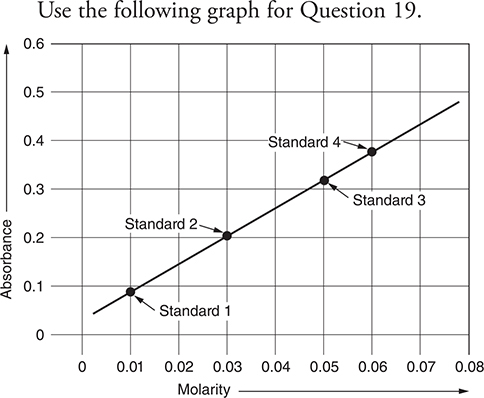
19. A student is studying the absorbance of an unknown indicator versus concentration. The student constructs the above graph. To test the accuracy of the calibration curve, another student prepares a different solution of the same indicator. The solution prepared by the other student has an absorbance of 0.44. Will the other student’s solution be a fair test of the calibration curve?
(A) Yes, the solution will work fine.
(B) Unknown until the other student’s calculations are checked.
(C) No, the absorbance is outside the range established by the standards.
(D) Unknown until the temperature is measured.
20. It is possible to determine the calcium in water (hardness) by titrating the water with Na2EDTA (EDTA is an abbreviation for ethylenediaminetetraacetic acid). This titration requires Eriochrome black as an indicator. This is an example of a complexometric titration as the calcium forms a complex with EDTA. To analyze a hard water sample a sample is pipetted into a flask, the indicator is added, a pH = 10 buffer and a small amount of magnesium ion are added. Theis solution is titrated as normal until the indicator changes from wine-red to blue. The net-ionic equation for the reaction is:
Ca2+(aq) + EDTA4-(aq) → CaEDTA2+(aq)
Historically, the concentration was reported as the titer (mg CaCO3 L-1) instead of M (mole L-1). Today ppm (parts per million) are normally used; however, ppm is numerically equal to the titer. A student conducts a titration of a hard-water sample and determines the Ca2+ concentration to be 0.0100 M. What is the titer of this solution?
(A) 4.00 × 102 mg CaCO3 L-1
(B) 1.00 × 103 mg CaCO3 L-1
(C) 1.00 mg CaCO3 L-1
(D) 1.00 × 10-3 mg CaCO3 L-1
![]() Answers and Explanations
Answers and Explanations
1. A—Separating materials in solution normally involves a physical change such as removing the solvent through evaporation. B and C will work on a heterogeneous mixture but not a solution (homogeneous mixture). D is a chemical change.
2. A—Molarity is moles per liter. The moles are readily determined from the grams of NaOH. Then, simply measuring the volume (and converting to liters) is all that is needed to determine the molarity. B may seem like a good option because titration is a common method for determining the molarity of a solution; however, one base (KOH) cannot be used to titrate another base (NaOH), as an acid is needed. C could be used to determine the molality of the solution; however, it is easier to determine the molality from the mass of NaOH and the mass of water; to convert to molarity, another measurement would be required. The vapor pressure would help determine the molarity, but it would not be easy to get to the molarity from the vapor pressure.
3. C—If the solute contains an electrolyte, the solution will conduct electricity and the van’t Hoff factor, i, will be greater than 1. The choices do not include any conductivity measurements; therefore, it is necessary to determine the van’t Hoff factor. It is possible to determine the van’t Hoff by measuring the osmotic pressure, the boiling-point elevation, or the freezing-point depression. The freezing-point depression may be found by measuring the freezing point of the solution and comparing the measured freezing-point depression to that expected for a nonelectrolyte.
4. A—To calculate the molarity, the moles of urea and the volume of the solution are necessary. It is possible to calculate the volume of the solution from the density of the solution and the mass of the solution (110 g). The mass of urea and the molar mass of urea give the moles of urea.
5. A—The units on each side must match. The rate has the units of M/s (or M s-1 or mol L-1 s-1). Substituting units for symbols changes the rate law from Rate = k [Br-] [BrO-] [H+]2 to M/s = k [M] [M] [M2] or M/s = k[M4]. From this, k must have the units 1/(s M3) or s-1M -3.
