CHEMISTRY THE CENTRAL SCIENCE
1 INTRODUCTION: MATTER AND MEASUREMENT
1.2 CLASSIFICATIONS OF MATTER
Let's begin our study of chemistry by examining some fundamental ways in which matter is classified. Two principal ways of classifying matter are according to physical state (gas, liquid, or solid) and according to composition (element, compound, or mixture).
States of Matter
A sample of matter can be a gas, a liquid, or a solid. These three forms, called the states of matter, differ in some of their observable properties. A gas (also known as vapor) has no fixed volume or shape; rather, it conforms to the volume and shape of its container. A gas can be compressed to occupy a smaller volume, or it can expand to occupy a larger one. A liquid has a distinct volume independent of its container but has no specific shape. It assumes the shape of the portion of the container it occupies. A solid has both a definite shape and a definite volume. Neither liquids nor solids can be compressed to any appreciable extent.
The properties of the states of matter can be understood on the molecular level (![]() FIGURE 1.4). In a gas the molecules are far apart and moving at high speeds, colliding repeatedly with one another and with the walls of the container. Compressing a gas decreases the amount of space between molecules and increases the frequency of collisions between molecules but does not alter the size or shape of the molecules. In a liquid the molecules are packed closely together but still move rapidly. The rapid movement allows the molecules to slide over one another; thus, a liquid pours easily. In a solid the molecules are held tightly together, usually in definite arrangements in which the molecules can wiggle only slightly in their otherwise fixed positions. Thus, the distances between molecules are similar in the liquid and solid states, but the two states differ in how free the molecules are to move around. Changes in temperature and/or pressure can lead to conversion from one state of matter to another, illustrated by such familiar processes as ice melting or water vapor condensing.
FIGURE 1.4). In a gas the molecules are far apart and moving at high speeds, colliding repeatedly with one another and with the walls of the container. Compressing a gas decreases the amount of space between molecules and increases the frequency of collisions between molecules but does not alter the size or shape of the molecules. In a liquid the molecules are packed closely together but still move rapidly. The rapid movement allows the molecules to slide over one another; thus, a liquid pours easily. In a solid the molecules are held tightly together, usually in definite arrangements in which the molecules can wiggle only slightly in their otherwise fixed positions. Thus, the distances between molecules are similar in the liquid and solid states, but the two states differ in how free the molecules are to move around. Changes in temperature and/or pressure can lead to conversion from one state of matter to another, illustrated by such familiar processes as ice melting or water vapor condensing.
![]() GO FIGURE
GO FIGURE
In which form of water are the water molecules farthest apart?
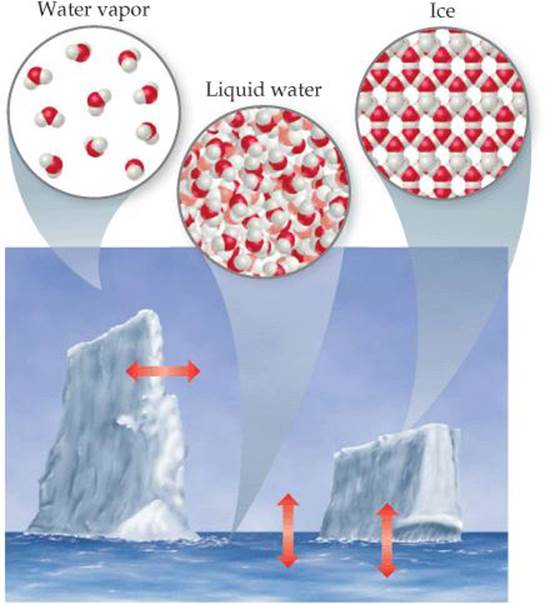
![]() FIGURE 1.4 The three physical states of water—water vapor, liquid water, and ice. We see the liquid and solid states but cannot see the gas (vapor) state. When we look at steam or clouds, we see tiny droplets of liquid water dispersed in the atmosphere. The red arrows show that the three states of matter interconvert.
