5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
Appendixes
Notes
5 Steps to Teaching AP Chemistry

John T. Moore, EdD
Richard H. Langley, PhD
Thanks to Greg Jacobs, an AP physics teacher at Woodberry Forest School in Virginia, for developing the 5-step approach used in this teaching guide.
Introduction to the Teacher’s Manual
Nowadays, teachers have no shortage of resources for the AP Chemistry class. No longer limited to just the textbook, today’s teachers can utilize online simulations, apps, computer-based homework, video lectures, and so on. Even the College Board itself provides so much material related to the AP Chemistry Exam that the typical teacher—and student—can easily become overwhelmed by the excess of teaching materials and resources.
This book is a vital resource for your class because it explains in straight forward language exactly what a student needs to know for the AP Chemistry Exam and provides a review program that students can use to review for the test.
This teacher’s manual will take you through the five steps of teaching AP Chemistry. These 5 steps are:
1. Prepare a strategic plan for the course
2. Hold an interesting class every day
3. Evaluate your students’ progress
4. Get students ready to take the AP exam
5. Become a better teacher every year
We will discuss each of these steps, providing suggestions and ideas that we use in our classes. We present them here because, over the years, we have found that they work. You may have developed a different course strategy, teaching activities, and evaluation techniques. That is fine; different things work for different teachers. But we hope you find in this teacher’s manual something that will be useful to you.
STEP 1
Prepare a Strategic Plan for the Course
The Course and Exam Description (CED) from the College Board, which can be found at: https://apcentral.collegeboard.org/courses/ap-chemistry/course, provides a suggested scope and sequence for the AP Chemistry class. If you are a new teacher (or new to AP Chemistry), this scope and sequence will prove to be invaluable. Later on, after you have taught the course a few times and feel comfortable with the content, you may want to modify the suggested scope and sequence to better fit your style of teaching and your students.
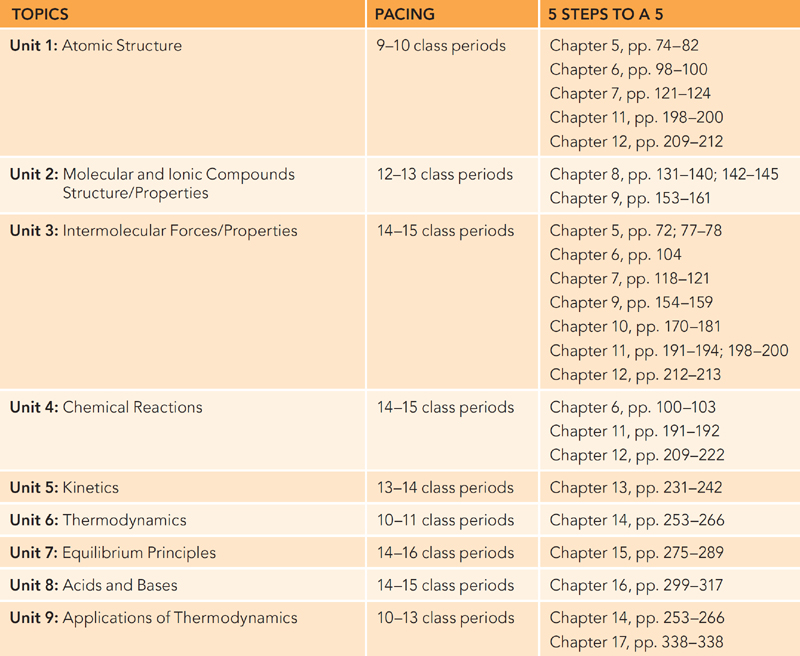
When looking at our 5 Steps to a 5 AP Chemistry, please note that the individual AP unit topics are not located in a single book chapter. This is because the book was designed for individual review, and we feel that our choice for grouping individual topics is better for individual review. As a teacher, please feel free to skip around to best suit your individual class. Also, we provide a diagnostic exam that is useful to determine the student’s readiness for your AP course.
