March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 4. Stereochemistry and Conformation
4.B. What Kinds of Molecules Display Optical Activity?
Although the ultimate criterion is, of course, nonsuperimposability on the mirror image (chirality), other tests may be used that are simpler to apply, but not always accurate. One such test is the presence of a plane of symmetry.17 A plane of symmetry18 (also called a mirror plane) is a plane passing through an object such that the part on one side of the plane is the exact reflection of the part on the other side (the plane acting as a mirror). Compounds possessing such a plane are always optically inactive, but there are a few cases known in which compounds lack a plane of symmetry and are nevertheless inactive. Such compounds possess a center of symmetry (e.g., in α-truxillic acid), or an alternating axis of symmetry as in 1.19 A center of symmetry18 is a point within an object such that a straight line drawn from any part or element of the object to the center and extended an equal distance on the other side encounters an equal part or element. An alternating axis of symmetry18 of order n is an axis such that when an object containing such an axis is rotated by 360°/n about the axis and then reflection is effected across a plane at right angles to the axis, a new object is obtained that is indistinguishable from the original one. Compounds that lack an alternating axis of symmetry are always chiral.
A molecule that contains just one stereogenic carbon atom (defined as a carbon atom connected to four different groups; also called a chiral atom or an asymmetric carbon atom) is always chiral, and hence optically active.20 As seen in Fig. 4.1, such a molecule cannot have a plane of symmetry, whatever the identity of the four atoms or groups, as long as they are all different. However, optical activity may be present in molecules with no stereogenic atom.21 Some molecules with two or more steroegenic carbon atoms, however, are superimposable on their mirror images (called meso compounds), and hence inactive principally because there is symmetry. Examples of such compounds will be discussed subsequently.
Optically active compounds may be classified into several categories.
1. Compounds with a Stereogenic Carbon Atom. If there is only one such atom, the molecule must be optically active, no matter how slight the differences are among the four groups. An example is 1,12-dibromo-6-methyldodecane, which has one stereogenic carbon and will be optically active. Optical activity has been detected even in cases22 (e.g., 1-butanol-1-d), where one group is hydrogen and another deuterium:23 The stereogenic carbon is connected to OH, H, D, and a propyl group.
Although enantiomers will exhibit specific rotation of equal magnitude but opposite sign, the difference may be too small to be measured accurately. In optically active compounds, the amount of rotation is greatly dependent on the nature of the four groups, in general increasing with increasing differences in polarizabilities among the groups. Alkyl groups have very similar polarizabilities24 and the optical activity of 5-ethyl-5-propylundecane is too low to be measurable at any wavelength between 280 and 580 nm.25
2. Compounds with Other Quadrivalent Stereogenic Atoms.26 Any molecule with an atom that has four bonds pointing to the corners of a tetrahedron will be optically active if the four groups are different. Among atoms in this category are Si,27 Ge, Sn,28 and N (in quaternary salts or N-oxides).29 In sulfones, the sulfur bonds have a tetrahedral array, but since two of the groups are always oxygen, no chirality results. However, the preparation30 of an optically active sulfone (2) in which one oxygen is 16O and the other is 18O illustrates the point that slight differences in groups are all that is necessary. This point has been taken even further with the preparation of ester 3, both enantiomers of which have been prepared.31 Optically active chiral phosphates (4) have similarly been made.32
3. Compounds with Tervalent Stereogenic Atoms. Atoms with pyramidal bonding33 might be expected to give rise to optical activity if the atom is connected to three different groups, since the unshared pair of electrons is analogous to a fourth group, necessarily different from the others. For example, a secondary or tertiary amine
