Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
4. Automation in Microreactor Systems
4.3. Automated Optimization with HPLC Sampling
In one recent example of automated optimization, a Knoevenagel condensation reaction (Scheme 4.1) was investigated with in-line high-performance liquid chromatography (HPLC) monitoring, sampling the reactor effluent after a set number of residence times [35]. An objective function based upon production throughput and yield (Equation 4.2a) was optimized using three feedback algorithms: simplex, steepest descent, and SNOBFIT. For this reversible reaction, this objective searches for conditions that rapidly approach equilibrium.
(4.3) ![]()
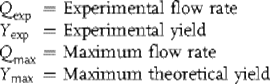
Results for the three optimization methods are shown in Figure 4.2 and summarized in Table 4.1.
Figure 4.2 Optimization results for Knoevenagel example with different optimization algorithms. Boundaries on the reaction variables are denoted by red dashed lines. (a) Simplex method. (b) SNOBFIT. (c) Steepest descent method. Source: Reprinted with permission from the American Chemical Society [35].
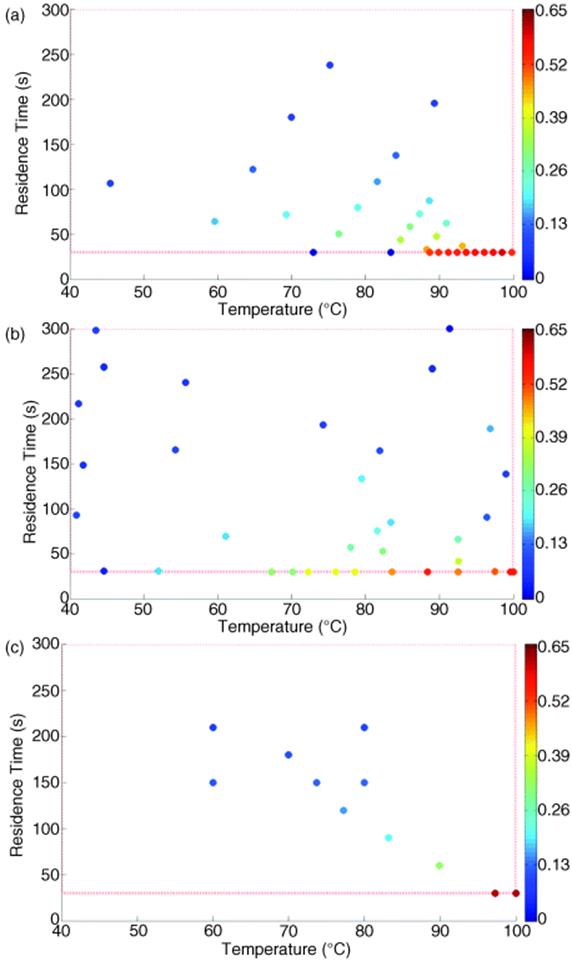
Scheme 4.1 Knoevenagel condensation [36].
Table 4.1 Summary of single-trajectory optimization algorithm performance.
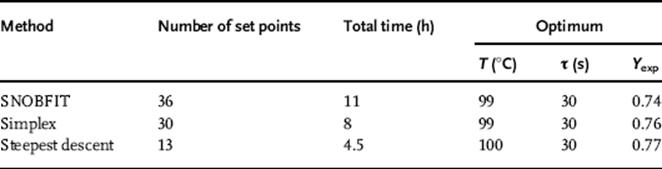
Both the simplex and steepest descent optimizations began at 70 °C and 180 s. From there, the simplex method moved toward conditions at higher temperature and lower residence time. Upon reaching the minimum residence time constraint, the simplex contracted, ultimately becoming a one-dimensional search until it reached the optimum of 99 °C and 30 s near the corner of the design space after 30 experiments. The SNOBFIT algorithm initially performed a randomized, space-filling search, allowing it to examine better the overall design space before focusing on experiments at higher temperature and lower residence time. After 36 experiments, the algorithm reached the same optimum as the simplex method at 99 °C and 30 s. The steepest descent optimization performed an initial DoE (Design of Experiments) to determine the gradient near the initial conditions. The algorithm then performed a line search along the gradient, until becoming constrained and reaching an optimum at the corner of the design space at 100 °C and 30 s after 13 experiments.
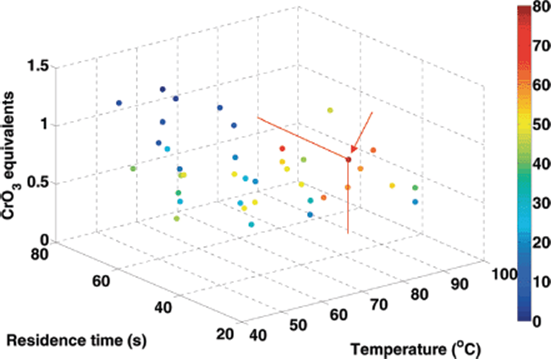
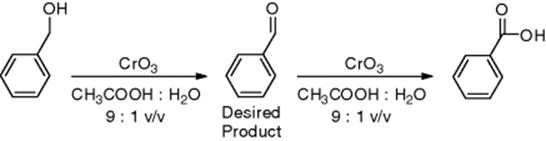
The partial oxidation of benzyl alcohol to benzaldehyde by CrO3 (Scheme 4.2) has been optimized using the simplex method in a parameter space of four variables: temperature, residence time, initial reactant concentration, and CrO3 equivalents. This method began at the specified initial conditions of 50 °C, 60 s residence time, 8 mM benzyl alcohol, and 1 CrO3 equivalent, which resulted in a yield of 21.0%. After 46 experiments, an optimum benzaldehyde yield of 80% was found at 88 °C, 48 s residence time, 8.2 mM benzyl alcohol, and 0.65 CrO3 equivalents (Figure 4.3). These optimal conditions favored shorter residence times at higher temperatures, in contrast to the typical batch conditions of longer times at lower temperatures due to the more facile and tight control of conditions allowed in microreactors.
Figure 4.3 Benzaldehyde yield during four-dimensional simplex optimization. Benzyl alcohol reactant concentration not shown for clarity. Source: Reprinted with permission from the American Chemical Society [35].
Scheme 4.2 Oxidation of benzyl alcohol and benzaldehyde [37].
In another example of application of the simplex method, McMullen et al. [38] demonstrated the rapid optimization and scaling of a Heck reaction using an automated microreactor system with HPLC monitoring and feedback control. Optimal reaction conditions in the microreactor were determined after 19 automated experiments and required a relatively small amount of starting material. The reaction was then successfully scaled up 50-fold from a microreactor to a Corning meso-scale glass reactor using the optimal conditions determined by the microreactor system.