The chart below shows the units and pacing suggested for each unit. The number of class periods is based on a typical 45-minute class. However, if your school is on some form of block scheduling or some other type of nontraditional schedule, feel free to adjust the pacing to fit the needs of your students.
STEP 2
Hold an Interesting Class Every Day
While direct instruction (sage on the stage) can be the most efficient way to deliver content, long daily lectures with copious note taking is not very effective with this age group. Make the most of lectures by trying some of the following ideas:
![]() Include demonstrations to excite and engage students while making content concrete. With a little planning and practice, this can be an easy way to encourage students to look forward to class and to visualize ideas. Demonstrations that are also discrepant events serve as great anchors for introducing new units of study.
Include demonstrations to excite and engage students while making content concrete. With a little planning and practice, this can be an easy way to encourage students to look forward to class and to visualize ideas. Demonstrations that are also discrepant events serve as great anchors for introducing new units of study.
![]() Include student input in every lecture. Ask probing questions along the way to help students open meaningful discussions about content. When possible, answer questions with more leading questions to allow the students to discover trends, processes, and answers themselves. Allow some lectures to be student-led discussions based on homework problems or reading assignments.
Include student input in every lecture. Ask probing questions along the way to help students open meaningful discussions about content. When possible, answer questions with more leading questions to allow the students to discover trends, processes, and answers themselves. Allow some lectures to be student-led discussions based on homework problems or reading assignments.
![]() Infuse mini breakout labs within a lecture. Get students out of their seats and moving when possible.
Infuse mini breakout labs within a lecture. Get students out of their seats and moving when possible.
![]() Make interesting presentations if you use them. Chemistry is beautiful! Include well-crafted diagrams, short animations, other video clips, and pictures when possible.
Make interesting presentations if you use them. Chemistry is beautiful! Include well-crafted diagrams, short animations, other video clips, and pictures when possible.
In addition to weekly laboratory activities, use a variety of instructional modes each week:
![]() Take advantage of technology to utilize things like Kahoot, Quizizz, Quizlet, and Sporcle for reviews and quick formative assessments.
Take advantage of technology to utilize things like Kahoot, Quizizz, Quizlet, and Sporcle for reviews and quick formative assessments.
![]() Establish traditions in class by doing one or two short activities on a regular, weekly basis. Activities like “The Monday Molecule Minute” or “The Burning Question of the Week” give students something else interesting to anticipate.
Establish traditions in class by doing one or two short activities on a regular, weekly basis. Activities like “The Monday Molecule Minute” or “The Burning Question of the Week” give students something else interesting to anticipate.
![]() Utilize cooperative working groups for skills practice, especially for new content that involves mathematics.
Utilize cooperative working groups for skills practice, especially for new content that involves mathematics.
![]() Provide opportunities for students to draw models to represent processes throughout the course. As students grow used to drawing dynamic models that can be manipulated to show chemical events as a function of time, their ability to reason using models will develop into a tool they can utilize to understand and visualize things they cannot see.
Provide opportunities for students to draw models to represent processes throughout the course. As students grow used to drawing dynamic models that can be manipulated to show chemical events as a function of time, their ability to reason using models will develop into a tool they can utilize to understand and visualize things they cannot see.
![]() Dynamic model drawing can also be used to check for understanding when minutes count.
Dynamic model drawing can also be used to check for understanding when minutes count.
Homework is an essential part of success in this course:
![]() A set of homework problems is assigned at the start of each unit of study along with due dates.
A set of homework problems is assigned at the start of each unit of study along with due dates.
![]() Students must turn in each assignment on time to receive full credit.
Students must turn in each assignment on time to receive full credit.
![]() Randomly selected problems/questions from the original set are graded, for example, at 20 points each. This saves time on grading while holding students accountable for needed practice.
Randomly selected problems/questions from the original set are graded, for example, at 20 points each. This saves time on grading while holding students accountable for needed practice.
