5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 4 Review the Knowledge You Need to Score High
7 Spectroscopy, Light, and Electrons
IN THIS CHAPTER
In this chapter, the following AP topics are covered:
1.5 Atomic Structure and Electron Configuration
1.6 Photoelectron Spectroscopy
3.11 Spectroscopy and the Electromagnetic Spectrum
Summary: In the development of the model of the atom, it was thought initially that all subatomic particles obeyed the laws of classical physics—that is, they were tiny bits of matter behaving like macroscopic pieces of matter. Later, however, it was discovered that this particle view of the atom could not explain many of the observations being made. About this time, the dual particle/wave model of matter began to gain favor. It was discovered that in many cases, especially when dealing with the behavior of electrons, describing some of their behavior in terms of waves explained the observations much better. Thus, the quantum mechanical model of the atom was born.
Keywords and Equations

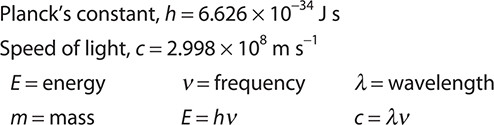
The Nature of Light
Light is a part of the electromagnetic spectrum—radiant energy composed of gamma rays, X-rays, ultraviolet light, visible light, and so on. Figure 7.1 shows the electromagnetic spectrum.
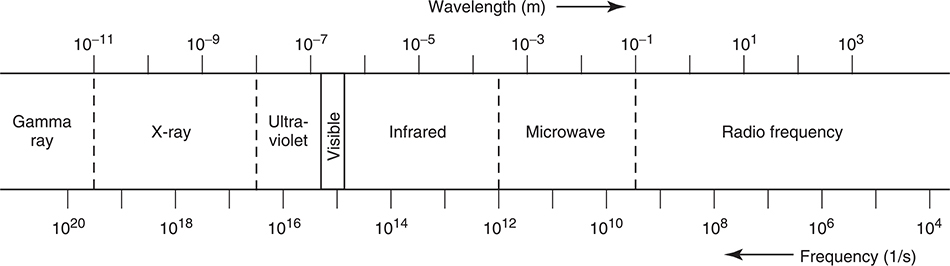
Figure 7.1 The electromagnetic spectrum.
The energy of the electromagnetic spectrum moves through space as waves that have three associated variables—frequency, wavelength, and amplitude. The frequency, ν, is the number of waves that pass a point per second. Wavelength (λ) is the distance between two identical points on a wave. Amplitude is the height of the wave and is related to the intensity (or brightness, for visible light) of the wave. Figure 7.2 shows the wavelength and amplitude of a wave.
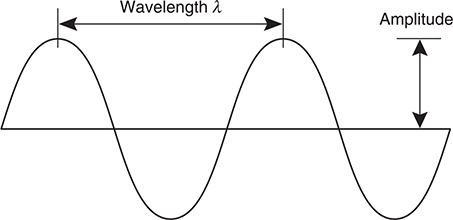
Figure 7.2 Wavelength and amplitude of a wave.
The energy associated with a certain frequency of light is related by the equation:
E = hv, where h is Planck’s constant = 6.626 × 10—34 J s
In developing the quantum mechanical model of the atom, it was found that the electrons can have only certain distinct quantities of energy associated with them, and that for an electron in an atom to change its energy it must absorb or emit a certain amount of energy. The energy that is emitted or absorbed is really the difference in the two energy states and can be calculated by:
E = hv
All electromagnetic radiation travels at about the same speed in a vacuum, 2.998 × 108 m s—1. This constant is called the speed of light (c). The product of the frequency and the wavelength is the speed of light:
C = vλ
Let’s apply some of the relationships. What wavelength of radiation has photons of energy 5.32 × 10—19 J?
