Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION II. Bioenergetics & the Metabolism of Carbohydrates & Lipids
Chapter 12. Biologic Oxidation
Kathleen M. Botham, PhD, DSc & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Understand the meaning of redox potential and explain how it can be used to predict the direction of flow of electrons in biologic systems.
Understand the meaning of redox potential and explain how it can be used to predict the direction of flow of electrons in biologic systems.
![]() Identify the four classes of enzymes (oxidoreductases) involved in oxidation and reduction reactions.
Identify the four classes of enzymes (oxidoreductases) involved in oxidation and reduction reactions.
![]() Describe the action of oxidases and provide examples of where they play an important role in metabolism.
Describe the action of oxidases and provide examples of where they play an important role in metabolism.
![]() Indicate the two main functions of dehydrogenases and explain the importance of NAD- and riboflavin-linked dehydrogenases in metabolic pathways such as glycolysis, the citric acid cycle, and the respiratory chain.
Indicate the two main functions of dehydrogenases and explain the importance of NAD- and riboflavin-linked dehydrogenases in metabolic pathways such as glycolysis, the citric acid cycle, and the respiratory chain.
![]() Identify the two types of enzymes classified as hydroperoxidases; indicate the reactions they catalyze and explain why they are important.
Identify the two types of enzymes classified as hydroperoxidases; indicate the reactions they catalyze and explain why they are important.
![]() Give the two steps of reactions catalyzed by oxygenases and identify the two subgroups of this class of enzymes.
Give the two steps of reactions catalyzed by oxygenases and identify the two subgroups of this class of enzymes.
![]() Appreciate the role of cytochrome P450 in drug detoxification and steroid synthesis.
Appreciate the role of cytochrome P450 in drug detoxification and steroid synthesis.
![]() Describe the reaction catalyzed by superoxide dismutase and explain how it protects tissues from oxygen toxicity.
Describe the reaction catalyzed by superoxide dismutase and explain how it protects tissues from oxygen toxicity.
BIOMEDICAL IMPORTANCE
Chemically, oxidation is defined as the removal of electrons and reduction as the gain of electrons. Thus, oxidation is always accompanied by reduction of an electron acceptor. This principle of oxidation-reduction applies equally to biochemical systems and is an important concept underlying understanding of the nature of biologic oxidation. Note that many biologic oxidations can take place without the participation of molecular oxygen, eg, dehydrogenations. The life of higher animals is absolutely dependent upon a supply of oxygen for respiration, the process by which cells derive energy in the form of ATP from the controlled reaction of hydrogen with oxygen to form water. In addition, molecular oxygen is incorporated into a variety of substrates by enzymes designated as oxygenases; many drugs, pollutants, and chemical carcinogens (xenobiotics) are metabolized by enzymes of this class, known as the cytochrome P450 system. Administration of oxygen can be lifesaving in the treatment of patients with respiratory or circulatory failure.
FREE ENERGY CHANGES CAN BE EXPRESSED IN TERMS OF REDOX POTENTIAL
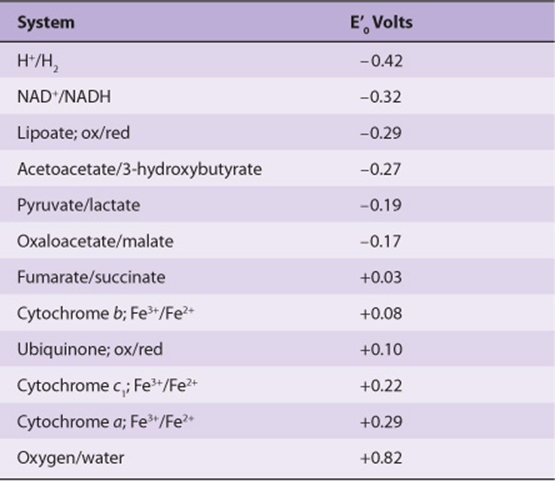
In reactions involving oxidation and reduction, the free energy change is proportionate to the tendency of reactants to donate or accept electrons. Thus, in addition to expressing free energy change in terms of ΔG0’ (Chapter 11), it is possible, in an analogous manner, to express it numerically as an oxidation-reduction or redox potential (E’0). The redox potential of a system (E0) is usually compared with the potential of the hydrogen electrode (0.0 V at pH 0.0). However, for biologic systems, the redox potential (E’0) is normally expressed at pH 7.0, at which pH the electrode potential of the hydrogen electrode is -0.42 V. The redox potentials of some redox systems of special interest in mammalian biochemistry are shown in Table 12-1. The relative positions of redox systems in the table allow prediction of the direction of flow of electrons from one redox couple to another.