FIGURE 1.4 The three physical states of water—water vapor, liquid water, and ice. We see the liquid and solid states but cannot see the gas (vapor) state. When we look at steam or clouds, we see tiny droplets of liquid water dispersed in the atmosphere. The red arrows show that the three states of matter interconvert.
Pure Substances
Most forms of matter we encounter—the air we breathe (a gas), the gasoline we burn in our cars (a liquid), and the sidewalk we walk on (a solid)—are not chemically pure. We can, however, separate these forms of matter into pure substances. A pure substance (usually referred to simply as a substance) is matter that has distinct properties and a composition that does not vary from sample to sample. Water and table salt (sodium chloride), the primary components of seawater, are examples of pure substances.
All substances are either elements or compounds. Elements are substances that cannot be decomposed into simpler substances. On the molecular level, each element is composed of only one kind of atom [![]() FIGURE 1.5 (a and b)]. Compounds are substances composed of two or more elements; they contain two or more kinds of atoms [Figure 1.5(c)]. Water, for example, is a compound composed of two elements: hydrogen and oxygen. Figure 1.5(d) shows a mixture of substances. Mixtures are combinations of two or more substances in which each substance retains its chemical identity.
FIGURE 1.5 (a and b)]. Compounds are substances composed of two or more elements; they contain two or more kinds of atoms [Figure 1.5(c)]. Water, for example, is a compound composed of two elements: hydrogen and oxygen. Figure 1.5(d) shows a mixture of substances. Mixtures are combinations of two or more substances in which each substance retains its chemical identity.
Elements
Currently, 118 elements are known, though they vary widely in abundance. For example, only five elements—oxygen, silicon, aluminum, iron, and calcium—account for over 90% of Earth's crust (including oceans and atmosphere) and only three—oxygen, carbon, and hydrogen—account for over 90% of the mass of the human body (![]() FIGURE 1.6).
FIGURE 1.6).
![]() GO FIGURE
GO FIGURE
How do the molecules of a compound differ from the molecules of an element?
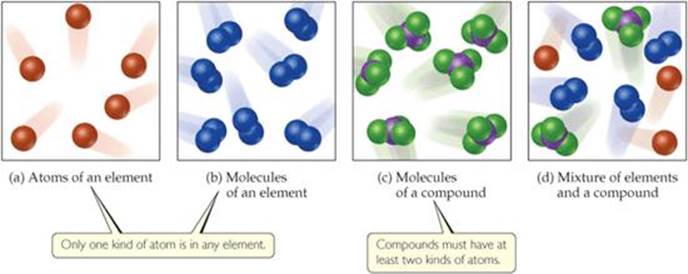
![]() FIGURE 1.5 Molecular comparison of elements, compounds, and mixtures.
FIGURE 1.5 Molecular comparison of elements, compounds, and mixtures.
![]() GO FIGURE
GO FIGURE
Can you name two significant differences between the elemental composition of Earth's crust and the elemental composition of the human body?
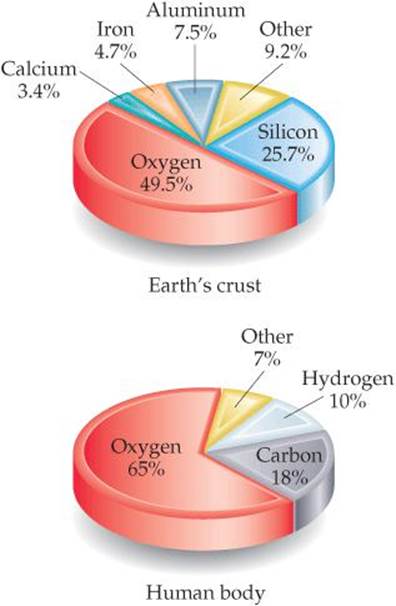
![]() FIGURE 1.6 Relative abundances of elements. Elements in percent by mass in Earth's crust (including oceans and atmosphere) and the human body.