![]() Ungraded problems can often be utilized for classroom discussions.
Ungraded problems can often be utilized for classroom discussions.
A wealth of classroom activities and ideas may be found on the AP Central website for chemistry: https://apcentral.collegeboard.org/courses/ap-chemistry/classroom-resources
There you will find:
![]() A list of daily videos for AP Chemistry
A list of daily videos for AP Chemistry
![]() Topic questions to help determine student understanding
Topic questions to help determine student understanding
![]() Progress checks to help evaluate student knowledge and skills
Progress checks to help evaluate student knowledge and skills
![]() A question bank of searchable database of real AP Chemistry questions
A question bank of searchable database of real AP Chemistry questions
![]() An AP Chemistry lab manual resource
An AP Chemistry lab manual resource
![]() Guided inquiry classroom activities
Guided inquiry classroom activities
![]() A webcast describing photoelectron spectroscopy (PES)
A webcast describing photoelectron spectroscopy (PES)
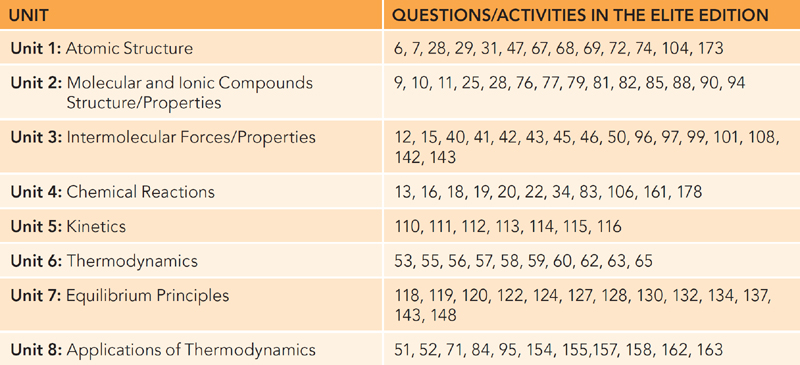
The 5 Steps to a 5 AP Chemistry: Elite Edition provides additional questions that can be used in your class. It contains 180 activities and questions that require five minutes a day. While they are primarily intended to be used by students studying for the test, you can use these as daily warm-ups in your course. To do this you will need the table below that organizes these questions and activities by unit since they do not follow the course in chronological order.
STEP 3
Evaluate Your Students’ Progress
The best way to prepare your students for the AP Chemistry Exam is to use AP-style questions for your own quizzes and exams. You can incorporate released AP questions from the College Board (found on AP Classroom) in your own exams. In addition, AP-style questions can be found in this book or you can use questions from your textbook or ones you make up on your own. However, there is nothing more authentic than released questions for quizzes and exams.
We highly suggest you incorporate a free-response question (FRQ) on each of your unit exams. Typically, these questions are more difficult for the students. These short-answer questions in the exam format are a great way to familiarize your students with the FRQs that they will find on the AP Chemistry Exam. The FRQs count for 40 percent of the students’ grades on the AP Exam, so practicing these on every unit exam is critical to their success.
STEP 4
Get Students Ready to Take the AP Exam
Hopefully, you will be able to have two or even three weeks to review with your students right before they take the AP Chemistry Exam. This is the time to review the more difficult concepts; there isn’t time to start from scratch.
Now is the time to help your students find the weak points in their knowledge in hopes that there is time to bolster their knowledge. This can be best done by utilizing the two Practice Exams found in the Build Your Test-Taking Confidence section of this book. Break each exam up into two or three class periods. They should count toward the students’ grades in the class; if they are not graded, the students will not take them seriously.
Tips for the Multiple-Choice Questions
Students have undoubtedly had many years of experience with multiple-choice exams. If on your unit exams you have used multiple-choice questions from previous AP exams, they will be familiar with the exact style of questions found on the AP exam. But there are several strategies that are important if students want to get their best score possible. Here are the most important ones that need to be reviewed to make sure all students understand them:
![]() Eliminate incorrect answers and guess. When students don’t know the answer, they should eliminate the answers they know are wrong and guess among the remaining choices.