TABLE 12–1 Some Redox Potentials of Special Interest in Mammalian Oxidation Systems
Enzymes involved in oxidation and reduction are called oxidoreductases and are classified into four groups: oxidases, dehydrogenases, hydroperoxidases, and oxygenases.
OXIDASES USE OXYGEN AS A HYDROGEN ACCEPTOR
Oxidases catalyze the removal of hydrogen from a substrate using oxygen as a hydrogen acceptor.* They form water or hydrogen peroxide as a reaction product (Figure 12–1).
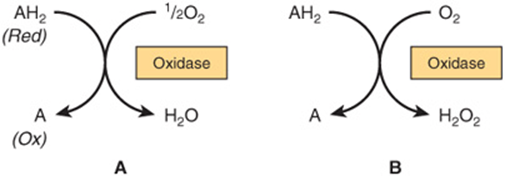
FIGURE 12–1 Oxidation of a metabolite catalyzed by an oxidase (A) forming H2O and (B) forming H2O2.
Some Oxidases Contain Copper
Cytochrome oxidase is a hemoprotein widely distributed in many tissues, having the typical heme prosthetic group present in myoglobin, hemoglobin, and other cytochromes (Chapter 6). It is the terminal component of the chain of respiratory carriers found in mitochondria (Chapter 13) and transfers electrons resulting from the oxidation of substrate molecules by dehydrogenases to their final acceptor, oxygen. The action of the enzyme is blocked by carbon monoxide, cyanide, and hydrogen sulfide, and this causes poisoning by preventing cellular respiration. It has also been termed “cytochrome a3.” However, it is now known that the heme a3 is combined with another heme, heme a, in a single protein to form the cytochrome oxidase enzyme complex, and so it is more correctly termed cytochrome aa3. It contains two molecules of heme, each having one Fe atom that oscillates between Fe3+ and Fe2+ during oxidation and reduction. Furthermore, two atoms of Cu are present, each associated with a heme unit.
Other Oxidases Are Flavoproteins
Flavoprotein enzymes contain flavin mononucleotide (FMN) or flavin adenine dinucleotide (FAD) as prosthetic groups. FMN and FAD are formed in the body from the vitamin riboflavin (Chapter 44). FMN and FAD are usually tightly—but not covalently—bound to their respective apoenzyme proteins. Metalloflavoproteins contain one or more metals as essential cofactors. Examples of flavoprotein enzymes include L-amino acid oxidase, an FMN-linked enzyme found in kidney with general specificity for the oxidative deamination of the naturally occurring L-amino acids; xanthine oxidase, which contains molybdenum and plays an important role in the conversion of purine bases to uric acid (Chapter 33), and is of particular significance in uricotelic animals (Chapter 28); and aldehyde dehydrogenase, an FAD-linked enzyme present in mammalian livers, which contains molybdenum and non-heme iron and acts upon aldehydes and N-heterocyclic substrates. The mechanisms of oxidation and reduction of these enzymes are complex. Evidence suggests a two-step reaction as shown in Figure 12–2.