Answer:
Rearranging the equations:
![]()
gives:
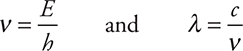
Insert the appropriate values:
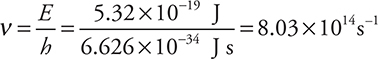
Then:
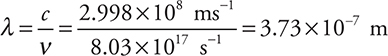
This answer could have been calculated more quickly by combining the original two equations to give:
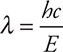
While this equation does not appear on the exam, it can save time by combining the two equations given on the exam into one calculation.
Spectroscopy and the Electromagnetic Spectrum
Information about a substance may be determined by spectroscopy. There are a number of spectroscopic methods, each of which supplies different information. Some of the methods, and their uses, are in Table 7.1. Depending on the region of the electromagnetic spectrum, the absorption or emission of radiation results in a transition between the ground state and an excited state or vice versa.
Table 7.1 Some spectroscopic methods and their uses.
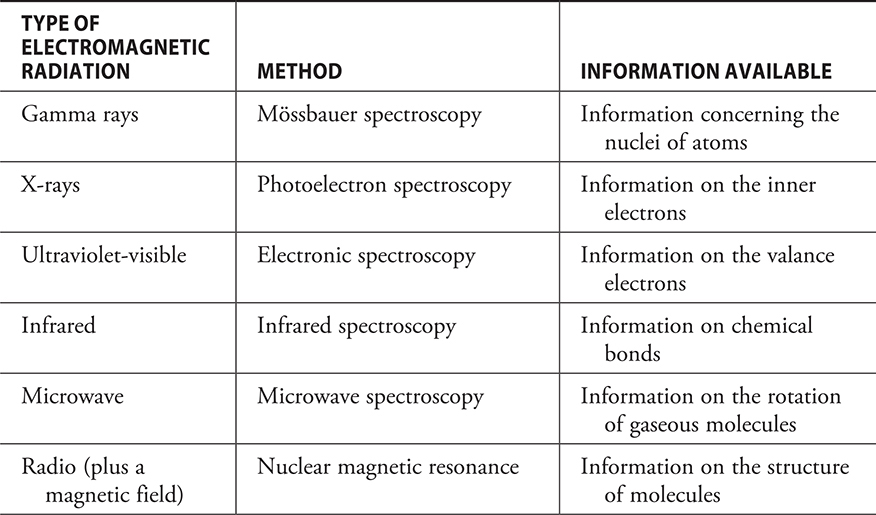
In addition to the structural information from the methods mentioned in the table, it is possible to obtain information on the concentration of a species. The greater the concentration, the higher the percentage of radiation absorbed.
Beer-Lambert Law
In general, the concentration of a chemical may be related to the amount of radiation absorbed through the Beer—Lambert law, or simply Beer’s law. In addition to the concentration, it is necessary to know the thickness (pathlength), through which the light passes, and the molar absorptivity, which depends upon the transition being observed and the wavelength of the radiation. The Beer—Lambert law is normally expressed by the following equation:
A = εbc
A is the amount of radiation absorbed (as an alternative, the percent of light transmitted can be measured). The symbol ε is the molar absorptivity, b is the pathlength, and c is the concentration of the absorbing species. For a particular experiment, the molar absorptivity and the pathlength are fixed. A commonly used instrument for observing Beer’s law is a spectrophotometer. A specially calibrated test tube, called a cuvette, holds the sample. Using the cuvette fixes the pathlength to the diameter of the cuvette. The wavelength to be absorbed can be adjusted, and normally, the wavelength chosen is the one with the greatest molar absorptivity for the transition of interest. The amount of radiation absorbed is measured, and the concentration calculated. However, the concentration is usually not calculated directly but determined through the preparation of a calibration curve.