FIGURE 1.6 Relative abundances of elements. Elements in percent by mass in Earth's crust (including oceans and atmosphere) and the human body.
![]() TABLE 1.2 lists some common elements, along with the chemical symbols used to denote them. The symbol for each element consists of one or two letters, with the first letter capitalized. These symbols are derived mostly from the English names of the elements, but sometimes they are derived from a foreign name instead (last column in Table 1.2). You will need to know these symbols and learn others as we encounter them in the text.
TABLE 1.2 lists some common elements, along with the chemical symbols used to denote them. The symbol for each element consists of one or two letters, with the first letter capitalized. These symbols are derived mostly from the English names of the elements, but sometimes they are derived from a foreign name instead (last column in Table 1.2). You will need to know these symbols and learn others as we encounter them in the text.
All of the known elements and their symbols are listed on the front inside cover of this text in a table known as the periodic table. In the periodic table the elements are arranged in columns so that closely related elements are grouped together. We describe the periodic table in more detail in Section 2.5 and consider the periodically repeating properties of the elements in Chapter 7.
Compounds
Most elements can interact with other elements to form compounds. For example, when hydrogen gas burns in oxygen gas, the elements hydrogen and oxygen combine to form the compound water. Conversely, water can be decomposed into its elements by passing an electrical current through it (![]() FIGURE 1.7). Pure water, regardless of its source, consists of 11% hydrogen and 89% oxygen by mass. This macroscopic composition corresponds to the molecular composition, which consists of two hydrogen atoms combined with one oxygen atom:
FIGURE 1.7). Pure water, regardless of its source, consists of 11% hydrogen and 89% oxygen by mass. This macroscopic composition corresponds to the molecular composition, which consists of two hydrogen atoms combined with one oxygen atom:
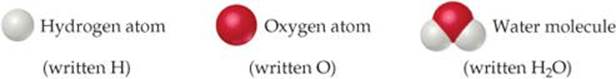
TABLE 1.2 • Some Common Elements and Their Symbols
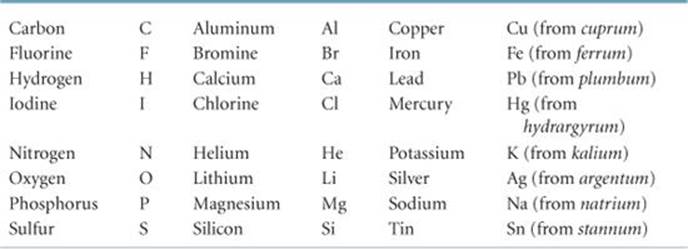
![]() GO FIGURE
GO FIGURE
What is the connection between the relative gas volumes collected in the two tubes and the relative number of gas molecules in the tubes?
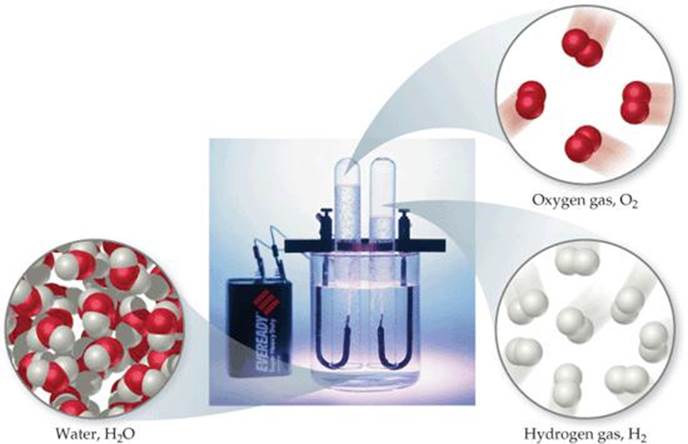
![]() FIGURE 1.7 Electrolysis of water. Water decomposes into its component elements, hydrogen and oxygen, when an electrical current is passed through it. The volume of hydrogen, collected in the right test tube, is twice the volume of oxygen.