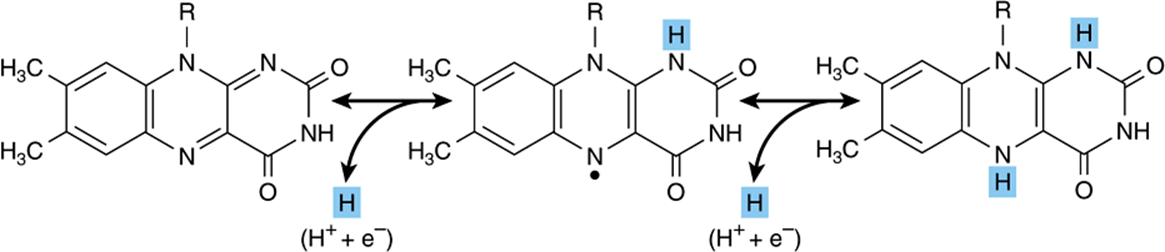
FIGURE 12–2 Oxidoreduction of isoalloxazine ring in flavin nucleotides via a semiquinone (free radical) intermediate (center).
DEHYDROGENASES CANNOT USE OXYGEN AS A HYDROGEN ACCEPTOR
There are a large number of enzymes in the dehydrogenase class. They perform the following two main functions:
1. Transfer of hydrogen from one substrate to another in a coupled oxidation-reduction reaction (Figure 12–3). These dehydrogenases are specific for their substrates but often utilize common coenzymes or hydrogen carriers, eg, NAD+. Since the reactions are reversible, these properties enable reducing equivalents to be freely transferred within the cell. This type of reaction, which enables one substrate to be oxidized at the expense of another, is particularly useful in enabling oxidative processes to occur in the absence of oxygen, such as during the anaerobic phase of glycolysis (Figure 18–2).
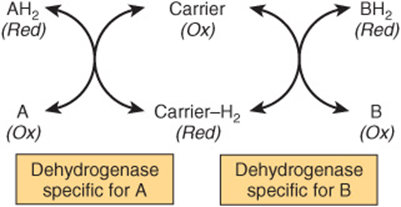
FIGURE 12–3 Oxidation of a metabolite catalyzed by coupled dehydrogenases.
2. Transfer of electrons in the respiratory chain of electron transport from substrate to oxygen (Figure 13–3).
Many Dehydrogenases Depend on Nicotinamide Coenzymes
These dehydrogenases use nicotinamide adenine dinucleotide (NAD+) or nicotinamide adenine dinucleotide phosphate (NADP+)—or both—which are formed in the body from the vitamin niacin (Chapter 44). The coenzymes are reduced by the specific substrate of the dehydrogenase and reoxidized by a suitable electron acceptor (Figure 12–4). They are able to freely and reversibly dissociate from their respective apoenzymes.
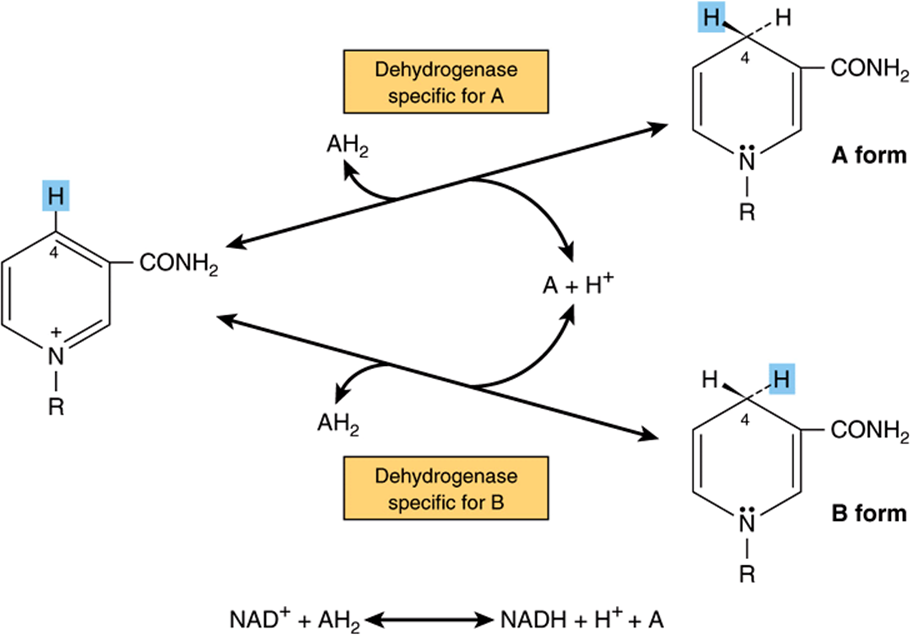
FIGURE 12–4 Mechanism of oxidation and reduction of nicotinamide coenzymes. There is stereospecificity about position 4 of nicotinamide when it is reduced by a substrate AH2. One of the hydrogen atoms is removed from the substrate as a hydrogen nucleus with two electrons (hydride ion, H–) and is transferred to the 4 position, where it may be attached in either the A or the B form according to the specificity determined by the particular dehydrogenase catalyzing the reaction. The remaining hydrogen of the hydrogen pair removed from the substrate remains free as a hydrogen ion.