Eliminate incorrect answers and guess. When students don’t know the answer, they should eliminate the answers they know are wrong and guess among the remaining choices.
![]() Mark an answer for every question. Tell students not to leave any questions blank; there may not be time to come back. Answering every question also eliminates the possibility that they will get confused and end up putting answers on the wrong line. They want to come back to the question at the end if they have time.
Mark an answer for every question. Tell students not to leave any questions blank; there may not be time to come back. Answering every question also eliminates the possibility that they will get confused and end up putting answers on the wrong line. They want to come back to the question at the end if they have time.
![]() Pace yourself. The student’s goal should be to answer every question correctly for which they know the answer. The questions on the AP exam are arranged in random order. So, the easiest question could be the last question on the test. Students need to pace themselves so that they have enough time to get to all the questions. They should not spend a lot of time thinking about difficult questions for which they don’t know the answer. Make their best guess and move on.
Pace yourself. The student’s goal should be to answer every question correctly for which they know the answer. The questions on the AP exam are arranged in random order. So, the easiest question could be the last question on the test. Students need to pace themselves so that they have enough time to get to all the questions. They should not spend a lot of time thinking about difficult questions for which they don’t know the answer. Make their best guess and move on.
![]() There is no penalty for wrong answers. Remind students of this. If they are running out of time, they should make sure to answer every question even if they have to guess at random.
There is no penalty for wrong answers. Remind students of this. If they are running out of time, they should make sure to answer every question even if they have to guess at random.
![]() Don’t make stray marks on the answer sheet. Make sure erasures are complete and that there are no marks on the page that could cause a machine to read it wrong.
Don’t make stray marks on the answer sheet. Make sure erasures are complete and that there are no marks on the page that could cause a machine to read it wrong.
Tips for the Free-Response Questions
Students will also be familiar with the type of FRQ found on the exam if you have included at least one previously released FRQ on each unit exam. But many students do not get all the points simply because of careless mistakes. We have been grading the free-response part of the AP Chemistry Exam for quite a while. Between the two of us, we have almost 35 years of grading experience—that’s more than 150,000 exams! Over the years, we have seen quite a number of careless mistakes made by students. These mistakes resulted from not being careful rather than not being prepared for the exam. Here are some practical tips to avoid the most common careless errors. (This section may also be found in the Appendix of this book, but we felt is worth repeating here.) You can incorporate a tip or so in each class period so that it becomes second nature to the students and not just something to memorize. Then during your review days, you can take a few of these tips and discuss them.
![]() Don’t forget to state the units of measurement. Many students would have gotten more credit if they had shown the units, both in the calculations and in the final answer. The units help you stay on the right track and help the grader determine if (or where) you went wrong.
Don’t forget to state the units of measurement. Many students would have gotten more credit if they had shown the units, both in the calculations and in the final answer. The units help you stay on the right track and help the grader determine if (or where) you went wrong.
![]() Use the formula given. If the exam gives you a chemical formula, don’t use a different formula in your answer. In general, do not alter anything given to you on the exam. For example, we have seen Ba(NO3)2 become Ba(NO2)2.
Use the formula given. If the exam gives you a chemical formula, don’t use a different formula in your answer. In general, do not alter anything given to you on the exam. For example, we have seen Ba(NO3)2 become Ba(NO2)2.
![]() Be careful with the math. We have seen many errors involving the simplest math such as 12 mL + 3 mL = 0.042 L (rather than 0.015 L).
Be careful with the math. We have seen many errors involving the simplest math such as 12 mL + 3 mL = 0.042 L (rather than 0.015 L).
![]() Don’t confuse molarity and moles. The units M and [ ] are identical (molarity) and are completely different from moles.
Don’t confuse molarity and moles. The units M and [ ] are identical (molarity) and are completely different from moles.