6. A—At G, all the C2O42- has been converted to HC2O4-, and the of HC2O4- will equal the moles of C2O42- originally present. It will require an equal volume of acid to titrate an equal number of moles of HC2O4-, as required for the C2O42-.
7. B—The reaction with HCl converts all the C2O42- to HC2O4-. The Na+ has not reacted, so it is still present. The Cl- is from the HCl and remains in solution because it has not reacted. The H+ from the acid reacted with the C2O42- to form HC2O4- and is no longer present (in a significant amount). Other than water, all species are strong electrolytes and exist as ions in solution.
8. B—A source of cations and anions is necessary for the operation of a cell to keep the charges in each compartment neutral. If there is no salt bridge, there is no ion source, and the cell cannot operate (zero voltage).
9. A—The size of the electrode is irrelevant to the cell voltage.
10. B—The radioactive decay process follows first-order kinetics; only B will give a linear graph for a first-order process. A applies to zero-order kinetics. C applies to second-order kinetics. D is not a kinetics graph.
11. A—The molar mass and the density are irrelevant to the task the student has been assigned. The dilutions are necessary to construct a calibration curve; however, it is necessary to know the wavelength of maximum absorbance first.
12. B—Cuvettes are carefully calibrated to make sure they always behave the same in a spectrophotometer. Test tubes are not carefully calibrated, so a series of different test tubes will each behave differently and disrupt the measurements. Unless the experiment was conducted at a temperature other than room temperature, there is no need to keep the temperature constant. An indicator will produce a linear calibration curve. If the concentrations of the dilutions were too high, the results would appear to be a straight line at maximum absorbance (across the top of the graph).
13. C—The student forgot than one should never add water to acid.
14. D—The Ksp is too large to give good results in a gravimetric analysis experiment. The relatively large Ksp value will lead to low results. Washing the precipitate with sodium acetate solution reduces the quantity of precipitate that dissolves through Le Châtelier’s principle (a final wash with distilled water should remove any residual sodium acetate in the sample and dissolve a minimal quantity of silver acetate). Washing with distilled water several times will dissolve some silver precipitate with each washing.
15. A—The results were consistent; therefore, something occurring in one sample would not lead to consistent results.
To check the H2SO4 answer, you need to start by determining the moles of Na2C2O4:

Now calculating the moles H2SO4 required:
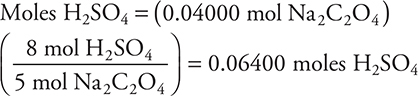 needed
needed
The moles H2SO4 the student used was:
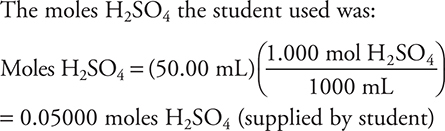
The moles of H2SO4 the student added was less than the moles required for the reaction to go to completion.
Note: the molarity was written as

16. B—The equation needed for this problem is q = mCΔT. This needs to be rearranged to solve for C:
![]()
It is now necessary to determine the value of each of the quantities used in this equation.
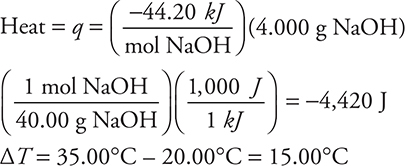
Mass = m = 100.0 g H2O + 4.00 g NaOH = 104.0 g solution
Since the dissolving of the NaOH caused 4,450 J to be released into the solution, the solution absorbed 4,420 J.
Entering the values into the equation:
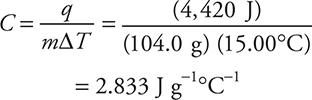
To save time, round the values:
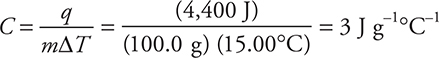
The 73.67 J g—1°C—1 results if only the mass of NaOH is used instead of the mass of the solution.