Generally, NAD-linked dehydrogenases catalyze oxidoreduction reactions in the oxidative pathways of metabolism, particularly in glycolysis (Chapter 18), in the citric acid cycle (Chapter 17), and in the respiratory chain of mitochondria (Chapter 13). NADP-linked dehydrogenases are found characteristically in reductive syntheses, as in the extramitochondrial pathway of fatty acid synthesis (Chapter 23) and steroid synthesis Chapter 26)—and also in the pentose phosphate pathway (Chapter 21).
Other Dehydrogenases Depend on Riboflavin
The flavin groups associated with these dehydrogenases are similar to FMN and FAD occurring in oxidases. They are generally more tightly bound to their apoenzymes than are the nicotinamide coenzymes. Most of the riboflavin-linked dehydrogenases are concerned with electron transport in (or to) the respiratory chain (Chapter 13). NADH dehydrogenase acts as a carrier of electrons between NADH and the components of higher redox potential (Figure 13–3). Other dehydrogenases such as succinate dehydrogenase, acyl-CoA dehydrogenase, and mitochondrial glycerol-3-phosphate dehydrogenase transfer reducing equivalents directly from the substrate to the respiratory chain (Figure 13–5). Another role of the flavin-dependent dehydrogenases is in the dehydrogenation (by dihydrolipoyl dehydrogenase) of reduced lipoate, an intermediate in the oxidative decarboxylation of pyruvate and α-ketoglutarate (Figures 13-5 & 18-5). The electron-transferring flavoprotein (ETF) is an intermediary carrier of electrons between acyl-CoA dehydrogenase and the respiratory chain (Figure 13–5).
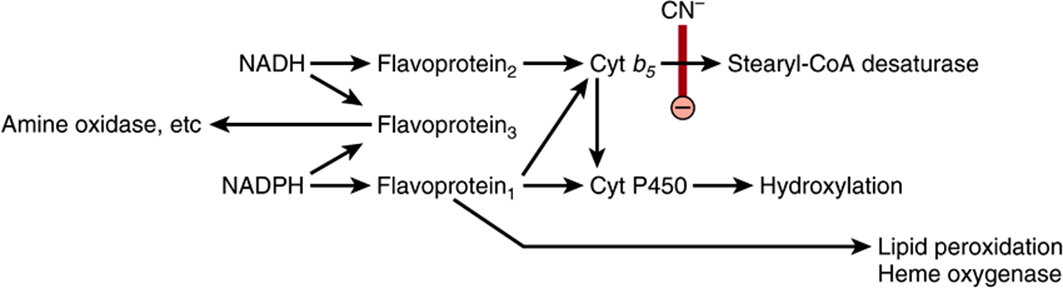
FIGURE 12–5 Electron transport chain in the endoplasmic reticulum. Cyanide (CN–) inhibits the indicated step.
