Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION II. Bioenergetics & the Metabolism of Carbohydrates & Lipids
Chapter 14. Carbohydrates of Physiologic Significance
David A. Bender, PhD & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Explain what is meant by the terms monosaccharide, disaccharide, oligosaccharide and polysaccharide.
Explain what is meant by the terms monosaccharide, disaccharide, oligosaccharide and polysaccharide.
![]() Explain the different ways in which the structures of glucose and other monosaccharides can be represented, and describe the various types of isomerism of sugars and the pyranose and furanose ring structures.
Explain the different ways in which the structures of glucose and other monosaccharides can be represented, and describe the various types of isomerism of sugars and the pyranose and furanose ring structures.
![]() Describe the formation of glycosides and the structures of the important disaccharides and polysaccharides.
Describe the formation of glycosides and the structures of the important disaccharides and polysaccharides.
![]() Explain what is meant by the glycemic index of a carbohydrate.
Explain what is meant by the glycemic index of a carbohydrate.
![]() Describe the roles of carbohydrates in cell membranes and lipoproteins.
Describe the roles of carbohydrates in cell membranes and lipoproteins.
BIOMEDICAL IMPORTANCE
Carbohydrates are widely distributed in plants and animals; they have important structural and metabolic roles. In plants, glucose is synthesized from carbon dioxide and water by photosynthesis and stored as starch or used to synthesize the cellulose of the plant cell walls. Animals can synthesize carbohydrates from amino acids, but most are derived ultimately from plants. Glucose is the most important carbohydrate; most dietary carbohydrate is absorbed into the bloodstream as glucose formed by hydrolysis of dietary starch and disaccharides, and other sugars are converted to glucose in the liver. Glucose is the major metabolic fuel of mammals (except ruminants) and a universal fuel of the fetus. It is the precursor for synthesis of all the other carbohydrates in the body, including glycogen for storage; ribose and deoxyribose in nucleic acids; galactose for synthesis of lactose in milk, in glycolipids, and in combination with protein in glycoproteins and proteoglycans. Diseases associated with carbohydrate metabolism include diabetes mellitus, galactosemia, glycogen storage diseases, and lactose intolerance.
CARBOHYDRATES ARE ALDEHYDE OR KETONE DERIVATIVES OF POLYHYDRIC ALCOHOLS
Carbohydrates are classified as follows:
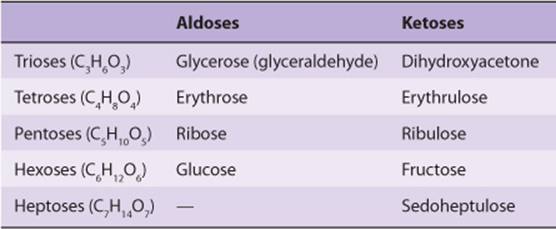
1. Monosaccharides are those sugars that cannot be hydrolyzed into simpler carbohydrates. They may be classified as trioses, tetroses, pentoses, hexoses, or heptoses, depending upon the number of carbon atoms (3-7), and as aldoses or ketoses, depending upon whether they have an aldehyde or ketone group. Examples are listed in Table 14-1. In addition to aldehydes and ketones, the polyhydric alcohols (sugar alcohols or polyols), in which the aldehyde or ketone group has been reduced to an alcohol group, also occur naturally in foods. They are synthesized by reduction of monosaccharides for use in the manufacture of foods for weight reduction and for diabetics. They are poorly absorbed, and have about half the energy yield of sugars.
TABLE 14–1 Classification of Important Sugars
2. Disaccharides are condensation products of two monosaccharide units, for example, lactose, maltose, sucrose, and trehalose.
3. Oligosaccharides are condensation products of three to ten monosaccharides. Most are not digested by human enzymes.
4. Polysaccharides are condensation products of more than ten monosaccharide units; examples are the starches and dextrins, which may be linear or branched polymers. Polysaccharides are sometimes classified as hexosans or pentosans, depending on the identity of the constituent monosaccharides (hexoses and pentoses, respectively). In addition to starches and dextrins, foods contain a wide variety of other polysaccharides that are collectively known as nonstarch polysaccharides; they are not digested by human enzymes, and are the major component of dietary fiber. Examples are cellulose from plant cell walls (a glucose polymer) and inulin, the storage carbohydrate in some plants (a fructose polymer).
BIOMEDICALLY, GLUCOSE IS THE MOST IMPORTANT MONOSACCHARIDE
The Structure of Glucose Can Be Represented in Three Ways
The straight-chain structural formula (aldohexose; Figure 14–1A) can account for some of the properties of glucose, but a cyclic structure (a hemiacetal formed by reaction between the aldehyde group and a hydroxyl group) is thermodynamically favored and accounts for other properties. The cyclic structure is normally drawn as shown in Figure 14–1B, the Haworth projection, in which the molecule is viewed from the side and above the plane of the ring; the bonds nearest to the viewer are bold and thickened, and the hydroxyl groups are above or below the plane of the ring. The six-membered ring containing one oxygen atom is actually in the form of a chair (Figure 14–1C).