The negative answers result if you forget that the solution absorbs the heat (+).
17. B—The resultant solution from mixing the titrated and untitrated solution is a buffer with equal concentrations of the conjugate acid and base. Such a solution has pH = pKa. Then Ka = or 10-pH. (Note that such a solution also results at the midpoint of a weak acid—strong base titration.)
18. B—While it is true that both acids are strong, only nitric acid is an oxidizing acid. It takes an oxidizing acid to react with copper metal or any less active metal. The student could have substituted another strong oxidizing acid such as sulfuric acid.
Note: the potential required to oxidize copper must be greater than 0.34 V. Nonoxidizing acids, such as hydrochloric acid, have a potential of 0.0 V. The potential for nitric acid is 0.90 V.
19. C—The graph shows that the absorption versus concentration of the unknown indicator is linear from Standard 1 to Standard 4. There is no indication of the relationship outside this range. The first student should not have extended the calibration curve beyond this range. There is a lower limit and an upper limit to the region on linearity for any solution. An absorbance of 0.44 is outside the known linear range; therefore, it would be improper to assume that it is.
20. B—This is a unit conversion problem.
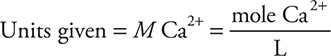
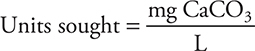
As with any unit conversion problem, the conversion may be done in any order:
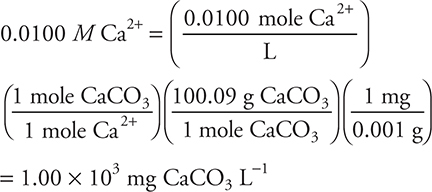
The 4.00 × 102 mg CaCO3 L-1 answer comes from using the molar mass of Ca2+ instead of the molar mass of CaCO3. The 1.00 mg CaCO3 L-1 comes from forgetting the gram-to-milligram conversion; we have seen this commonly when students do not write their units when working problems. The 1.00 × 10-3 mg CaCO3 L-1 comes from doing the gram-to-milligram conversion incorrectly, as seen on many of the exams we have graded.
The use of titers and ppm illustrates that the entire world is still not metric. For example, the author’s local water quality report from the city lists an average alkalinity for 2020 as 82.45 ppm.
![]() Free-Response Questions
Free-Response Questions
You have 15 minutes to answer each of the following two long questions and 10 minutes each for each of the four short questions. You may use a calculator and the tables in the back of the book.
Question 1
A sample of a solid weak monoprotic acid, HA, is supplied, along with solid sodium hydroxide, NaOH, a phenolphthalein solution, and primary standard potassium hydrogen phthalate (KHP).
(a) Describe how a standardized sodium hydroxide solution may be prepared for the titration.
(b) Sketch a graph of pH versus volume of base added for the titration.
(c) Sketch the titration curve if the unknown acid were really a diprotic acid.
(d) Describe the steps necessary to determine Ka for HA.
(e) What factor determines which indicator should be chosen for this titration?
Question 2
A student is studying the kinetics of the decomposition of a blue dye. Aqueous solutions of this dye will decompose to colorless products when exposed to air. For this reason, aqueous solutions are normally stored in tightly sealed containers. The decomposition of the blue dye is thought to follow first-order kinetics. If this is true, a graph of the natural log of the concentration (ln[A]) versus time will give a straight line with a slope proportional to the rate of the reaction.
In one experiment, she collects or calculates the data in the following table:

(a) What is the average slope for the ln[A] versus time line between Experiments 3 through 7? Include the units in your answer.
(b) Why did the student calculate the entries in the “ln[A]” column instead of using the [A] values directly?
(c) Using the integrated first-order rate law equation, calculate the value of k using the data from Experiments 3 and 7. Include the units in your answer.
(d) Based on your answer for part (a), calculate the first-order half-life for this reaction. Assume the reaction is first order. Include correct units.
(e) The assumption was made that the reaction followed first-order kinetics. Use the data for Experiments 2 and 6 from the data table to support or refute this assumption. The answer must contain an explanation of how you can determine how the data from Experiments 1 and 6 can be used.