A calibration curve is prepared by measuring the absorbance of several solutions of known concentration and plotting the observed absorbances versus concentration. Any concentration unit may be used, and, in most cases, the most convenient unit is chosen. Figure 7.3 shows a calibration curve for solutions containing the permanganate ion, MnO4—. Over the limited region shown in the calibration curve, the graph is linear. Care must be taken when using the graph not to go beyond the region experimentally shown to be linear. For example, this graph is not useful for a solution with an absorbance of 0.800 or higher, or below 0.850. At very low or very high concentrations the calibration curve is not linear. Deviations at low concentrations are the result of the concentration of the absorbing species being too low to result in a statistically significant probability of absorbing a photon of ration. When the concentration is too high, not every absorbing species will have a chance to absorb a photon because some of the species will be behind the ones that do absorb photons.
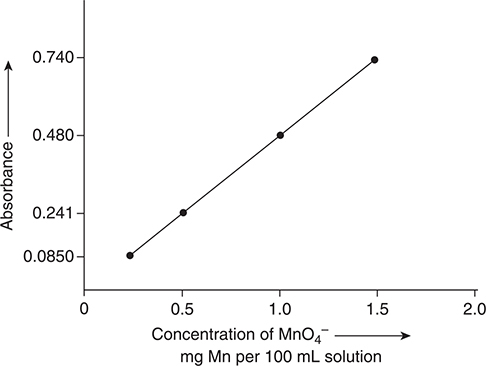
Figure 7.3 A calibration curve for MnO4— at 545 nm. The region shown is from 0.200 mg Mn per 100 mL to 1.50 mg Mn per 100 mL.
Wave Properties of Matter
The concept that matter possesses both particle and wave properties was first postulated by de Broglie in 1925. He introduced the equation 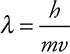 , which indicates a mass (m) moving with a certain velocity (v) would have a specific wavelength (λ) associated with it. (Note that this v is the velocity, not ν the frequency.) If the mass is very large (a locomotive), the associated wavelength is insignificant. However, if the mass is very small (an electron), the wavelength is measurable. The denominator may be replaced with the momentum of the particle (p = mv).
, which indicates a mass (m) moving with a certain velocity (v) would have a specific wavelength (λ) associated with it. (Note that this v is the velocity, not ν the frequency.) If the mass is very large (a locomotive), the associated wavelength is insignificant. However, if the mass is very small (an electron), the wavelength is measurable. The denominator may be replaced with the momentum of the particle (p = mv).
Atomic Spectra
Late in the nineteenth century, scientists discovered that when the vapor of an element was heated it gave off a line spectrum, a series of fine lines of colors, instead of a continuous spectrum like a rainbow. This was used in the developing quantum mechanical model as evidence that the energy of the electrons in an atom was quantized; that is, there could only be certain distinct energies (lines) associated with the atom. Niels Bohr developed the first modern atomic model for hydrogen using the concepts of quantized energies. The Bohr model postulated a ground state for the electrons in the atom, an energy state of lowest energy, and an excited state, an energy state of higher energy. For an electron to go from its ground state to an excited state, it must absorb a certain amount of energy (a quantum). If the electron dropped back from that excited state to its ground state, that same amount of energy would be emitted. Bohr’s model also allowed scientists to develop a method of calculating the energy associated with a specific energy level for the electron in the hydrogen atom:
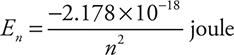
where n is the energy state. This equation can then be modified to calculate the energy difference between any two energy levels:
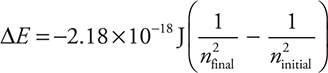
Atomic Orbitals
Bohr’s model worked well for hydrogen, the simplest atom, but didn’t work very well for any others. In the early 1900s, Schrödinger developed a more involved model and set of equations that better described atoms by using quantum mechanical concepts. His model introduced a mathematical description of the electron’s motion called a wave function or atomic orbital. Squaring the wave function (orbital) gives the volume of space in which the probability of finding the electron is high. This is commonly referred to as the electron cloud.
Schrödinger’s equation required the use of three quantum numbers to describe each electron within an atom, corresponding to the orbital size, shape, and orientation in space. It was also found that a quantum number concerning the spin of the electron was needed.