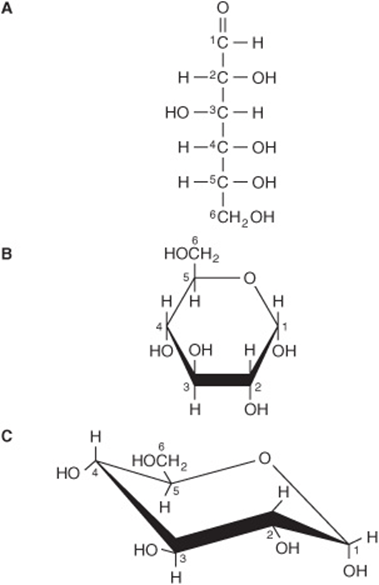
FIGURE 14–1 D-Glucose. (A) Straight-chain form. (B) α-D-glucose; Haworth projection. (C) α-D-glucose; chair form.
Sugars Exhibit Various Forms of Isomerism
Glucose, with four asymmetric carbon atoms, can form 16 isomers. The more important types of isomerism found with glucose are as follows.
1. D and L isomerism: The designation of a sugar isomer as the D form or of its mirror image as the L form is determined by its spatial relationship to the parent compound of the carbohydrates, the three-carbon sugar glycerose (glyceraldehyde). The L and D forms of this sugar, and of glucose, are shown in Figure 14–2. The orientation of the—H and—OH groups around the carbon atom adjacent to the terminal primary alcohol carbon (carbon 5 in glucose) determines whether the sugar belongs to the Dor Lseries. When the—OH group on this carbon is on the right (as seen in Figure 14–2), the sugar is the Disomer; when it is on the left, it is the Lisomer. Most of the naturally occurring monosaccharides are Dsugars, and the enzymes responsible for their metabolism are specific for this configuration.
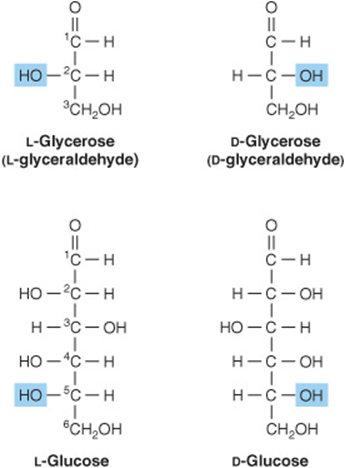
FIGURE 14–2 D-and L-isomerism of glycerose and glucose.
The presence of asymmetric carbon atoms also confers optical activity on the compound. When a beam of planepolarized light is passed through a solution of an optical isomer, it rotates either to the right, dextrorotatory (+), or to the left, levorotatory (-). The direction of rotation of polarized light is independent of the stereochemistry of the sugar, so it may be designated D(-), D(+), L(-), or For example, the naturally occurring form of fructose is the D(-) isomer. In solution, glucose is dextrorotatory, and glucose solutions are sometimes known as dextrose.
2. Pyranose and furanose ring structures: The ring structures of monosaccharides are similar to the ring structures of either pyran (a six-membered ring) or furan (a five-membered ring) (Figures 14-3 and 14-4). For glucose in solution, more than 99% is in the pyranose form.
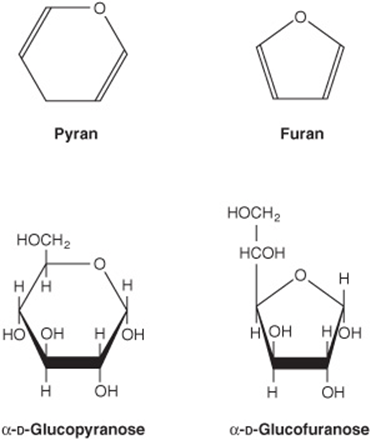
FIGURE 14–3 Pyranose and furanose forms of glucose.
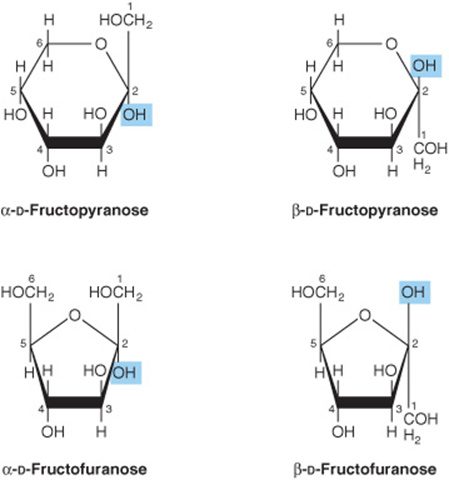
FIGURE 14–4 Pyranose and furanose forms of fructose.
3. Alpha and beta anomers: The ring structure of an aldose is a hemiacetal, since it is formed by combination of an aldehyde and an alcohol group. Similarly, the ring structure of a ketose is a hemiketal. Crystalline glucose is α-D-glucopyranose. The cyclic structure is retained in the solution, but isomerism occurs about position 1, the carbonyl or anomeric carbon atom, to give a mixture of α-glucopyranose (38%) and β-glucopyranose (62%). Less than 0.3% is represented by α and β anomers of glucofuranose.
4. Epimers: Isomers differing as a result of variations in configuration of the—OH and—H on carbon atoms 2, 3, and 4 of glucose are known as epimers. Biologically, the most important epimers of glucose are mannose (epimerized at carbon 2) and galactose (epimerized at carbon 4) (Figure 14–5).
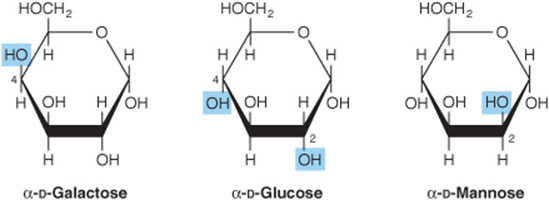
FIGURE 14–5 Epimers of glucose.