![]()
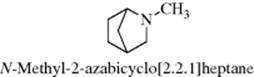
where X, Y, and Z are different and the fourth group is the electron pair would be expected to be chiral and thus resolvable. Many attempts have been made to resolve such compounds, but until 1968 all of them failed because of pyramidal inversion (also called fluxional inversion), which is a rapid oscillation of the unshared pair from one side of the XYZ plane to the other, thus converting the molecule into its enantiomer.34 For ammonia, there are 2 × 1011 inversions every second. The inversion is less rapid in substituted ammonia derivatives35 (amines, amides, etc.). The interconversion barrier for endo versus exo methyl in N-methyl-2-azabicyclo[2.2.1]heptane, for example, is 0.3 kcal mol−1 (1.26 kJ mol−1).36 In this case, torsional strain plays a significant role, along with angle strain, in determining inversion barriers. Two types of nitrogen atom invert particularly slowly, namely, a nitrogen atom in a three-membered ring and a nitrogen atom connected to another atom bearing an
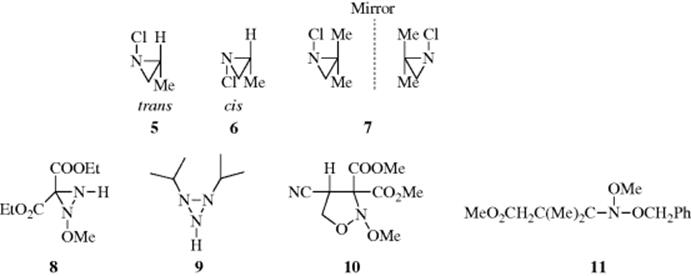
unshared pair. Even in such compounds, however, pyramidal inversion proved too rapid to permit isolation of separate isomers for many years. This goal was accomplished29 only when compounds were synthesized in which both features are combined: a nitrogen atom in a three-membered ring connected to an atom containing an unshared pair. For example, the two isomers of 1-chloro-2-methylaziridine (5 and 6) were separated and do not interconvert at room temperature.37 In suitable cases, this barrier to inversion can result in compounds that are optically active solely because of a chiral tervalent nitrogen atom. For example, 7 has been resolved into its separate enantiomers.38 Note that in this case too, the nitrogen is connected to an atom with an unshared pair. Conformational stability has also been demonstrated for oxaziridines,39 diaziridines (e.g., 8),40 triaziridines (e.g., 9),41 and 1,2-oxazolidines (e.g., 10),42 even though in this case the ring is five membered. However, note that the nitrogen atom in 10 is connected to two oxygen atoms.
Compound 11 is another example in which nitrogen is connected to two oxygen atoms. In this case there is no ring at all, but it has been resolved into (+) and (−) enantiomers ![]() .43 This compound and several similar ones reported in the same paper are the first examples of compounds whose optical activity is solely due to an acyclic tervalent chiral nitrogen atom. However, 11 is not optically stable and racemizes at 20°C with a half-life of 1.22 h. A similar compound (11, with OCH2Ph replaced by OEt) has a longer half-life, 37.5 h at 20°C.
.43 This compound and several similar ones reported in the same paper are the first examples of compounds whose optical activity is solely due to an acyclic tervalent chiral nitrogen atom. However, 11 is not optically stable and racemizes at 20°C with a half-life of 1.22 h. A similar compound (11, with OCH2Ph replaced by OEt) has a longer half-life, 37.5 h at 20°C.
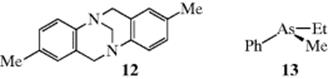
In molecules in which the nitrogen atom is at a bridgehead, pyramidal inversion is of course prevented. Such molecules, if chiral, can be resolved even without the presence of the two structural features noted above. For example, optically active 12 (Tröger's base) has been prepared.44 Phosphorus inverts more slowly and arsenic still more slowly.45 Nonbridgehead phosphorus,46 arsenic, and antimony compounds have also been resolved (e.g., 13).47 Sulfur exhibits pyramidal bonding in sulfoxides, sulfinic esters, sulfonium salts, and sulfites. Examples of each of these have been resolved.48 An interesting example is (+)-Ph12CH2SO13CH2Ph, a sulfoxide in which the two alkyl groups differ only in 12C versus 13C, but which has [α]280 = +0.71°.49 A computational study indicates that base-catalyzed inversion at sulfur in sulfoxides is possible via a tetrahedral intermediate.50
![]()
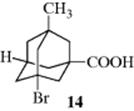
4. Suitably Substituted Adamantanes. Adamantanes bearing four different substituents at the bridgehead positions are chiral and optically active and 14, for example, has been resolved.51 This type of molecule is a kind of expanded tetrahedron and has the same symmetry properties as any other tetrahedron.