The first quantum number is the principal quantum number (n). It describes the energy (related to size) of the orbital and relative distance from the nucleus. The allowed (by the mathematics of the Schrödinger equation) values are positive integers (1, 2, 3, 4, etc.). The smaller the value of n, the closer the orbital is to the nucleus. The number n is sometimes called the atom’s shell.
The second quantum number is the angular momentum quantum number (l). Its value is related to the principal quantum number and has allowed values of 0 up to (n — 1). For example, if n = 3, then the possible values of l would be 0, 1, and 2 (3 — 1). This value of l defines the shape of the orbital:
• If l = 0, the orbital is called an s orbital and has a spherical shape with the nucleus at the center of the sphere. The greater the value of n, the larger the sphere.
• If l = 1, the orbital is called a p orbital and has two lobes of high electron density on either side of the nucleus. This makes for an hourglass or dumbbell shape.
• If l = 2, the orbital is a d orbital and can have a variety of shapes.
• If l = 3, the orbital is an f orbital, with more complex shapes.
Figure 7.4 shows the shapes of the s, p, and d orbitals. These are sometimes called sublevels or subshells.
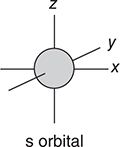
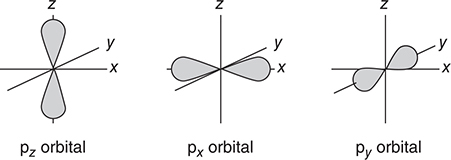
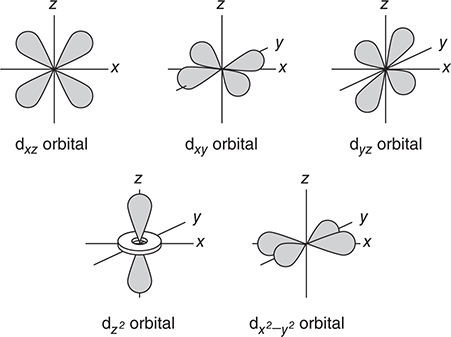
Figure 7.4 The shapes of the s, p, and d atomic orbitals.
The third quantum number is the magnetic quantum number (ml). It describes the orientation of the orbital around the nucleus. The possible values of ml depend on the value of the angular momentum quantum number, l. The allowed values for ml are —l through zero to +l. For example, for l = 2 the possible values of ml would be —2, —1, 0, +1, +2. This is why, for example, if l = 1 (a p orbital), then there are three p orbitals corresponding to ml values of —1, 0, +1. This is also shown in Figure 7.4.
The fourth quantum number, the spin quantum number (ms), indicates the direction the electron is spinning. There are only two possible values for ms, + ½ and —½.
The quantum numbers for the six electrons in carbon would be:
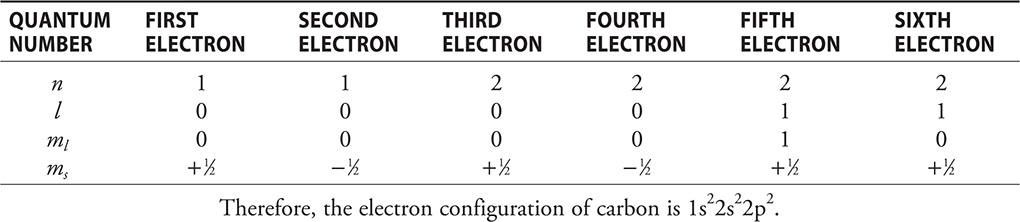
Photoelectron (Photoemission) Spectroscopy (PES)
Photoelectron spectroscopy is one of a group of related techniques where high-energy photons remove an electron from an atom in a photoelectric effect process. The method relies on a measurement of the kinetic energy of the emitted electron. The kinetic energy is equal to the energy of the photon minus the binding energy of the electron. The binding energy is the energy holding the electron in the atom and can be rather difficult to measure.