![]() Show your work for conversions. For example, if you are changing grams to moles and make a simple mistake, showing your work (labeled) may get you partial credit.
Show your work for conversions. For example, if you are changing grams to moles and make a simple mistake, showing your work (labeled) may get you partial credit.
![]() Don’t argue with the test. This is an argument you cannot win. For example, if the question asks for calculations, you are unlikely to get full credit without any calculations even if you have the right answer. It won’t help to write that you feel the calculations are unnecessary.
Don’t argue with the test. This is an argument you cannot win. For example, if the question asks for calculations, you are unlikely to get full credit without any calculations even if you have the right answer. It won’t help to write that you feel the calculations are unnecessary.
![]() Be careful in applying gas laws. Gas laws can be very useful. However, they should never be used when there is not a gas in the problem. Having a volume included in the question information doesn’t necessarily mean you are dealing with a gas.
Be careful in applying gas laws. Gas laws can be very useful. However, they should never be used when there is not a gas in the problem. Having a volume included in the question information doesn’t necessarily mean you are dealing with a gas.
![]() Be careful making comparisons. We have seen many students incorrectly say that 10—8 is smaller than 10—12 and actually write 10—8 < 10—12. We have even seen students write the relationship correctly (10—8 > 10—12) but still state that 10—8 is smaller.
Be careful making comparisons. We have seen many students incorrectly say that 10—8 is smaller than 10—12 and actually write 10—8 < 10—12. We have even seen students write the relationship correctly (10—8 > 10—12) but still state that 10—8 is smaller.
![]() Be careful using 22.4 L/mol. You will probably not need to use this on the exam. But if you do want to use this value, you must have a gas and this gas must be at 0°C (273 K) and 1 atm (STP). If you forget the values for STP, they can be found on the exam. We have seen quite a few students incorrectly use this value at 298 K.
Be careful using 22.4 L/mol. You will probably not need to use this on the exam. But if you do want to use this value, you must have a gas and this gas must be at 0°C (273 K) and 1 atm (STP). If you forget the values for STP, they can be found on the exam. We have seen quite a few students incorrectly use this value at 298 K.
![]() There are no trick questions on the exam. If you think you have found a trick question, you need to reevaluate your thinking and reread the question.
There are no trick questions on the exam. If you think you have found a trick question, you need to reevaluate your thinking and reread the question.
![]() Don’t confuse solutions and precipitates in solution. They are different phases and are not interchangeable. The color of one is not necessarily the color of the other.
Don’t confuse solutions and precipitates in solution. They are different phases and are not interchangeable. The color of one is not necessarily the color of the other.
![]() Be careful describing reactions. If the problem gives you, for example, a sodium nitrate solution, part of your answer describing a reaction cannot be “the sodium nitrate dissolves.” You already have a solution, so the process of dissolving happened before you got to the problem. Furthermore, dissolving should not be treated as a reaction.
Be careful describing reactions. If the problem gives you, for example, a sodium nitrate solution, part of your answer describing a reaction cannot be “the sodium nitrate dissolves.” You already have a solution, so the process of dissolving happened before you got to the problem. Furthermore, dissolving should not be treated as a reaction.
![]() Be careful using positive and negative charges. In the following equation, each reactant and product are wrong: NH4 + NO3 → NH4+NO3—, and will not substitute for the correct NH4+ + NO3— → NH4NO3. Remember, ionic equations, of any type, have ions (with charges) on one or both sides of the reaction arrow.
Be careful using positive and negative charges. In the following equation, each reactant and product are wrong: NH4 + NO3 → NH4+NO3—, and will not substitute for the correct NH4+ + NO3— → NH4NO3. Remember, ionic equations, of any type, have ions (with charges) on one or both sides of the reaction arrow.
![]() Don’t do a calculator dump (write down every number displayed by your calculator). For example, your final answer will not be 3.27584827 g.
Don’t do a calculator dump (write down every number displayed by your calculator). For example, your final answer will not be 3.27584827 g.