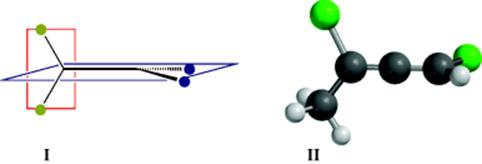
5. Restricted Rotation Giving Rise to Perpendicular Disymmetric Planes. Certain compounds that do not contain asymmetric atoms are nevertheless chiral, as illustrated in Fig. 4.2. For such compounds, there are two perpendicular planes (see I), neither of which can be bisected by a plane of symmetry (as illustrated by II). If either plane could be so bisected, the molecule would be superimposable on its mirror image, since such a plane would be a plane of symmetry. These points will be illustrated by examples.
Biphenyls that contain four large groups in the ortho positions cannot freely rotate about the central bond because of steric hindrance.52 For example, the activation energy (rotational barrier) for the enantiomerization process of the chiral 2-carboxy-2′-methoxy-6-nitrobiphenyl was determined, ΔG‡ = 21.8 ± 0.1 kcal mol−1(91.3 kJ mol−1).53 In such compounds, the two rings are in perpendicular planes. If either ring is symmetrically substituted, the molecule has a plane of symmetry. For example, consider the biaryls:
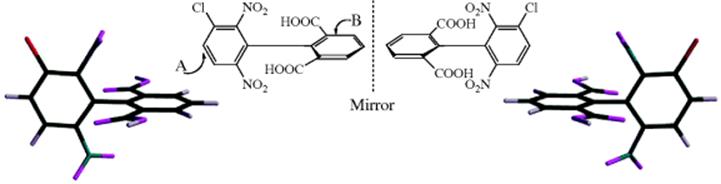
Ring B is symmetrically substituted. A plane drawn perpendicular to ring B contains all the atoms and groups in ring A; hence, it is a plane of symmetry and the compound is achiral. On the other hand, consider
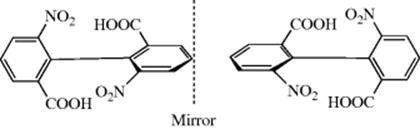
In this molecule, there is no plane of symmetry and the molecule is chiral; many such compounds have been resolved. Note that groups in the para position cannot cause lack of symmetry. Isomers that can be separated only because rotation about single bonds is prevented or greatly slowed are called atropisomers.54 9,9′-Bianthryls also show hindered rotation and exhibit atropisomers.55 Low-temperature NMR is sometimes used to detect atropisomers in certain systems [1,2,4,5-tetra(o-tolyl)benzene, e.g.].56 Configurationally stable atropisomers are known.57
It is not always necessary for four large ortho groups to be present in order for rotation to be prevented. Compounds with three and even two groups, if large enough, can have hindered rotation and, if suitably substituted, can be resolved. An example is biphenyl-2,2′-bis-sulfonic acid.58 In some cases, the groups may be large enough to slow rotation greatly, but not to prevent it completely. In such cases, optically active compounds can be prepared that slowly racemize on standing. Thus, 15 loses its optical activity with a half-life of 9.4 min in ethanol at 25°C.59 Compounds with greater rotational stability can often be racemized if higher temperatures are used to supply the energy necessary to force the groups past each other.60
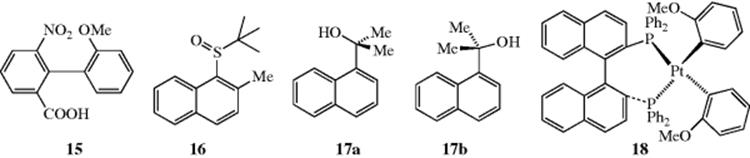
Atropisomerism occurs in other systems as well, including monopyrroles.61 Sulfoxide (16), for example, forms atropisomers with an interconversion barrier with its atropisomer of 18–19 kcal mol−1 (75.4–79.5 kJ mol−1).62The atropisomers of hindered naphthyl alcohols (e.g., 17) exist as the sp-atropisomer (17a) and the ap-atropisomer (17b).63 Atropisomers can also be formed in organometallic compounds, [e.g., the bis(phosphinoplatinum) complex (see 18)] generated by reaction with (R)-BINAP [(2R1 3S)-2,2′-bis(diphenylphosphino)-1,1′-binaphyl, see Reaction 19-36].64