Figure 7.5 shows the PES spectrum of lithium. Lithium has three electrons; two are 1s electrons and one is a 2s electron. The 2s is less tightly bound (right peak—lower binding energy) than the 1s electrons (left peak—higher binding energy). The left peak is twice as high as the right peak because there are two 1s electrons compared to one 2s electron. X-ray photons can excite core electrons. For example, it is possible to focus on the 1s electrons of an oxygen atom. The binding energy is in part related to the effective nuclear charge experienced by the electron. In compounds, other atoms bonded to the atom of interest can influence the effective nuclear charge. Atoms donating electron density to the atom of interest decrease the effective nuclear charge, while electron-withdrawing atoms lead to an increase in the effective nuclear charge. An important factor in whether an atom donates or withdraws electron density is the relative electronegativity of the two atoms. This experimental method can be used to give information on which atoms are bonded to each other.
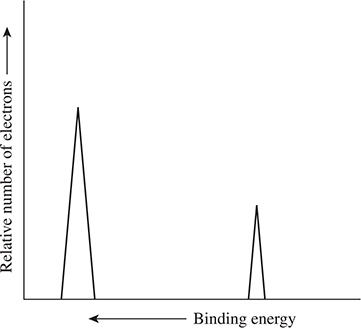
Figure 7.5 PES spectrum of lithium.
Experiments

No experimental questions related to this chapter have appeared on the AP Exam in recent years.
Common Mistakes to Avoid
1. Be sure not to confuse wavelength and frequency.
2. The speed of light is 3.0 × 108 m/s. The exponent is positive.
3. The value of n is never zero.
4. The values of l and ml include zero.
5. Do not confuse velocity (v) and frequency (ν).
6. The units of Planck’s constant are J s, not J/s.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. First are 20 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. There are questions included to review prior knowledge of material. Following those is a long free-response question like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. Which of the following represents the electron arrangement for the least reactive element of the four?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
2. Which of the following might refer to a transition element?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
3. Which of the following electron arrangements refers to the most chemically reactive element of the four?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
4. Which of the following electron arrangements represents an atom in an excited state?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
5. The ground-state configuration of Fe2+ is which of the following?
(A) 1s22s22p63s23p63d54s1
(B) 1s22s22p63s23p63d6
(C) 1s22s22p63s23p63d64s2
(D) 1s22s22p63s23p63d84s2
6. Which of the following contains only atoms that are diamagnetic in their ground state?
(A) Kr, Ca, and P
(B) Ne, Be, and Zn
(C) Ar, K, and Ba
(D) He, Sr, and C
7. Which of the following is the electron configuration of a halogen?
(A) 1s21p62s22p3
(B) 1s22s22p63s23p64s23d104p65s24d1
(C) 1s22s22p63s23p63d3
(D) 1s22s22p5
8. Which of the following is a possible configuration for a transition metal atom?
(A) 1s21p62s22p3
(B) 1s22s22p63s23p64s23d104p65s24d1
(C) 1s22s22p63s23p63d3
(D) 1s22s22p5
9. The following are some electron configurations reported by four students. Which of the following electron configurations is not possible?
(A) 1s22s32p3
(B) 1s22s22p63s23p64s23d104p6
(C) 1s22s22p63s23p63d3
(D) 1s22s22p5
10. Which of the following is a possible configuration for a transition metal ion?
(A) 1s21p62s22p3
(B) 1s22s22p63s23p64s23d104p65s24d1
(C) 1s22s22p63s23p63d3
(D) 1s22s22p5
11. If all the electrons are present in pairs, a substance is said to be diamagnetic. If there is at least one electron by itself in an orbital, a substance is said to be paramagnetic. In which of the following groups are all atoms diamagnetic?