Cytochromes May Also Be Regarded as Dehydrogenases
The cytochromes are iron-containing hemoproteins in which the iron atom oscillates between Fe3+ and Fe2+ during oxidation and reduction. Except for cytochrome oxidase (previously described), they are classified as dehydrogenases. In the respiratory chain, they are involved as carriers of electrons from flavoproteins on the one hand to cytochrome oxidase on the other (Figure 13–5). Several identifiable cytochromes occur in the respiratory chain, ie, cytochromes b, c1, c, and cytochrome oxidase. Cytochromes are also found in other locations, eg, the endoplasmic reticulum (cytochromes P450 and b5), and in plant cells, bacteria, and yeasts.
HYDROPEROXIDASES USE HYDROGEN PEROXIDE OR AN ORGANIC PEROXIDE AS SUBSTRATE
Two type of enzymes found both in animals and plants fall into this category: peroxidases and catalase.
Hydroperoxidases protect the body against harmful peroxides. Accumulation of peroxides can lead to generation of free radicals, which in turn can disrupt membranes and perhaps cause diseases including cancer and atherosclerosis (see Chapters 15 and 44).
Peroxidases Reduce Peroxides Using Various Electron Acceptors
Peroxidases are found in milk and in leukocytes, platelets, and other tissues involved in eicosanoid metabolism (Chapter 23). The prosthetic group is protoheme. In the reaction catalyzed by peroxidase, hydrogen peroxide is reduced at the expense of several substances that will act as electron acceptors, such as ascorbate, quinones, and cytochrome c. The reaction catalyzed by peroxidase is complex, but the overall reaction is as follows:
![]()
In erythrocytes and other tissues, the enzyme glutathione peroxidase, containing selenium as a prosthetic group, catalyzes the destruction of H2O2 and lipid hydroperoxides through the conversion of reduced glutathione to its oxidized form, protecting membrane lipids and hemoglobin against oxidation by peroxides (Chapter 21).
Catalase Uses Hydrogen Peroxide as Electron Donor & Electron Acceptor
Catalase is a hemoprotein containing four heme groups. In addition to possessing peroxidase activity, it is able to use one molecule of H2O2 as a substrate electron donor and another molecule of H2O2 as an oxidant or electron acceptor.
![]()
Under most conditions in vivo, the peroxidase activity of cat-alase seems to be favored. Catalase is found in blood, bone marrow, mucous membranes, kidney, and liver. It functions to destroy hydrogen peroxide formed by the action of oxidases. Peroxisomes are found in many tissues, including liver. They are rich in oxidases and in catalase. Thus, the enzymes that produce H2O2 are grouped with the enzyme that breaks it down. However, mitochondrial and microsomal electron transport systems as well as xanthine oxidase must be considered as additional sources of H2O2.
OXYGENASES CATALYZE THE DIRECT TRANSFER & INCORPORATION OF OXYGEN INTO A SUBSTRATE MOLECULE
Oxygenases are concerned with the synthesis or degradation of many different types of metabolites. They catalyze the incorporation of oxygen into a substrate molecule in two steps: (1) oxygen is bound to the enzyme at the active site and (2) the bound oxygen is reduced or transferred to the substrate. Oxy-genases may be divided into two subgroups, dioxygenases and monooxygenases.
Dioxygenases Incorporate Both Atoms of Molecular Oxygen into the Substrate
The basic reaction catalyzed by dioxygenases is shown below:
![]()
Examples include the liver enzymes, homogentisate dioxygenase (oxidase) and 3-hydroxyanthranilate dioxygenase (oxidase), which contain iron; and L-tryptophan dioxygenase (tryptophan pyrolase) (Chapter 29), which utilizes heme.