5. Aldose-ketose isomerism: Fructose has the same molecular formula as glucose but differs in its structure, since there is a potential keto group in position 2, the anomeric carbon of fructose (Figures 14-4 and 14-6), whereas in glucose there is a potential aldehyde group in position 1, the anomeric carbon (Figures 14-2 and 14-7).
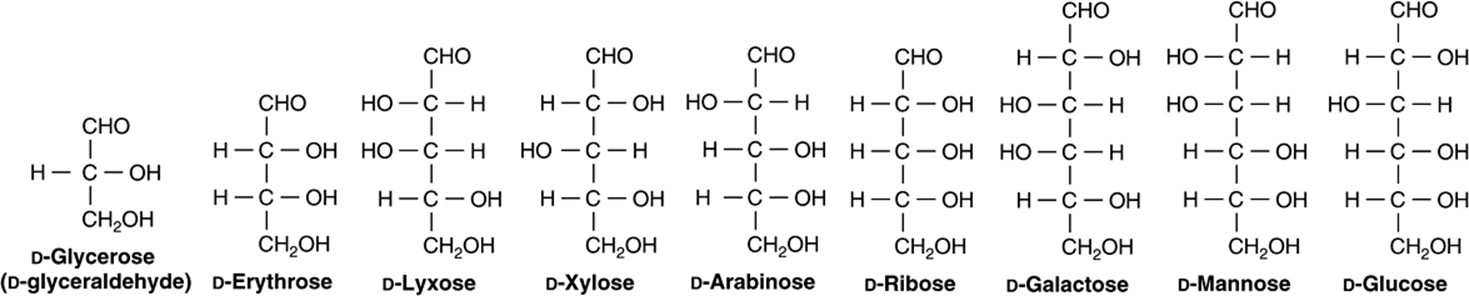
FIGURE 14–6 Examples of aldoses of physiologic significance.
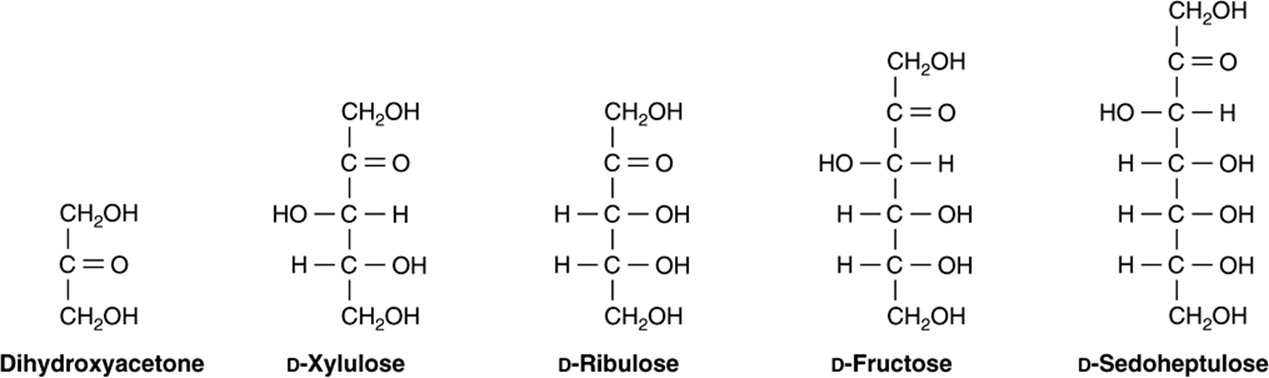
FIGURE 14–7 Examples of ketoses of physiologic significance.
Many Monosaccharides Are Physiologically Important
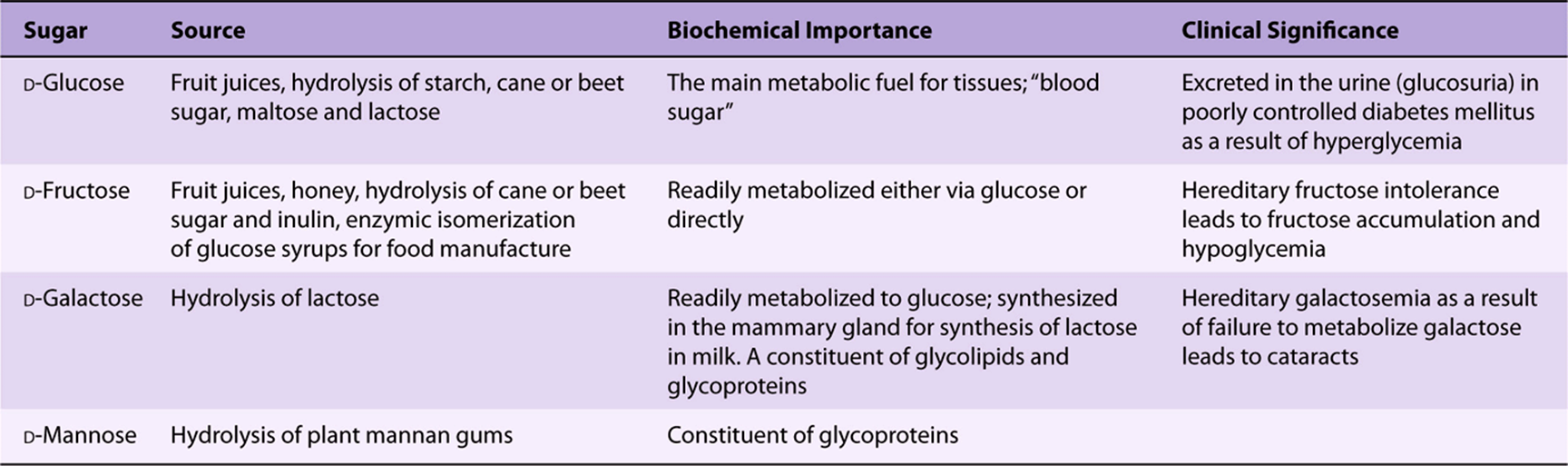
Derivatives of trioses, tetroses, and pentoses and of a seven-carbon sugar (sedoheptulose) are formed as metabolic intermediates in glycolysis (Chapter 18) and the pentose phosphate pathway (Chapter 21). Pentoses are important in nucleotides, nucleic acids, and several coenzymes (Table 14-2). Glucose, galactose, fructose, and mannose are physiologically the most important hexoses (Table 14-3). The biochemically important ketoses are shown in Figure 14–6, and aldoses in Figure 14–7.