Question 3
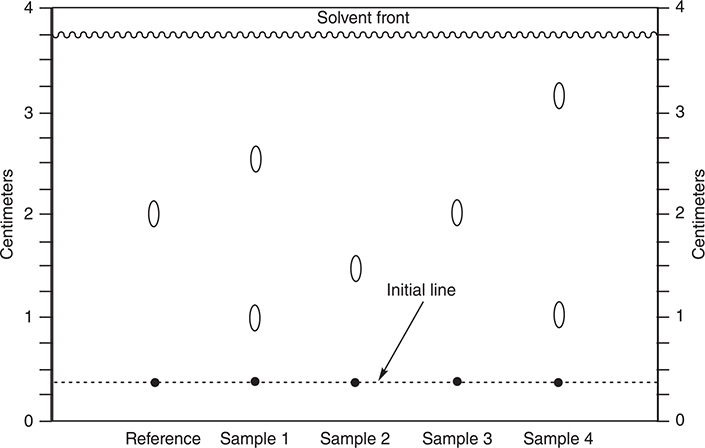
During a chemistry lab to identify the FD&C—approved dyes being used in coloring candy, a student uses paper chromatography to separate the dyes. The student obtains the chromatogram pictured above. He produced each “dot” on the initial line by moistening a piece of colored candy and rubbing it with a toothpick and repeatedly transferring some of the dye to the dot. Each of the samples was a different color of candy. The reference was FD&C—approved yellow dye #6 (the other FD&C—approved yellow dye is yellow dye #5), which causes an allergic reaction in some people. After creating all the dots, the student places the paper in a large beaker with about 0.2 cm of 0.1% aqueous NaCl. After about 20 minutes, the solvent had moved to the position indicated by the solvent front. The following table lists the colors of the candy used by this student.

(a) Determine the Rf value for each of the spots arising from Sample 4.
(b) Assume that a green color arises from a combination of a blue and a yellow dye. Is the yellow component of Sample 1 likely to be FD&C yellow dye #5 or #6? Explain why you made the choice you did.
(c) Orange is a combination of red and yellow. Sample 4 shows one way of producing orange. What is another?
(d) In paper chromatography, it is quite usual for the spots to smear while they are traveling up the paper. What could the student have done to minimize this smearing?
Question 4
A student is performing a chemistry lab to determine the formula of a hydrate. She heats a crucible and lid over a Bunsen burner until it is red-hot, allows it to cool, and weighs the crucible and lid. The lid is kept ajar during the heating and put back on the crucible when the crucible is cooling and being weighed. She then repeats the procedure and obtains the same mass (12.125 g). Next, she adds a sample of a hydrated salt to the crucible, replaces the lid, and weighs the crucible, lid, and hydrate on a balance and finds that the combination weighs 15.245 g. In the next step, she carefully heats the crucible with the lid ajar. After a few minutes, she removes the crucible from the heat, replaces the lid on the crucible, and allows the crucible and contents to cool. After the crucible has cooled to room temperature, she weighs the crucible and contents. She then repeats the heating of the hydrate procedure two more times to get a constant mass of 13.650 g for the crucible, lid, and anhydrous salt.
The anhydrous salt was magnesium sulfate, MgSO4, with a molar mass of 120.371 g mol -1. The molar mass of water, H2O, is 18.015 g mol -1.
(a) Determine the masses of the hydrate, anhydrous salt, and the water lost by the hydrate during heating. Show your work.
(b) Determine the moles of anhydrous salt and water. Show your work.
(c) Why did the student heat the empty crucible until it was red-hot, but carefully heat the crucible plus hydrate?
(d) Why did she leave the lid of the crucible ajar while heating the hydrate and in place during cooling?