Monooxygenases (Mixed-Function Oxidases, Hydroxylases) Incorporate Only One Atom of Molecular Oxygen into the Substrate
The other oxygen atom is reduced to water, an additional electron donor or cosubstrate (Z) being necessary for this purpose:
![]()
Cytochromes P450 Are Monooxygenases Important for the Detoxification of Many Drugs & for the Hydroxylation of Steroids
Cytochromes P450 are an important superfamily of heme-containing monooxgenases, and >50 such enzymes have been found in the human genome. These cytochromes are located mainly in the endoplasmic reticulum in the liver and intestine, but are also found in the mitochondria in some tissues. Both NADH and NADPH donate reducing equivalents for the reduction of these cytochromes (Figure 12–5), which in turn are oxidized by substrates in a series of enzymatic reactions collectively known as the hydroxylase cycle (Figure 12–6). In the endoplasmic reticulum of the liver, cytochromes P450 are found together with cytochrome b5 and have a major role in drug metabolism and detoxification; they are responsible for about 75% of the modification and degradation of drugs which occurs in the body. The rate of detoxification of many medicinal drugs by cytochromes P450 determines the duration of their action. Benzpyrene, aminopyrine, aniline, morphine, and benzphetamine are hydroxylated, increasing their solubility and aiding their excretion. Many drugs such as phenobarbital have the ability to induce the synthesis of cytochromes P450.
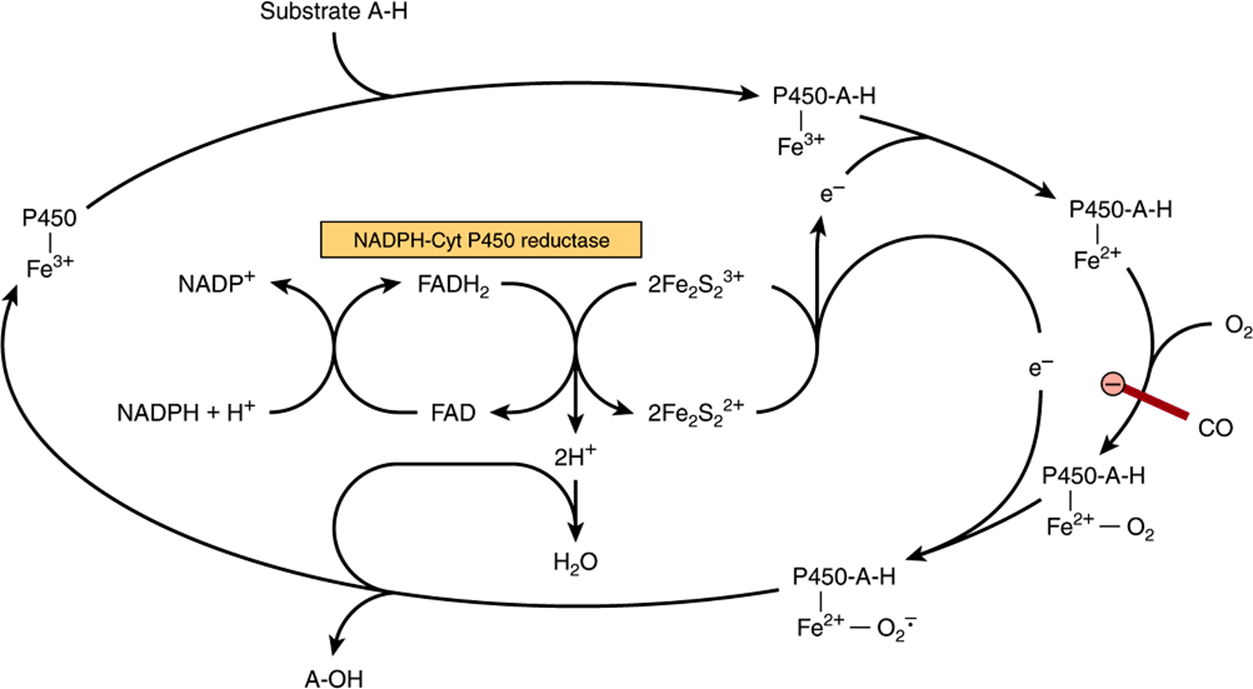
FIGURE 12–6 Cytochrome P450 hydroxylase cycle. The system shown is typical of steroid hydroxylases of the adrenal cortex. Liver microsomal cytochrome P450 hydroxylase does not require the iron-sulfur protein Fe2S2. Carbon monoxide (CO) inhibits the indicated step.