TABLE 14–2 Pentoses of Physiologic Importance
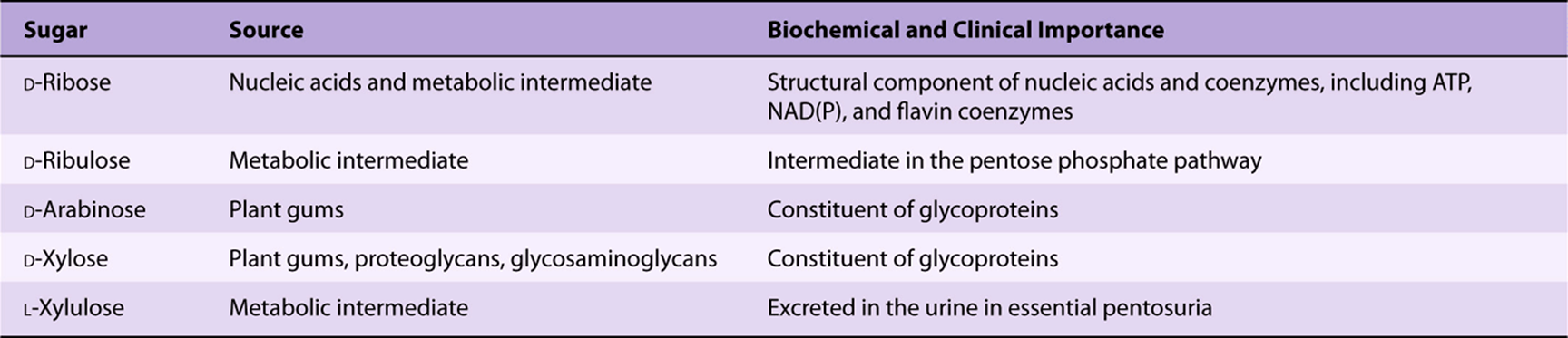
TABLE 14–3 Hexoses of Physiologic Importance
In addition, carboxylic acid derivatives of glucose are important, including D-glucuronate (for glucuronide formation and in glycosaminoglycans) and its metabolic derivative, L-iduronate (in glycosaminoglycans) (Figure 14–8)and L-gulonate (an intermediate in the uronic acid pathway; see Figure 21–4).
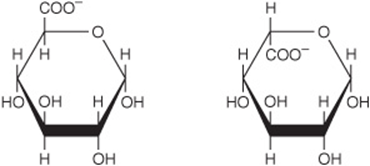
FIGURE 14–8 α-D-Glucuronate (left) and β-L-iduronate (right).
Sugars Form Glycosides with Other Compounds & with Each Other
Glycosides are formed by condensation between the hydroxyl group of the anomeric carbon of a monosaccharide, and a second compound that may or may not (in the case of an aglycone) be another monosaccharide. If the second group is a hydroxyl, the O-glycosidic bond is an acetal link because it results from a reaction between a hemiacetal group (formed from an aldehyde and an—OH group) and another—OH group. If the hemiacetal portion is glucose, the resulting compound is a glucoside; if galactose, a galactoside; and so on. If the second group is an amine, an N-glycosidic bond is formed, for example, between adenine and ribose in nucleotides such as ATP (Figure 11–4).
Glycosides are widely distributed in nature; the aglycone may be methanol, glycerol, a sterol, a phenol, or a base such as adenine. The glycosides that are important in medicine because of their action on the heart (cardiac glycosides) all contain steroids as the aglycone. These include derivatives of digitalis and strophanthus such as ouabain, an inhibitor of the Na+-K+-ATPase of cell membranes. Other glycosides include antibiotics such as streptomycin.
Deoxy Sugars Lack an Oxygen Atom
Deoxy sugars are those in which one hydroxyl group has been replaced by hydrogen. An example is deoxyribose (Figure 14–9) in DNA. The deoxy sugar L-fucose (Figure 14–13) occurs in glycoproteins; 2-deoxyglucose is used experimentally as an inhibitor of glucose metabolism.
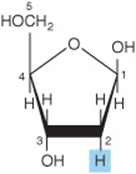
FIGURE 14–9 2-Deoxy-D-ribofuranose (βform).