Question 5
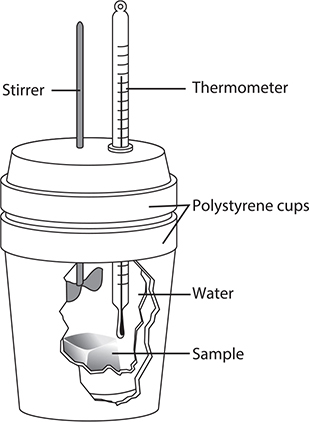
The calorimeter pictured above was used to study the enthalpy of neutralization for the reaction between sulfuric acid, H2SO4, with potassium hydroxide, KOH. A 50.0-mL sample of 0.500 M H2SO4 (at 22.3°C) was placed in a calorimeter. A 50.0-mL sample of 1.00 M KOH (at 22.3°C) was added. The temperature of the solution increased from 22.3 to 29.1°C.
(a) Assuming the reaction goes to completion, write a net ionic equation for this reaction.
(b) Determine the heat energy, q, produced in the reaction. The solutions are primarily water, so assume the density is 1.00 g mL-1 and that the specific heat, c, is 4.18 J g-1 K-1. Show your work.
(c) Determine the enthalpy change, ∆H, for the reaction in terms of kJ/mol H+. Show your work.
(d) The theoretical value for ∆H is —55.83 kJ/mol H+. Calculate the percent error in your experimental value.
Question 6
A student wishes to determine the percent of potassium chlorate, KClO3, in a sample of potassium chlorate mixed with potassium chloride, KCl. She wishes to determine the percentage through the decomposition of the KClO3 in the sample. The decomposition reaction is:
2 KClO3(s) → 2 KCl(s) + 3 O2(g)
This decomposition is catalyzed by manganese(IV) oxide, MnO2.
The experiment involves the addition of impure KClO3 to a test tube containing some MnO2. The mixture is then heated to decompose the KClO3. The test tube and contents are cooled and weighed, then reheated, cooled, and weighed again. Depending upon the results from the second heating, there may be a third or even a fourth heating, cooling, and weighing cycle.
The chemist collected the following data:
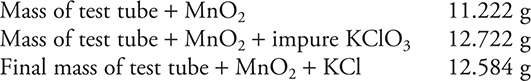
(a) What mass of O2 was produced? Show your work.
(b) What is the percentage of KClO3 in the sample? Show your work.
(c) Why is it necessary to heat the sample more than once?
(d) A separate analysis showed that the percent KClO3 was incorrect. Assuming there was adequate heating, give a reason why the percentage was incorrect. Explain.
![]() Answers and Explanations
Answers and Explanations
Long Questions
Question 1
(a) A sample of sodium hydroxide is weighed and dissolved in deionized water to give a solution of the approximate concentration desired. (Alternatively, a concentrated NaOH solution with unknown concentration could be diluted.) The process is as follows:
1. Weigh samples of dried KHP into flasks and dissolve in deionized water.
2. Add a few drops of the appropriate acid—base indicator (phenolphthalein) to each sample.
3. Rinse a buret with a little of the NaOH solution; then fill the buret with the NaOH solution.
4. Take the initial buret reading.
5. Titrate the NaOH solution into the KHP samples until the first permanent pink color appears.
6. Take the final buret reading.
7. Using the molar mass of KHP, determine the moles of KHP present. This is equal to the moles of NaOH.
8. The difference in the buret readings is the volume of NaOH solution added (convert this to liters).
9. The molarity of the NaOH solution is the moles of NaOH divided by the liters of NaOH solution added.
10. (Repeat the procedure for each sample.)
Give yourself 3 points for this full list if the items are in order. If three to five items are in the wrong order or missing, you get only 2 points. If five to seven items are in the wrong order or missing, you get only 1 point. You get 0 points for three or fewer items.
(b)
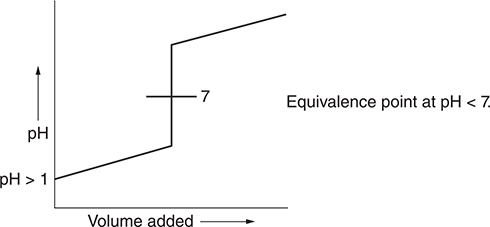
You get 1 point for this general graph. You get an additional point for noting that the equivalence point is greater than 7. The graph does not need to be perfect.