FIGURE 1.7 Electrolysis of water. Water decomposes into its component elements, hydrogen and oxygen, when an electrical current is passed through it. The volume of hydrogen, collected in the right test tube, is twice the volume of oxygen.
The elements hydrogen and oxygen themselves exist naturally as diatomic (twoatom) molecules:
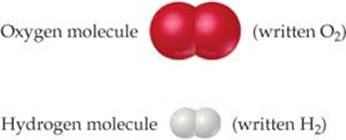
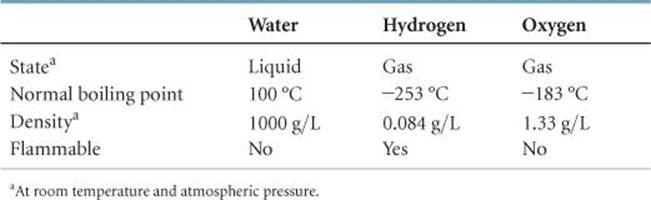
As seen in ![]() TABLE 1.3, the properties of water bear no resemblance to the properties of its component elements. Hydrogen, oxygen, and water are each a unique substance, a consequence of the uniqueness of their respective molecules.
TABLE 1.3, the properties of water bear no resemblance to the properties of its component elements. Hydrogen, oxygen, and water are each a unique substance, a consequence of the uniqueness of their respective molecules.
TABLE 1.3 • Comparison of Water, Hydrogen, and Oxygen
The observation that the elemental composition of a compound is always the same is known as the law of constant composition (or the law of definite proportions). French chemist Joseph Louis Proust (1754–1826) first stated the law in about 1800. Although this law has been known for 200 years, the belief persists among some people that a fundamental difference exists between compounds prepared in the laboratory and the corresponding compounds found in nature. However, a pure compound has the same composition and properties regardless of its source. Both chemists and nature must use the same elements and operate under the same natural laws. When two materials differ in composition or properties, either they are composed of different compounds or they differ in purity.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Hydrogen, oxygen, and water are all composed of molecules. What is it about a molecule of water that makes it a compound, whereas hydrogen and oxygen are elements?
Mixtures
Most of the matter we encounter consists of mixtures of different substances. Each substance in a mixture retains its chemical identity and properties. In contrast to a pure substance, which by definition has a fixed composition, the composition of a mixture can vary. A cup of sweetened coffee, for example, can contain either a little sugar or a lot. The substances making up a mixture are called components of the mixture.
Some mixtures do not have the same composition, properties, and appearance throughout. Rocks and wood, for example, vary in texture and appearance in any typical sample. Such mixtures are heterogeneous [![]() FIGURE 1.8 (a)]. Mixtures that are uniform throughout arehomogeneous. Air is a homogeneous mixture of nitrogen, oxygen, and smaller amounts of other gases. The nitrogen in air has all the properties of pure nitrogen because both the pure substance and the mixture contain the same nitrogen molecules. Salt, sugar, and many other substances dissolve in water to form homogeneous mixtures [Figure 1.8(b)]. Homogeneous mixtures are also called solutions. Although the term solution conjures an image of a liquid, solutions can be solids, liquids, or gases.
FIGURE 1.8 (a)]. Mixtures that are uniform throughout arehomogeneous. Air is a homogeneous mixture of nitrogen, oxygen, and smaller amounts of other gases. The nitrogen in air has all the properties of pure nitrogen because both the pure substance and the mixture contain the same nitrogen molecules. Salt, sugar, and many other substances dissolve in water to form homogeneous mixtures [Figure 1.8(b)]. Homogeneous mixtures are also called solutions. Although the term solution conjures an image of a liquid, solutions can be solids, liquids, or gases.
![]() FIGURE 1.9 summarizes the classification of matter into elements, compounds, and mixtures.