Amino Sugars (Hexosamines) Are Components of Glycoproteins, Gangliosides, & Glycosaminoglycans
The amino sugars include D-glucosamine, a constituent of hyaluronic acid (Figure 14–10), D-galactosamine (also known as chondrosamine), a constituent of chondroitin, and D-mannosamine. Several antibiotics (eg, erythromycin) contain amino sugars, which are important for their antibiotic activity.
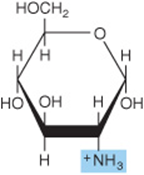
FIGURE 14–10 Glucosamine (2-amino-D-glucopyranose) (α form). Galactosamine is 2-amino-D-galactopyranose. Both glucosamine and galactosamine occur as N-acetyl derivatives in more complex carbohydrates, for example, glycoproteins.
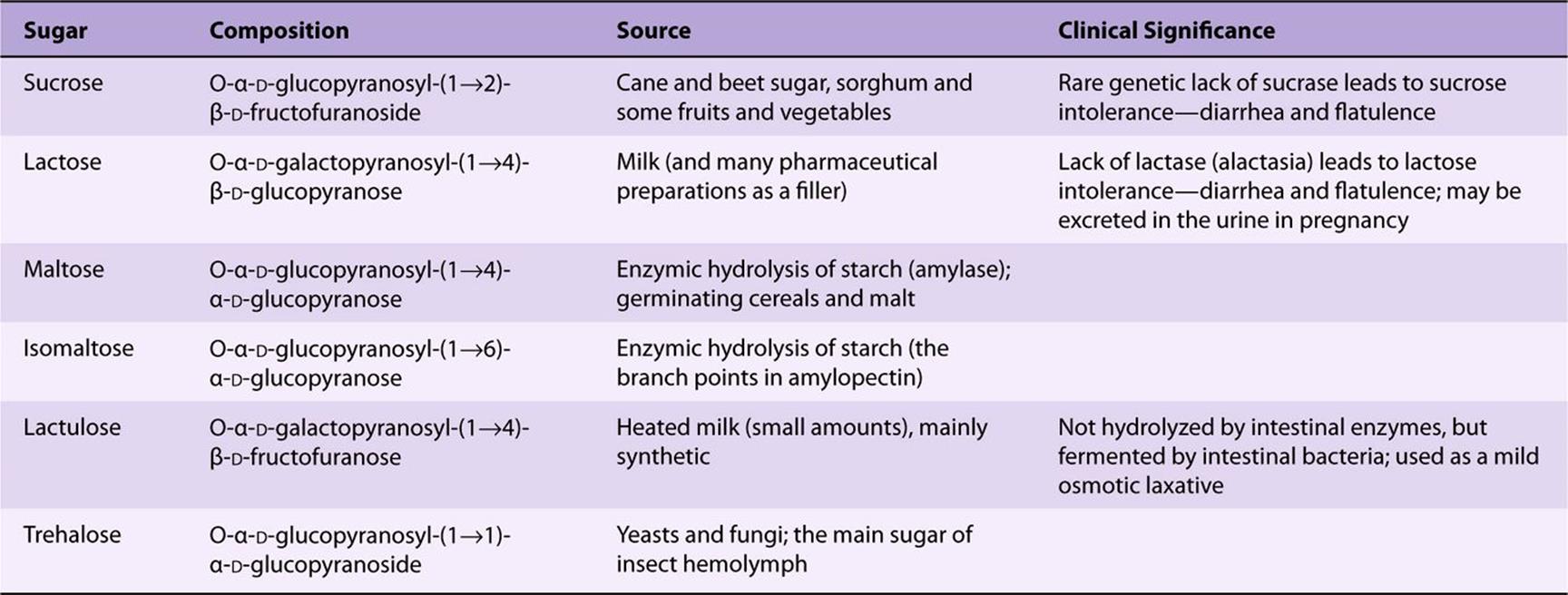
Maltose, Sucrose, & Lactose Are Important Disaccharides
The disaccharides are sugars composed of two monosaccharide residues linked by a glycoside bond (Figure 14–11). The physiologically important disaccharides are maltose, sucrose, and lactose (Table 14-4). Hydrolysis of sucrose yields a mixture of glucose and fructose called “invert sugar” because fructose is strongly levorotatory and changes (inverts) the weaker dextrorotatory action of sucrose.
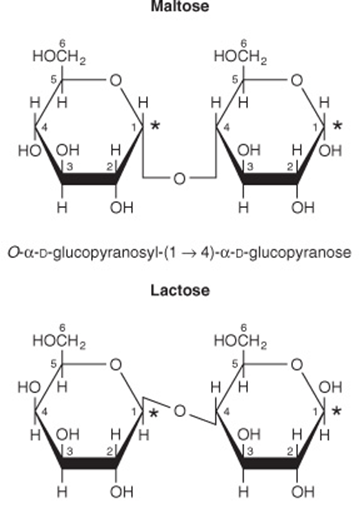
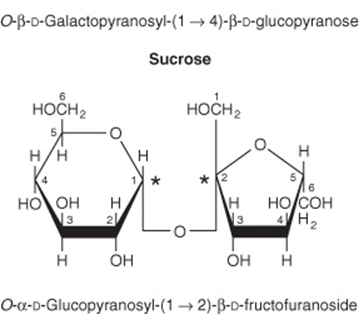
FIGURE 14–11 Structures of important disaccharides. α and β refer to the configuration at the anomeric carbon atom (*). When the anomeric carbon of the second residue takes part in the formation of the glycosidic bond, as in sucrose, the residue becomes a glycoside known as a furanoside or a pyranoside. As the disaccharide no longer has an anomeric carbon with a free potential aldehyde or ketone group, it no longer exhibits reducing properties. The configuration of the β-fructofuranose residue in sucrose results from turning the β-fructofuranose molecule depicted in Figure 14–4 through 180° and inverting it.