(c)
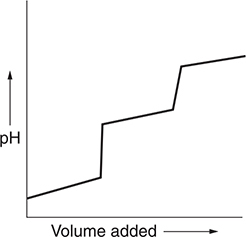
You get 2 points for this graph if you show both steps. The graph does not need to be perfect, but you need to show two steps. You get 1 point for showing only one step.
(d) There are several related ways to do this problem. One method is to split the sample into two portions. Titrate one portion to the equivalence point. Add the titrated sample to the untitrated solution and add a volume of deionized water equal to the volume of NaOH solution added. The pH of this mixture is equal to the pKa of the acid (this corresponds to a half-titrated sample).
You get 1 point for anything concerning a half-titrated sample and an additional 1 point for pH = pKa at the half-titrated point.
(e) The pH at the equivalence point must be close to the pKa of the indicator.
You get 1 point for this answer.
Total your points. There are 10 points possible.
Short Questions
Question 2
(a) Following the directions, the initial and final values necessary to calculate the slope are:
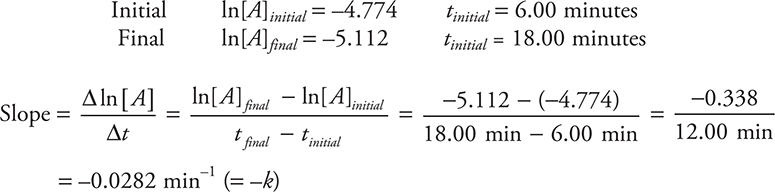
Optionally, you could convert the minutes to seconds, which will give —4.69 × 10—4 s—1.
You get 2 points for either of the correct answers (including correct units and significant figures). If only the number is correct, you get 1 point. If you wrote out the calculation and entered only the correct numbers as shown in the solution but miscalculated the answer, you still get 1 point. If you picked any values from the data table other than the ones entered in the solution shown above, you did not follow the directions, which means you get 0 points.
(b) The graph of ln[A] versus time will give a straight line if these data are truly a first-order reaction. You get 1 point for this answer. Note that many students lose points on an answer like this. This usually occurs because they do not simply answer the question shown but go on to add a large amount of additional information. Taking time to write unnecessarily long answers is taking time away from finishing the test.
(c) The integrated rate law for first-order kinetics may be written in many ways. The form given on the AP Exam is:
ln[A]t — ln[A]0 = —kt
If you use a different form of this equation correctly, you will still get the same points as if you used this form.
Using the table above:
[A]0 = 8.450 × 10—3 M (Experiment 3)
[A]t = 6.000 × 10—3 M (Experiment 7)
t = (18.00 — 6.00) minutes = 12.00 minutes
Rearranging the integrated rate law and solving for k gives:
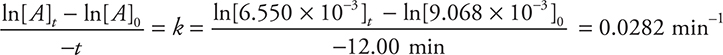
Optionally, you could convert the minutes to seconds, which will give 4.69 × 10—3 s—1.
You get 3 points for either of the correct answers (including correct units and significant figures). If only the number is correct, you get 1 point (1 more if either the correct units or significant figures are also correct). If you wrote out the calculation and entered only the correct numbers as shown in the solution but miscalculated the answer, you get 2 points. If you picked any values from the data table other than the ones entered in the solution shown above, you did not follow the directions, which means you get 0 points.
(d) You need to use the first-order half-life equation. If you do not remember this equation, it is given in the exam booklet. The equation for a first-order half-life (t1/2) is:
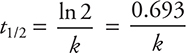
Note: you could use the integrated rate law instead with [A]0 = any value and [A]t = half the value you chose for [A]0.
Use your answer for k from part (c). If you miscalculated the answer for (c) but you use it correctly here, you may still get full credit for this part.