![]() Keep in mind the meaning of “observe.” If the problem asks about observation, tell what you would actually observe (see, hear, or smell). You will not see a compound separating into ions; usually you will not see the excess reagent, and you will not see the atoms forming bonds. In contrast, you might observe a compound dissolving.
Keep in mind the meaning of “observe.” If the problem asks about observation, tell what you would actually observe (see, hear, or smell). You will not see a compound separating into ions; usually you will not see the excess reagent, and you will not see the atoms forming bonds. In contrast, you might observe a compound dissolving.
![]() Remember, a solvent is usually not a reactant. Therefore, changing the grams of solvent to moles is probably wrong. (However, you will need to know the moles of solvent if you are looking for a mole fraction.)
Remember, a solvent is usually not a reactant. Therefore, changing the grams of solvent to moles is probably wrong. (However, you will need to know the moles of solvent if you are looking for a mole fraction.)
![]() Think before creating mole ratios. Since the solvent is not a reactant, a mole ratio relating the solvent to anything else in the problem is most likely wrong. We have seen many students change the grams of water to moles and then use these moles in a mole ratio to relate to some other substance in the problem.
Think before creating mole ratios. Since the solvent is not a reactant, a mole ratio relating the solvent to anything else in the problem is most likely wrong. We have seen many students change the grams of water to moles and then use these moles in a mole ratio to relate to some other substance in the problem.
![]() Don’t go off on a tangent. Stay focused on answering the original question.
Don’t go off on a tangent. Stay focused on answering the original question.
![]() Double-check the numbers you use. We have seen many cases where the problem gave a number like 2.75 × 10—18, and the student worked the problem with 2.75 × 10—8. If you show your work, it will be obvious to the grader that you miscopied the value and you might pick up some points; otherwise, you just have a wrong answer.
Double-check the numbers you use. We have seen many cases where the problem gave a number like 2.75 × 10—18, and the student worked the problem with 2.75 × 10—8. If you show your work, it will be obvious to the grader that you miscopied the value and you might pick up some points; otherwise, you just have a wrong answer.
![]() Remember that sometimes not all of the information given is needed to solve the problem. For example, in the equilibrium problem, many times the temperature is given but it is not actually part of the calculations.
Remember that sometimes not all of the information given is needed to solve the problem. For example, in the equilibrium problem, many times the temperature is given but it is not actually part of the calculations.
![]() Only round your final answer. Don’t round off the results of intermediate calculations; only use rounding after you’ve gotten your final answer.
Only round your final answer. Don’t round off the results of intermediate calculations; only use rounding after you’ve gotten your final answer.
![]() Be careful with math. Especially take care in reading the scales on graphs. We have seen students write down that 0.5 is between 1.0 and 2.0.
Be careful with math. Especially take care in reading the scales on graphs. We have seen students write down that 0.5 is between 1.0 and 2.0.
![]() Don’t confuse intermolecular and intramolecular forces. These are two different concepts and are not interchangeable.
Don’t confuse intermolecular and intramolecular forces. These are two different concepts and are not interchangeable.
In addition to avoiding the careless mistakes mentioned above, here are some easy ways to help improve your score on the free-response questions:
![]() Show your work. In most cases, no work, no credit.
Show your work. In most cases, no work, no credit.
![]() Use the space provided for answers. It helps you and the grader if you answer the question in the space provided instead of crowding the answers between the questions. You will have more than enough room on the following page(s). It also helps to label the parts (a, b, etc.) and to answer the parts in order.
Use the space provided for answers. It helps you and the grader if you answer the question in the space provided instead of crowding the answers between the questions. You will have more than enough room on the following page(s). It also helps to label the parts (a, b, etc.) and to answer the parts in order.
![]() Make sure your answer can be easily read. It will really help the grader—and your score—if you write legibly, in a normal size (not too small, please), and use a pencil or pen that writes dark enough to be easily read.