Mitochondrial cytochrome P450 systems are found in steroidogenic tissues such as adrenal cortex, testis, ovary, and placenta and are concerned with the biosynthesis of steroid hormones from cholesterol (hydroxylation at C22and C20 in side-chain cleavage and at the 11β and 18 positions). In addition, renal systems catalyzing 1α- and 24-hydroxylations of 25-hydroxycholecalciferol in vitamin D metabolism—and cholesterol 7α-hydroxylase and sterol 27-hydroxylase involved in bile acid biosynthesis from cholesterol in the liver (Chapter 26)—are P450 enzymes.
SUPEROXIDE DISMUTASE PROTECTS AEROBIC ORGANISMS AGAINST OXYGEN TOXICITY
Transfer of a single electron to O2 generates the potentially damaging superoxide anion free radical ![]() , which gives rise to free-radical chain reactions (Chapter 15), amplifying its destructive effects. The ease with which superoxide can be formed from oxygen in tissues and the occurrence of superoxide dismutase, the enzyme responsible for its removal in all aerobic organisms (although not in obligate anaerobes), indicate that the potential toxicity of oxygen is due to its conversion to superoxide.
, which gives rise to free-radical chain reactions (Chapter 15), amplifying its destructive effects. The ease with which superoxide can be formed from oxygen in tissues and the occurrence of superoxide dismutase, the enzyme responsible for its removal in all aerobic organisms (although not in obligate anaerobes), indicate that the potential toxicity of oxygen is due to its conversion to superoxide.
Superoxide is formed when reduced flavins—present, for example, in xanthine oxidase—are reoxidized univalently by molecular oxygen:
![]()
Superoxide can reduce oxidized cytochrome c
![]()
or be removed by superoxide dismutase.
In this reaction, superoxide acts as both oxidant and reductant. Thus, superoxide dismutase protects aerobic organisms against the potential deleterious effects of superoxide. The enzyme occurs in all major aerobic tissues in the mitochondria and the cytosol. Although exposure of animals to an atmosphere of 100% oxygen causes an adaptive increase in superoxide dismutase, particularly in the lungs, prolonged exposure leads to lung damage and death. Antioxidants, eg, α-tocopherol (vitamin E), act as scavengers of free radicals and reduce the toxicity of oxygen (Chapter 44).
SUMMARY
![]() In biologic systems, as in chemical systems, oxidation (loss of electrons) is always accompanied by reduction of an electron acceptor.
In biologic systems, as in chemical systems, oxidation (loss of electrons) is always accompanied by reduction of an electron acceptor.
![]() Oxidoreductases have a variety of functions in metabolism; oxidases and dehydrogenases play major roles in respiration; hydroperoxidases protect the body against damage by free radicals; and oxygenases mediate the hydroxylation of drugs and steroids.
Oxidoreductases have a variety of functions in metabolism; oxidases and dehydrogenases play major roles in respiration; hydroperoxidases protect the body against damage by free radicals; and oxygenases mediate the hydroxylation of drugs and steroids.
![]() Tissues are protected from oxygen toxicity caused by the superoxide free radical by the specific enzyme superoxide dismutase.
Tissues are protected from oxygen toxicity caused by the superoxide free radical by the specific enzyme superoxide dismutase.
REFERENCES
Babcock GT, Wikstrom M: Oxygen activation and the conservation of energy in cell respiration. Nature 1992;356:301.
Coon MJ: Cytochrome P450: Nature’s most versatile biological catalyst. Annu Rev Pharmacol Toxicol 2005;4:1.
Harris DA: Bioenergetics at a Glance: An Illustrated Introduction. Blackwell Publishing, 1995.
Johnson F, Giulivi C: Superoxide dismutases and their impact upon human health. Mol Aspects Med 2005;26.
Nicholls DG, Ferguson SJ: Bioenergetics3. Academic Press, London 2002.
Raha S, Robinson BH: Mitochondria, oxygen free radicals, disease and aging. Trends Biochem Sci 2000;25:502.
*The term “oxidase” is sometimes used collectively to denote all enzymes that catalyze reactions involving molecular oxygen.