Entering the answer for (c) into this equation gives:
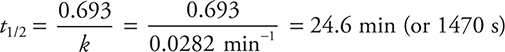
You get 2 points for either of the correct answers (including correct units and significant figures). If only the number is correct, you get 1 point. If you wrote out the calculation and entered only the correct numbers as shown in the solution but miscalculated the answer, you still get 1 point. If you picked any values from the data table other than the ones entered in the solution shown above, you did not follow the directions, which means you get 0 points.
(e) If the reaction is truly first order, the rate constant, k, should be constant (within rounding). So calculating k for any two experiments will support the assumption if the values match the answer to part (c) and refute the assumption if the value is different from that calculated in part (c).
Recalculating k using Experiments 1 and 6 gives:
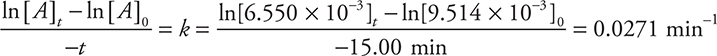
The calculated answer here does not match the answer for (c); therefore, the assumption is refuted.
You get 1 point for comments on k being a constant. You get 1 point for correctly calculating k again and stating your conclusion that the assumption is refuted. You do not get the second point if you incorrectly used data from the table above to calculate the new k.
Question 3
(a) To determine the Rf value it is necessary to determine how far a sample traveled relative to how far the solvent traveled. The distance the solvent traveled is the distance between the initial line and the solvent front.

Rf values
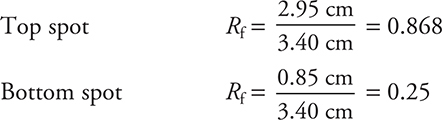
You get 1 point for correctly determining the two Rf values. In this solution, the tops of the spots were used; however, you can still get 1 point if you consistently used the bottoms or some other position of the spot. Due to variations in reading the scale, you do not need to get the exact same numbers. We cannot count the times we have not been able to award this point because the student writes what appears to be random numbers on the test. Make sure you identify things and show your work.
(b) If green is blue plus yellow, then one of the two dots for Sample 1 must be blue and the other must be yellow. Neither of these dots matches the reference (yellow #6); therefore, this dye cannot be present. The only other choice is yellow #5, which must be one of the dots.
You get 1 point for this answer.
(c) Another way to produce orange would be to use the dyes represented by Samples 2 and 3.
You get 1 point for this answer.
(d) The smaller the dots on the initial line are, the less smearing there will be.
You get 1 point for this answer.
Total your points. There is a maximum of 4 points possible. Subtract 1 point if any calculated answer does not have the correct number of significant figures.
Question 4
(a) 
(b) 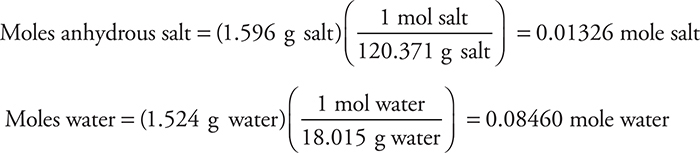
You earn 1 point total for both answers if you show your work. Both answers are necessary to receive this point. If you correctly used a wrong answer from part (a), you still get 1 point.
(c) It was necessary to make sure that all impurities were driven off the empty crucible; therefore, the higher the temperature the better. Heating the sample too much might lead to decomposition of the anhydrous salt.
You earn 1 point for this answer. You must explain the heating of each crucible.
(d) The lid was left ajar to allow volatile material (water vapor) to escape while heating. The lid was kept in place while cooling to minimize water vapor from air to be reabsorbed by the sample.
You earn 1 point for this answer.
Total your points. There is a maximum of 4 points possible. Subtract 1 point if any calculated answer does not have the correct number of significant figures.
Question 5
(a) The molecular equation is:
H2SO4(aq) + 2 KOH(aq) → K2SO4(aq) + 2 H2O(l)
The total ionic equation is:
2 H+(aq) + SO42-(aq) + 2 K+(aq) + 2 OH-(aq) → 2 K+(aq) + SO42-(aq) + 2 H2O(l)
The net ionic equation for this reaction is:
H+(aq) + OH-(aq) → H2O(l)
You get 1 point for this final answer. If you forgot to reduce the coefficients or you forgot ions have charges, you get 0 points. (The molecular and total ionic equations are only shown here to help with the explanation; they are not part of the answer required by you.)