TABLE 14–4 Disaccharides of Physiologic Importance
POLYSACCHARIDES SERVE STORAGE & STRUCTURAL FUNCTIONS
Polysaccharides include the following physiologically important carbohydrates:
Starch is a homopolymer of glucose forming an α-glucosidic chain, called a glucosan or glucan. It is the most important dietary carbohydrate in cereals, potatoes, legumes, and other vegetables. The two main constituents are amylose (13-20%), which has a nonbranching helical structure, and amylopectin (80-87%), which consists of branched chains composed of 24-30 glucose residues with α1 → 4 linkages in the chains and by α1 → 6 linkages at the branch points (Figure 14–12).
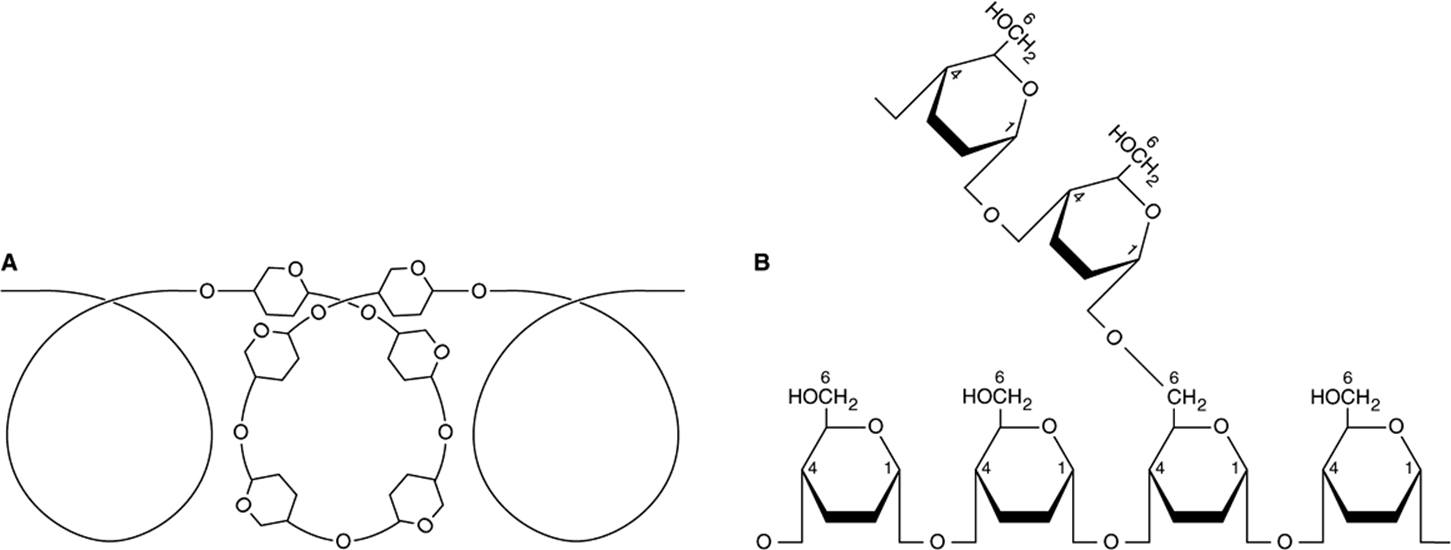
FIGURE 14–12 Structure of starch. (A) Amylose, showing helical coil structure. (B) Amylopectin, showing 1 → 6 branch point.
The extent to which starch in foods is hydrolyzed by amylase is determined by its structure, the degree of crystallization or hydration (the result of cooking), and whether it is enclosed in intact (and indigestible) plant cells walls. The glycemic index of a starchy food is a measure of its digestibility, based on the extent to which it raises the blood concentration of glucose compared with an equivalent amount of glucose or a reference food such as white bread or boiled rice. Glycemic index ranges from 1 (or 100%) for starches that are readily hydrolyzed in the small intestine to 0 for those that are not hydrolysed at all.
Glycogen (Figure 14–13) is the storage polysaccharide in animals and is sometimes called animal starch. It is a more highly branched structure than amylopectin with chains of 12-14 α-D-glucopyranose residues (in α1 → 4 glucosidic linkage) with branching by means of α1 → 6 glucosidic bonds. Muscle glycogen granules (β-particles) are spherical and contain up to 60,000 glucose residues; in liver there are similar granules and also rosettes of glycogen granules that appear to be aggregated β-particles.