Make sure your answer can be easily read. It will really help the grader—and your score—if you write legibly, in a normal size (not too small, please), and use a pencil or pen that writes dark enough to be easily read.
![]() Don’t use periodic trends and general rules as explanations. General rules such as “like dissolves like” are never explanations. They may help you in answering the multiple-choice part of the exam but will be of little benefit by themselves in the free-response section.
Don’t use periodic trends and general rules as explanations. General rules such as “like dissolves like” are never explanations. They may help you in answering the multiple-choice part of the exam but will be of little benefit by themselves in the free-response section.
![]() Don’t confuse “define” and “describe.” They are two different processes. If you are asked to describe or explain, simply giving a definition will earn you very few points.
Don’t confuse “define” and “describe.” They are two different processes. If you are asked to describe or explain, simply giving a definition will earn you very few points.
![]() Use only standard abbreviations. Your instructor may understand your abbreviations, but the grader may not. If you want to use abbreviations in a response, be sure to define them.
Use only standard abbreviations. Your instructor may understand your abbreviations, but the grader may not. If you want to use abbreviations in a response, be sure to define them.
![]() Don’t ramble. Normally an explanation or justification can be done in five sentences or less. Your answers should be clear, concise, and to the point.
Don’t ramble. Normally an explanation or justification can be done in five sentences or less. Your answers should be clear, concise, and to the point.
![]() The grader cannot see your calculator display or a graph/table that you do not include in your answer. Show all your work that you want graded; sometimes showing this will help you get a point that you might otherwise have lost.
The grader cannot see your calculator display or a graph/table that you do not include in your answer. Show all your work that you want graded; sometimes showing this will help you get a point that you might otherwise have lost.
![]() In general, the graders are not “mind readers.” Show your work and your reasoning. Don’t assume graders will know what you meant. They grade only what you have written down.
In general, the graders are not “mind readers.” Show your work and your reasoning. Don’t assume graders will know what you meant. They grade only what you have written down.
![]() Answer the question asked, not the question you wanted. You might have a great response to a question, but if that doesn’t answer the exact question asked, it is wrong!
Answer the question asked, not the question you wanted. You might have a great response to a question, but if that doesn’t answer the exact question asked, it is wrong!
![]() It is usually simpler to use the units given in the problem. It is less work on your part and less work on the grader.
It is usually simpler to use the units given in the problem. It is less work on your part and less work on the grader.
![]() Using extra calculation steps provides additional opportunities to make errors. Show all your pertinent calculation steps, but extra steps are spots for potential errors. This is especially true when you are tired.
Using extra calculation steps provides additional opportunities to make errors. Show all your pertinent calculation steps, but extra steps are spots for potential errors. This is especially true when you are tired.
STEP 5
Become a Better Teacher Every Year
A good AP teacher strives to improve every year. If there is anything that didn’t work as well as you had hoped this year, there’s always next year to try something different. Isn’t that great: a do-over every year!
How do you judge success? There is no right or wrong answer to this. We all teach at different schools with different students. You will have a few students with strong science and math skills. They could probably pass the exam without you. Most students may lack some of the skills or knowledge that is needed to do well on the AP exam. These students are the ones who need us most and when they succeed, it is because of their effort and your support.
If you are a new AP Chemistry teacher, keep the AP Central pages that are specific to chemistry bookmarked on both your school and personal computers. Take time to explore the resources available there. If there are experienced AP Chemistry teachers at your school or schools close by, reach out to them and pick their brains.