(b) The equation you need (q = mcΔT) is given on the AP Exam and in the back of this book. The variables are:
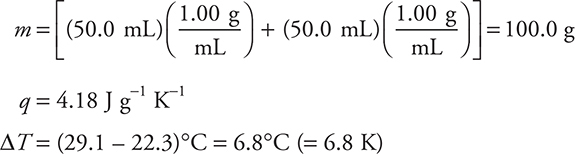
Entering these values into the equation gives:
q = (100.0 g) (4.18 J g-1 K-1) (6.8 K) = —2.8 × 103 J (or —2.8 kJ)
This is an exothermic process; therefore, the enthalpy change must be negative.
You get 1 point for this answer if you showed your work and remembered to change the sign.
(c) It is necessary to determine the moles of hydrogen ions in the reaction.
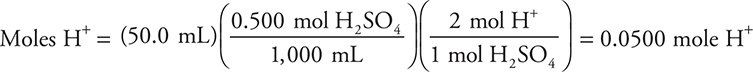
The moles of H+ plus your answer to part (b) gives:
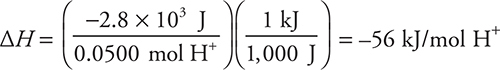
You get 1 point for this answer if you showed your work. If you correctly used an incorrect value from part (b), you still get 1 point.
(d) The theoretical value for ∆H is —55.83 kJ/mol H+. Calculate the percent error in your experimental value. Show your work.
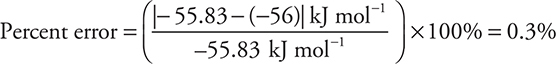
You get 1 point for this answer if you showed your work. If you correctly used an incorrect value from part (c), you still get 1 point.
Total your points. There is a maximum of 4 points. Deduct 1 point if any answer did not have the correct number of significant figures.
Question 6
(a) The mass of the oxygen produced is determined as:
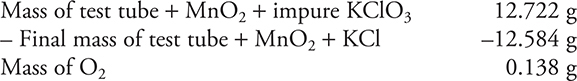
You get 1 point for this answer. If you did not show your work, you get 0 points.
(b) What is the percentage of KClO3 in the sample?
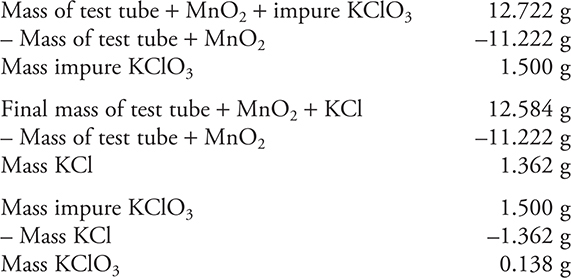
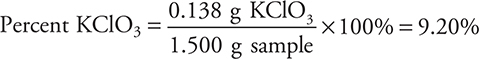
You get 1 point for this answer.
(c) The decomposition may not be complete after only one heating.
You get 1 point for this answer.
(d) One reason to get an erroneous percentage would be if the test tube and contents had not cooled completely to room temperature before weighing was done. Under these circumstances the mass would be incorrect.
You get 1 point for this answer with the explanation. If you said “experimental error,” you earn 0 points.
Total your points. There is a maximum of 4 points. Deduct 1 point if any answer did not have the correct number of significant figures.
![]() Rapid Review
Rapid Review
A review of the experiments should include looking at the synopsis, apparatus, calculations, and comments, as well as the appropriate concept chapters, if needed.
• Pay extra attention to any experiment you did not perform.
• Be familiar with the equipment used in each experiment (in many cases beakers may substitute for flasks).
• Know the basic measurements required in each experiment.
• Know what values are measured and which are calculated.
• Pay attention to significant figures.
• Balances are used to measure the mass of a substance, not the moles.