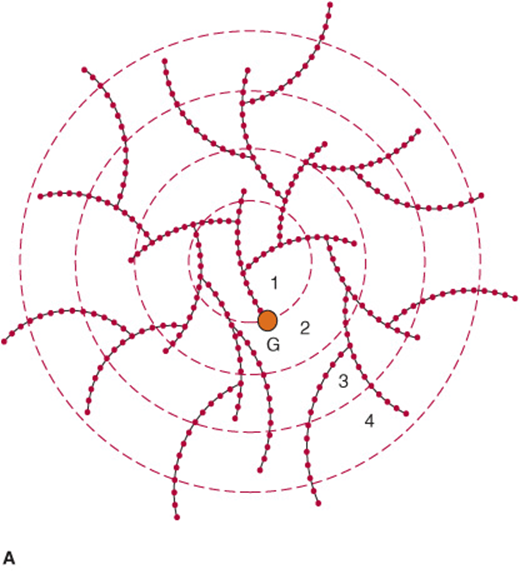
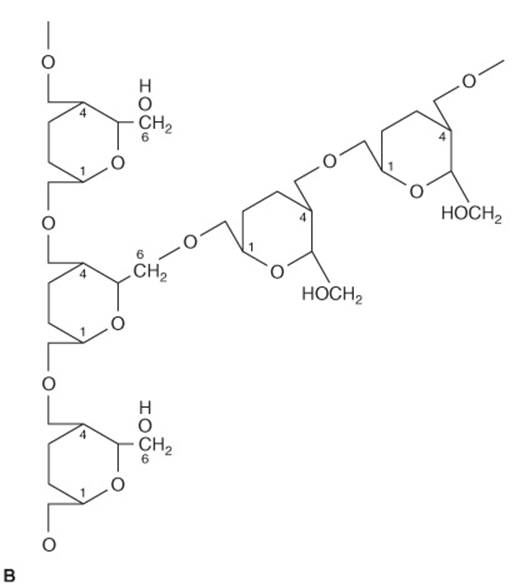
FIGURE 14–13 The glycogen molecule. (A) General structure. (B) Enlargement of structure at a branch point. The molecule is a sphere ~21 nm in diameter that can be seen in electron micrographs. It has a molecular mass of ~107Da and consists of polysaccharide chains, each containing about 13 glucose residues. The chains are either branched or unbranched and are arranged in 12 concentric layers (only four are shown in the figure). The branched chains (each has two branches) are found in the inner layers and the unbranched chains in the outer layer. (G, glycogenin, the primer molecule for glycogen synthesis.)
Inulin is a polysaccharide of fructose (and hence a fructosan) found in tubers and roots of dahlias, artichokes, and dandelions. It is readily soluble in water and is used to determine the glomerular filtration rate, but it is not hydrolyzed by intestinal enzymes. Dextrins are intermediates in the hydrolysis of starch. Cellulose is the chief constituent of plant cell walls. It is insoluble and consists of β-D-glucopyranose units linked by β1 → 4 bonds to form long, straight chains strengthened by cross-linking hydrogen bonds. Mammals lack any enzyme that hydrolyzes the β1 → 4 bonds, and so cannot digest cellulose. It is an important source of “bulk” in the diet, and the major component of dietary fiber. Microorganisms in the gut of ruminants and other herbivores can hydrolyze the linkage and ferment the products to short-chain fatty acids as a major energy source. There is some bacterial metabolism of cellulose in the human colon. Chitin is a structural polysaccharide in the exoskeleton of crustaceans and insects, and also in mushrooms. It consists of N-acetyl-D-glucosamine units joined by β1 → 4 glycosidic bonds (Figure 14–14).
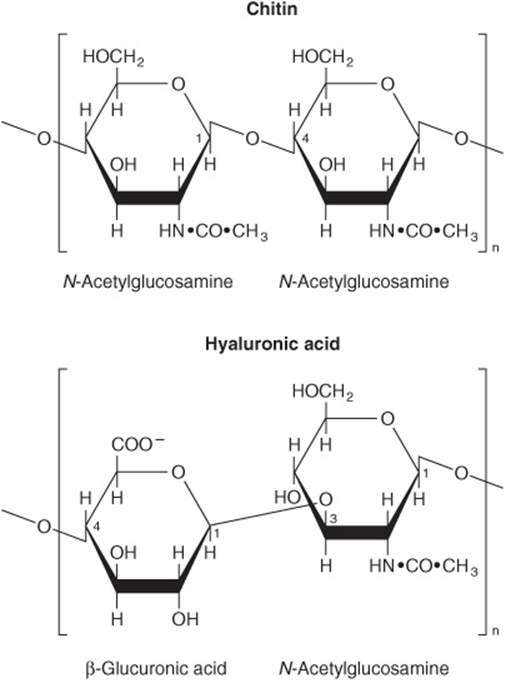
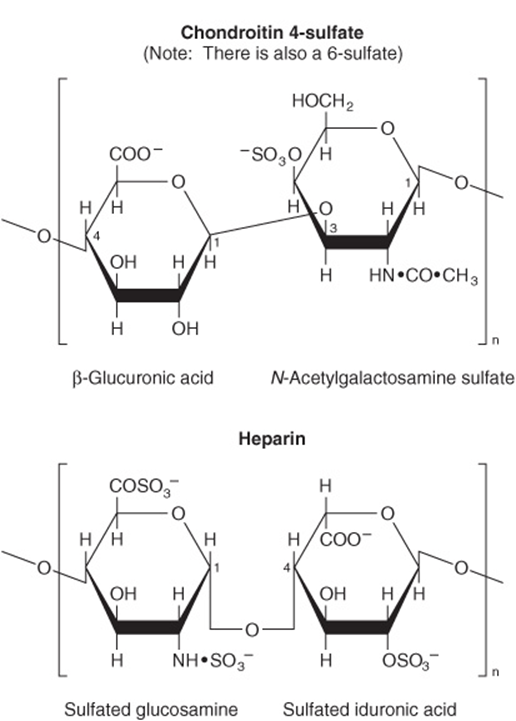
FIGURE 14–14 Structure of some complex polysaccharides and glycosaminoglycans.