FIGURE 1.9 summarizes the classification of matter into elements, compounds, and mixtures.
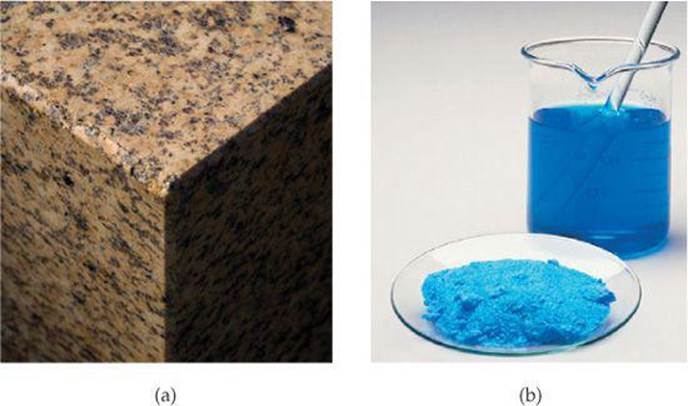
![]() FIGURE 1.8 Mixtures. (a) Many common materials, including rocks, are heterogeneous mixtures. This photograph of granite shows a heterogeneous mixture of silicon dioxide and other metal oxides. (b) Homogeneous mixtures are called solutions. Many substances, including the blue solid shown here [copper(II) sulfate], dissolve in water to form solutions.
FIGURE 1.8 Mixtures. (a) Many common materials, including rocks, are heterogeneous mixtures. This photograph of granite shows a heterogeneous mixture of silicon dioxide and other metal oxides. (b) Homogeneous mixtures are called solutions. Many substances, including the blue solid shown here [copper(II) sulfate], dissolve in water to form solutions.
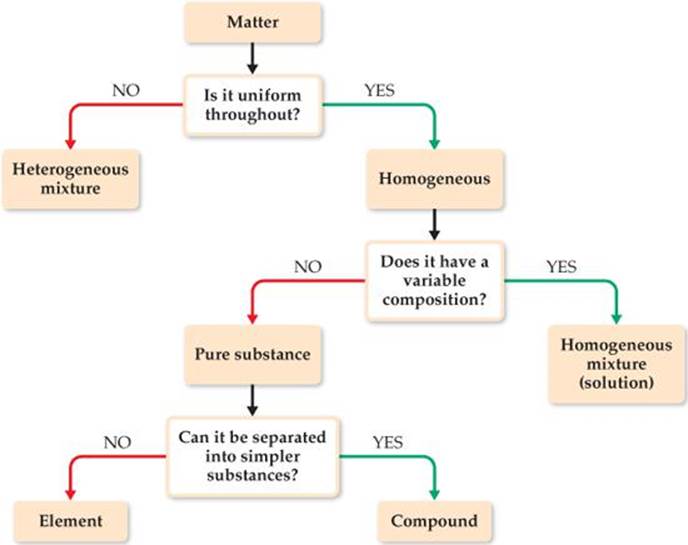
![]() FIGURE 1.9 Classification of matter. All pure matter is classified ultimately as either an element or a compound.
FIGURE 1.9 Classification of matter. All pure matter is classified ultimately as either an element or a compound.
SAMPLE EXERCISE 1.1 Distinguishing among Elements, Compounds, and Mixtures
“White gold” contains gold and a “white” metal, such as palladium. Two samples of white gold differ in the relative amounts of gold and palladium they contain. Both samples are uniform in composition throughout. Use Figure 1.9 to classify white gold.
SOLUTION
Because the material is uniform throughout, it is homogeneous. Because its composition differs for the two samples, it cannot be a compound. Instead, it must be a homogeneous mixture.
PRACTICE EXERCISE
Aspirin is composed of 60.0% carbon, 4.5% hydrogen, and 35.5% oxygen by mass, regardless of its source. Use Figure 1.9 to classify aspirin.
Answer: It is a compound because it has constant composition and can be separated into several elements.