5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
Appendixes
Exam Resources
Keywords and Equations: For Use with Free-Response Questions Only
Basics
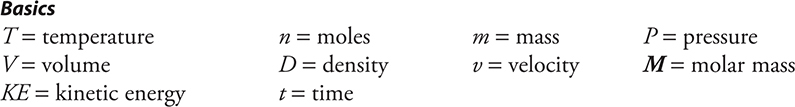
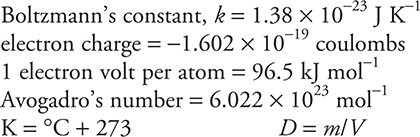
Gases
STP = 0.000° C and 1.000 atm
PV = nRT

![]()
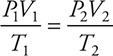
KE per molecule = ½ mv2
![]()
![]()

Thermodynamics

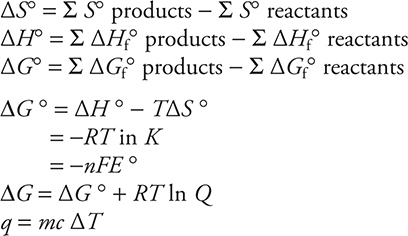
Light and Electrons
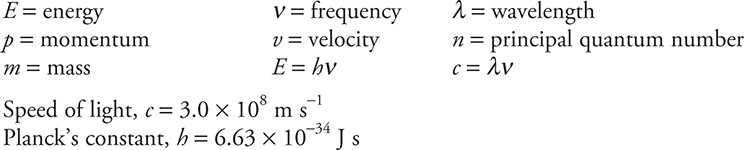
Solutions
Molarity, M = moles solute per liter solution
Kinetics
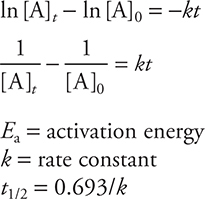
Electrochemistry

Faraday’s constant, F = 96,500 coulombs per mole of electrons
I = q/t
Equilibrium
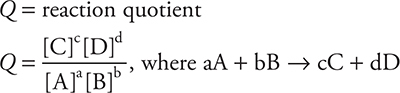
equilibrium constants:
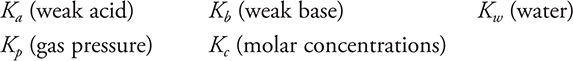
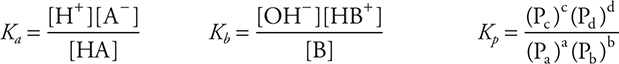
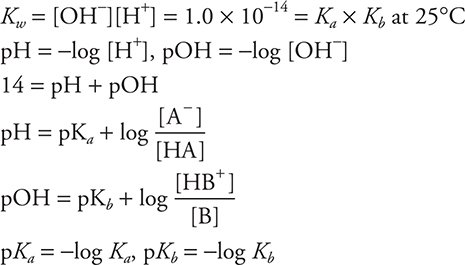
Experimental
Beer’s law: A = abc (A = absorbance; a = molar absorbtivity; b = path length;
c = concentration)
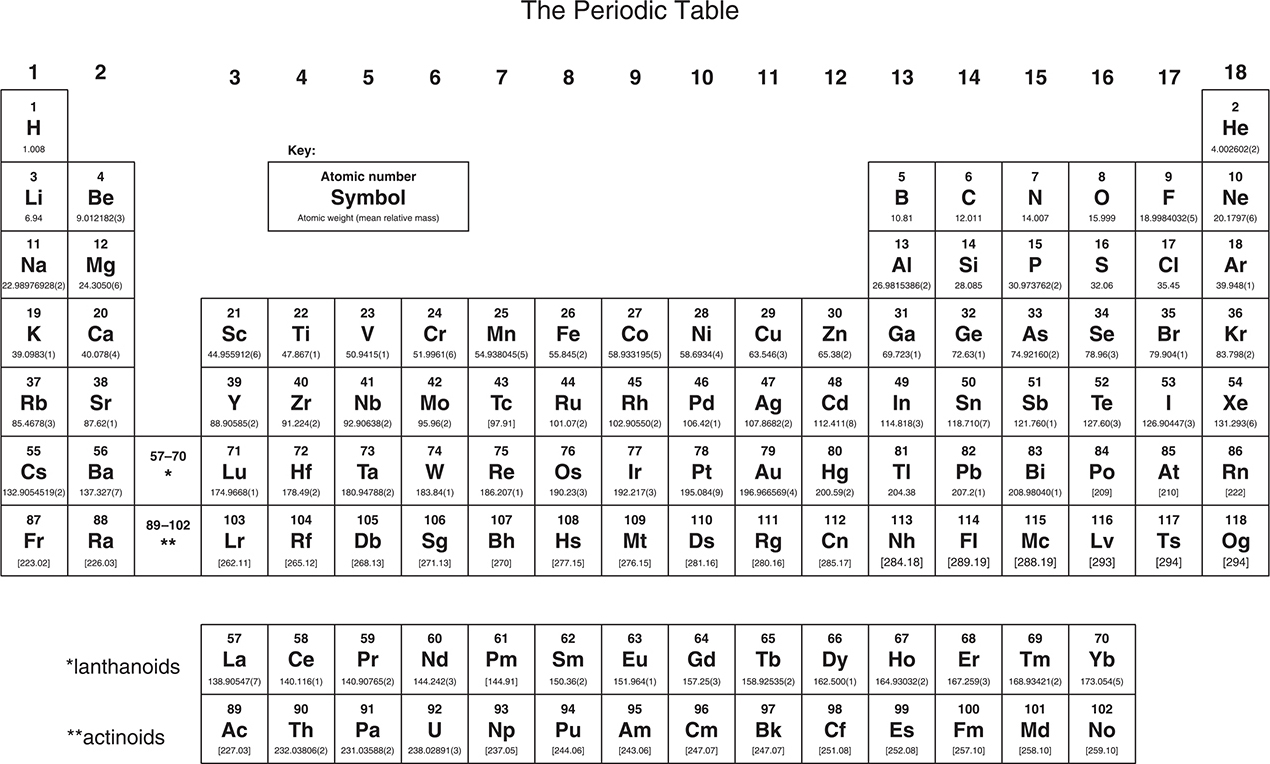
Periodic Table of the Elements
May be used with all questions.