(A) Be, O, and N
(B) Mg, Se, and Xe
(C) Kr, Be, and Zn
(D) At, Sn, and Ba
12. Which of the following explains why oxygen atoms, in their ground state, are paramagnetic?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
13. An atomic orbital can hold no more than two electrons; this is a consequence of which of the following?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
14. Why does the 4s orbital fill before the 3d orbital starts to fill?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
15. Calcium reacts with element Xto form an ionic compound. If the ground-state electron configuration of Xis 1s22s22p4, what is the simplest formula for this compound?
(A) CaX
(B) CaX2
(C) Ca4X2
(D) Ca2X2
16. In 1927, Clinton Davisson and Lester Germer experimentally confirmed what was known as the de Broglie hypothesis by observing the diffraction of electrons. This led to general acceptance of the de Broglie equation. Which of the following best explains the diffraction of electrons?
(A) Pauli exclusion principle
(B) Hund’s rule
(C) the wave properties of matter
(D) Heisenberg uncertainty principle
17. How many peaks are expected in the photoelectron spectrum (PES) of aluminum?
(A) 4
(B) 3
(C) 2
(D) 5
18. Which peak is expected to be the most intense in the photoelectron spectrum (PES) of chlorine?
(A) 3s
(B) 2p and 3p will be equally intense
(C) 2p
(D) 3p
19. The Beer’s law equation is A = εbc. A is the absorbance of the solution; what is the meaning of the other terms?
(A) ε = molar absorptivity, b = Beer’s constant, and c = concentration
(B) ε = molar absorptivity, b = path length, and c = concentration
(C) ε = extinction coefficient, b = path length, and c = speed of light
(D) ε = extinction coefficient, b = Beer’s constant, and c = speed of light
20. Determine the wavelength of light with and energy of 9.98 × 10—19 J.
(A) 1.99 × 10—7 m
(B) 5.03 × 106 m
(C) 4.51 × 1023 m
(D) 1.98 × 10—43 m
![]() Answers and Explanations
Answers and Explanations
1. D—This configuration represents a noble gas (neon). The outer s and p orbitals are filled. C is the electron configuration of Zr. A and B are both Li, with A being an excited state and B being the ground state. See questions 2—4.
2. C—Transition elements have partially filled d orbitals. This configuration is for the metal zirconium, Zr.
3. B—The single electron in the s orbital indicates that this is the very reactive alkali metal lithium.
4. A—The 1s orbital is not filled. One indication of excited states is one or more inner orbitals being unfilled.
5. B—The electron configuration for iron is 1s22s22p63s23p63d64s2. To produce an iron(II) ion, the two 4s electrons are removed first.
6. B—The elements that are normally diamagnetic are those in the same columns of the periodic table as Be, Zn, and He because all the electrons are paired. Atoms in all other columns are normally paramagnetic.
7. D—Halogens have a valence shell with s2p5.
8. B—Transition metals have partially filled d orbitals (d1—10), along with an s1 or s2. C represents a transition metal ion, not an atom.
9. A—A 2s3 configuration is not possible as s orbitals cannot accommodate more than two electrons.
10. C—The outer s-electrons are not present in most transition metal ions; however, d electrons may be present. C could be V2+, Cr3+, or Mn4+ (among other choices). B is a Y atom.
11. C—Atoms with only completely filled shells or subshells are diamagnetic; all others are paramagnetic. From the choices given, the elements with complete shells or subshells are Be, Mg, Xe, Kr, Zn, and Ba. Only one answer consists of atoms from this group. It might be helpful to consult the periodic table, as these elements are in groups 2, 12, and 18. All odd atomic number atoms are paramagnetic.
12. C—The four electrons in the oxygen 2p orbitals are arranged with one pair and two unpaired electrons with spins parallel. This makes the oxygen atom paramagnetic. This arrangement is due to Hund’s rule.