It is also very importance to attend an APSI (AP Summer Institute). This will keep you up-to-date on any changes to the AP Chemistry program. These summer institutes are a great place to meet other teachers and hear about how they teach, both as you begin to teach the course and also every few years as you continue. To get additional ideas and insights, we recommend that you take summer institutes from different instructors. There are also online workshops, free webinars and online sessions throughout the year. Links to these learning opportunities may be found here: https://apcentral.collegeboard.org/courses/ap-chemistry/professional-learning?course=ap-chemistry
For all AP teachers, both new and experienced, the best thing you can do to improve is to use the Instructional Planning Report you will receive after student scores are calculated. You can access this document in your AP Classroom. You will get a breakdown of scores by unit, by question type (multiple choice and FRQ), and so on. This information is what you need to adjust your course for the next school year. If you notice that students as a whole struggled with a particular unit, this is where you make changes. Maybe you will need to spend a little extra time on this unit or maybe you find new activities to use during class. Maybe you will need to be sure to review this unit in class before the next year’s AP exam. What if students did really well on the multiple choice but not the FRQs? How can you get some additional professional learning to better teach them how to write? It may take a few years to see results, but with attention to the Instructional Planning Reports and with continuous adjustments to your class, your student scores will increase.
We also encourage all AP teachers to apply to be a reader for the AP Exam after they have a few years of teaching the AP class under their belts. The AP reading is the best professional learning experience you can receive, and you will meet a group of like-minded teachers who will become your friends throughout the years to come. Who knows, you might find the authors of this book there! You can apply online through the College Board’s website:
https://apcentral.collegeboard.org/professional-learning/become-an-ap-reader
Additional Resources for Teachers
Teaching chemistry is just as much about how to teach as what to teach. The following resources can provide valuable content and pedagogy:
The American Association of Chemistry Teachers (AACT, teachchemistry.org) is an organization that is geared toward high school chemistry teachers. It publishes classroom resources, professional development, news, and so on. You get a lot of useful information for your dues.
The American Chemical Society (ACS, acs.org) is the premier organization devoted to chemistry. It has a division that is devoted to chemical education (divched.org). The ACS publishes ChemMatters magazine four times a year. It is devoted to clarifying chemistry for high school students and helping them see the connections between chemistry and everyday life. If you join AACT, you receive a complimentary subscription to ChemMatters and have access to the ChemMatters archive.
Another professional organization that deserves consideration is the National Science Teaching Association (NSTA, nsta.org). NSTA has a great deal of resources available for their members, including lesson plans, books, and so on. This organization not only covers chemistry but all the other sciences. This is invaluable for chemistry teachers who are also teaching physics, biology, and the like.
There is an online Facebook account for AP Chemistry Teachers, https://www.facebook.com/groups/866651346744793/about/. You must be an AP Chemistry teacher to join, but this would be an excellent way to remain in contact with other AP Chemistry teachers and to keep in touch with what topics are trending.
The CollegeBoard has an online resource called AP Teacher Community. It is an online presence that allows AP teachers and coordinators to connect with each other. There are discussion boards and a Resource Library to share class-ready resources. The CollegeBoard also maintains a page dedicated to online resources recommended by AP teachers at https://apcentral.collegeboard.org/courses/ap-chemistry/classroom-resources/teacher-recommended-resources.
Flinn Scientific (flinnsci.com) is a wonderful source for chemical supplies and chemicals. They have a real devotion for educating teachers in how to conduct safe science at the public school level. Their hard-copy catalog contains a wealth of information on chemical disposal and the safe handling of chemicals. It makes great bedtime reading!
In response to COVID-19 and the need to provide virtual instruction, many companies developed classroom instructional materials. These materials are also valuable in the face-to-face classroom. A simple Google search will generate dozens of useful hits. It is easy to get overwhelmed with the number of hits, so we suggest you specify the topic to narrow it down (i.e., teaching kinetics, teaching atomic structure). You can access a list and brief description of quite a few online resources here:
https://apcentral.collegeboard.org/courses/ap-chemistry/classroom-resources/teacher-recommended-resources?course=ap-chemistry
We hope that these suggestions and tips will help you in teaching AP Chemistry and that your student scores will benefit. One final word of advice: Have fun teaching AP Chemistry! To us nerds, the content is interesting and even exciting. The students are always an interesting group. Do your best to guide them through the material. It’s really rewarding to see them conquer the concepts.