Glycosaminoglycans (mucopolysaccharides) are complex carbohydrates containing amino sugars and uronic acids. They may be attached to a protein molecule to form a proteoglycan. Proteoglycans provide the ground or packing substance of connective tissue. They hold large quantities of water and occupy space, thus cushioning or lubricating other structures, because of the large number of—OH groups and negative charges on the molecule which, by repulsion, keep the carbohydrate chains apart. Examples are hyaluronic acid, chondroitin sulfate, and heparin (Figure 14–14).
Glycoproteins (also known as mucoproteins) are proteins containing branched or unbranched oligosaccharide chains (Table 14-5, Figure 14–15); they occur in cell membranes (Chapters 40 and 47) and many other situations; serum albumin is a glycoprotein. The sialic acids are N- or O-acyl derivatives of neuraminic acid (Figure 14–16). Neuraminic acid is a nine-carbon sugar derived from mannosamine (an epimer of glucosamine) and pyruvate. Sialic acids are constituents of both glycoproteins and gangliosides.
TABLE 14–5 Carbohydrates Found in Glycoproteins
FIGURE 14–15 β-L-Fucose (6-deoxy-β-L-galactose).
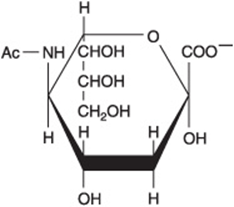
FIGURE 14–16 Structure of N-acetylneuraminic acid, a sialic acid ![]()
CARBOHYDRATES OCCUR IN CELL MEMBRANES & IN LIPOPROTEINS
Approximately 5% of the weight of cell membranes is carbohydrate in glycoproteins and glycolipids. Their presence on the outer surface of the plasma membrane (the glycocalyx) has been shown with the use of plant lectins, protein agglutinins that bind specific glycosyl residues. For example, concanavalin A binds α-glucosyl and α-mannosyl residues. Glycophorin is a major integral membrane glycoprotein of human erythrocytes. It has 130 amino acid residues and spans the lipid membrane, with polypeptide regions outside both the external and internal (cytoplasmic) surfaces. Carbohydrate chains are attached to the amino terminal portion outside the external surface. Carbohydrates are also present in apo-protein B of plasma lipoproteins.
SUMMARY
![]() Carbohydrates are major constituents of animal food and animal tissues. They are characterized by the type and number of monosaccharide residues in their molecules.
Carbohydrates are major constituents of animal food and animal tissues. They are characterized by the type and number of monosaccharide residues in their molecules.
![]() Glucose is the most important carbohydrate in mammalian biochemistry because nearly all carbohydrate in food is converted to glucose for metabolism.
Glucose is the most important carbohydrate in mammalian biochemistry because nearly all carbohydrate in food is converted to glucose for metabolism.
![]() Sugars have large numbers of stereoisomers because they contain several asymmetric carbon atoms.
Sugars have large numbers of stereoisomers because they contain several asymmetric carbon atoms.
![]() The physiologically important monosaccharides include glucose, the “blood sugar,” and ribose, an important constituent of nucleotides and nucleic acids.
The physiologically important monosaccharides include glucose, the “blood sugar,” and ribose, an important constituent of nucleotides and nucleic acids.
![]() The important disaccharides include maltose (glucosyl glucose), an intermediate in the digestion of starch; sucrose (glucosyl fructose), important as a dietary constituent containing fructose; and lactose (galactosyl glucose), in milk.
The important disaccharides include maltose (glucosyl glucose), an intermediate in the digestion of starch; sucrose (glucosyl fructose), important as a dietary constituent containing fructose; and lactose (galactosyl glucose), in milk.
![]() Starch and glycogen are storage polymers of glucose in plants and animals, respectively. Starch is the major source of energy in the diet.
Starch and glycogen are storage polymers of glucose in plants and animals, respectively. Starch is the major source of energy in the diet.
![]() Complex carbohydrates contain other sugar derivatives such as amino sugars, uronic acids, and sialic acids. They include proteoglycans and glycosaminoglycans, which are associated with structural elements of the tissues, and glycoproteins, which are proteins containing oligosaccharide chains; they are found in many situations including the cell membrane.
Complex carbohydrates contain other sugar derivatives such as amino sugars, uronic acids, and sialic acids. They include proteoglycans and glycosaminoglycans, which are associated with structural elements of the tissues, and glycoproteins, which are proteins containing oligosaccharide chains; they are found in many situations including the cell membrane.
REFERENCES
Champ M, Langkilde A-M, Brouns F, et al: Advances in dietary fibre characterisation. Nutrition Res Rev 2003;16:(1)71-82.
Davis BG, Fairbanks AJ: Carbohydrate Chemistry. Oxford University Press, 2002.
Kiessling LL, Splain RA: Chemical approaches to glycobiology. Ann Rev Biochem 2010;79:619-53.
Lindhorst TK, Thisbe K: Essentials of Carbohydrate Chemistry and Biochemistry, 3rd ed. Wiley-VCH, 2007.
Sinnott M: Carbohydrate Chemistry and Biochemistry: Structure and Mechanisms, Royal Society of Chemistry, 2007.