13. A—The Pauli exclusion principle restricts the number of electrons that can occupy a single orbital.
14. B—The d orbitals are shielded more efficiently than the s orbitals. Thus, the less-shielded d orbitals do not fill as readily as s orbitals with similar energy.
15. A—Calcium will form a +2 ion (Ca2+), and X will need to gain two electrons to fill its outer shell and become a —2 ion (X2—). The simplest formula for a compound containing a +2 ion and a —2 ion would be CaX. The other answers involve different charges or a formula that has not been simplified.
16. C—Diffraction is a wave phenomenon; therefore, the observation of diffraction proves the wave properties of electrons (matter).
17. D—The first step is to determine the complete electron configuration of aluminum, which is 1s22s22p63s23p1.
In the electron configuration of Al there are five different types of orbitals being used (1s, 2s, 2p, 3s, and 3p). Five types of orbitals means five peaks.
18. C—The first step is to determine the complete electron configuration of chlorine, which is 1s22s22p63s23p5.
There are five types of orbitals so there will be five peaks. The most intense peak is the one with the most electrons (2p). The 3p orbital, with only five electrons, will be 5/6 times as intense as the 2p with 6 electrons.
19. B—These are listed on the equation sheet accompanying the exam. While it is possible to find these terms on the equation sheet with the exam, on a timed exam, distractions such as looking for items can lead to running out of time on the exam and not completing the exam.
20. A—The AP Exam gives two equations for use in the question. These questions are: E = hν and c = λν. The constants are also listed. The constants are h = 6.626 × 10—34 J s and c = 2.998 × 108 m s—1.
Using these equations requires each to be rearranged to ν = E/h and λ = c/ν. The frequency calculated from the first of these equations is entered into the second to get the wavelength. The first calculation is
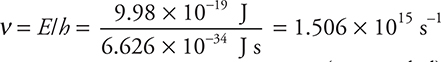
(unrounded)
Entering this calculated frequency into the second equation gives
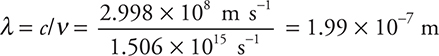
To save time on the exam, it will help if you combine the two equations before you begin calculating. This combined equation is λ = hc/E (you should prove to yourself that this is the correct rearranged equation).
Using the combined equation
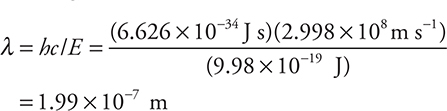
This saves doing two calculations and avoids the possibility of miscopying the answer from the first calculation into the second equation.
With the time constraints on a timed exam, it will help to round as much as possible. Rounding gives
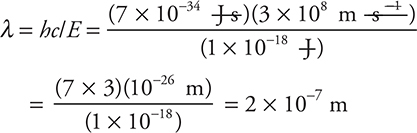
Note: you are calculating a wavelength; therefore, your answer MUST have a length unit (m). The 5.03 × 106 m answer comes by calculating through using the inverse of both rearranged equations. If you had written and watched all your units, you would have caught this because your answer would have m—1 as the unit, which is not a length unit.
The 4.51 × 1023 m answer comes by calculating through using the inverse of the first rearranged equation and the correct second equation. In this case, the calculation gives the units m s2, which is again not a length unit and obviously wrong (easily caught if you kept track of your units).
The 1.98 × 10—43 m answer comes when the two equations were incorrectly rearranged to ν = Eh and λ = cν. In this case, the calculation gives the units m J2, which is again not a length unit and obviously wrong (easily caught if you kept track of your units).
![]() Free-Response Question
Free-Response Question
You have 15 minutes to answer the following question. You may use a calculator and the tables in the back of the book.
Question
(a) The bond energy of fluorine is 159 kJ mol—1.
i. Determine the energy, in J, of a photon of light needed to break an F—F bond.
ii. Determine the frequency of this photon in s—1.
(b) Barium imparts a characteristic green color to a flame. The wavelength of this light is 551 nm. Determine the energy involved in kJ/mol.
![]() Answer and Explanation
Answer and Explanation
(a) If you do not remember them, several of the equations are given on the AP Exam and in the back of this book. In addition, the values of Planck’s constant, Avogadro’s number, and the speed of light are necessary. These constants are also given on the exam.
i. This is a simple conversion problem:
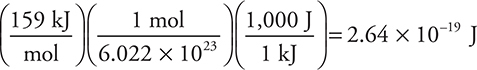
Give yourself 1 point if you got this answer. If you got the wrong units (not J), you made a mistake.
ii. This part requires the equation E = hν. (This equation is given on the equation page of the AP Exam.)
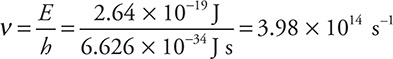
Give yourself 1 point for this answer. If you got the wrong answer in the preceding part but used it correctly here (in place of the 2.64 × 10—19 J), you still get 1 point. If you got the wrong units (not s—1), you made a mistake. You should realize that s ≠ s—1.
(b) This can be done as a one-step or two-step problem. The AP test booklet gives you the equations to solve this directly as a two-step problem. The two equations are c = λν and E = hν. This method will be followed here. The two equations may be combined to produce an equation (E = hc/λ) that will allow you to do the problem in one step (you can use the alternate one-equation method for practice and as a comparison).
Using c = λν:
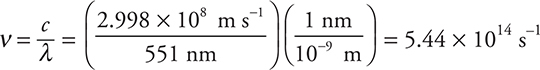
If you got the wrong units (not s—1), you made a mistake. You should realize that s ≠ s—1.
Using E = hν:
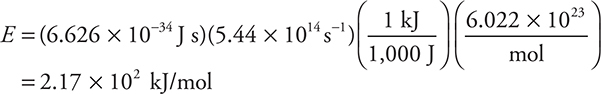
The information in the first two parentheses is a plug-in into the equation. The third parentheses include a unit conversion from joules to kilojoules. The final parentheses convert from photons to moles.
Give yourself 1 point for each of these answers. If you did the problem as a one-step problem, give yourself 2 points if you got the final answer correct or 1 point if you left out any of the conversions. We have seen numerous students lose points on the AP Exam because they either did not write the units or did not pay attention to the units they had. If the units are wrong, there is something else wrong with your answer. In many cases on the exams we have graded, students have thrown away points because of inconsistent units.
Total your points. There are 4 points possible. Subtract 1 point if any answer does not have the correct number of significant figures.
![]() Rapid Review
Rapid Review
• Know the general regions of the electromagnetic spectrum.
• The frequency, ν, is defined as the number of waves that pass a point per second.
• The wavelength, λ, is the distance between two identical points on a wave.
• The energy of light is related to the frequency by E = hν.
• The product of the frequency and wavelength of light is the speed of light: c = νλ.
• An orbital or wave function is a quantum mechanical, mathematical description of the electron.
• If all electrons in an atom are in their lowest possible energy level, then the atom is said to be in its ground state.
• If any electrons in an atom are in a higher energy state, then the atom is said to be in an excited state.
• The energy of an atom is quantized, existing in only certain distinct energy states.
• Quantum numbers are numbers used in Schrödinger’s equation to describe the orbital size, shape, and orientation in space and the spin of an electron.
• The principal quantum number, n, describes the size of the orbital. It must be a positive integer. It is sometimes referred to as the atom’s shell.
• The angular momentum quantum number, l, defines the shape of the electron cloud. If l = 0, it is an s orbital; if l = 1, it is a p orbital; if l = 2, it is a d orbital; if l = 3, it is an f orbital, etc.
• The magnetic quantum number, ml , describes the orientation of the orbital around the nucleus. It can be integer values ranging from —l through 0 to +l.
• The spin quantum number, ms, describes the spin of the electron and can only have values of +½ and —½.
• Be able to write the quantum numbers associated with the first 20 electrons.