It is possible to isolate isomers in some cases, often due to restricted rotation. In 9,10-bis(trifluorovinyl)phenanthrene (19) torsional diastereomers (see Sec. 4.G) are formed. The value of K for interconversion of 19a and 19bis 0.48, with ΔG0 = 15.1 kcal mol−1.65 The ability to isolate atropisomers can depend on interactions with solvent, as in the isolation of atropisomeric colchicinoid alkaloids, which have been isolated, characterized, and their dichroic behavior described.66
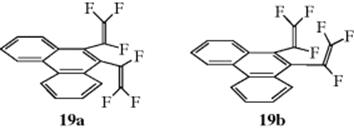
In allenes, the central carbon is sp hybridized. The remaining two p orbitals are perpendicular to each other and each overlaps with the p orbital of one adjacent carbon atom, forcing the two remaining bonds of each carbon into perpendicular planes. Thus allenes fall into the category represented by Fig. 4.2. Like biphenyls, allenes are chiral only if both sides are unsymmetrically substituted.67 These cases are completely different from the cis–trans isomerism of compounds with one double bond (Sec. 4.K). In the latter cases, the four groups are all in one plane, the isomers are not enantiomers, and neither is chiral, while in allenes the groups are in two perpendicular planes and the isomers are a pair of optically active enantiomers.
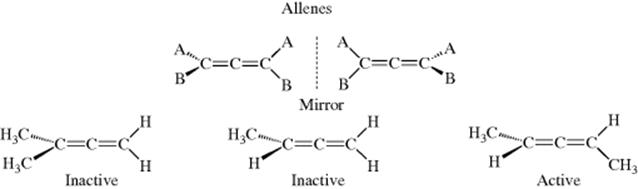
When a molecule has three, five, or any odd number of cumulative double bonds, orbital overlap causes the four groups to occupy one plane and cis–trans isomerism is observed. When four, six, or any even number of cumulative double bonds exist, the situation is analogous to that in the allenes and optical activity is possible. Compound 20 has been resolved.68
Other types of compounds that contain the system illustrated in Fig. 4.2 and that are similarly chiral if both sides are dissymmetric include spiranes (e.g., 21), and compounds with exocyclic double bonds (e.g., 22). Atropisomerism exists in (1,5)-bridgedcalix[8]arenes (see Sec. 3.C.ii).69
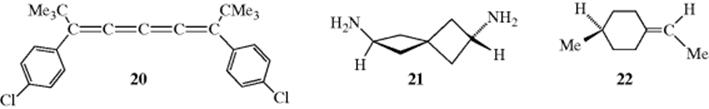
6. Chirality due to a Helical Shape.70 Several compounds have been prepared that are chiral because they have a shape that is actually helical and can therefore be left or right handed in orientation. The entire molecule is usually less than one full turn of the helix, but this does not alter the possibility of left- and right handedness. An example is hexahelicene,71 in which one side of the molecule must lie above the other because of crowding.72The rotational barrier for helicene is ~22.9 kcal mol−1(95.9 kJ mol−1), and significantly higher when substituents are present.73 It has been shown that the dianion of helicene retains its chirality.74 Chiral discrimination of helicenes is possible.75 1,16-Diazo[6]helicene has also been prepared and, interestingly, does not act as a proton sponge (see Sec. 8.F) because the helical structure leaves the basic nitrogen atoms too far apart. Heptalene is another compound that is not planar (Sec. 2.I.iii). Its twisted structure makes it chiral, but the enantiomers rapidly interconvert.76
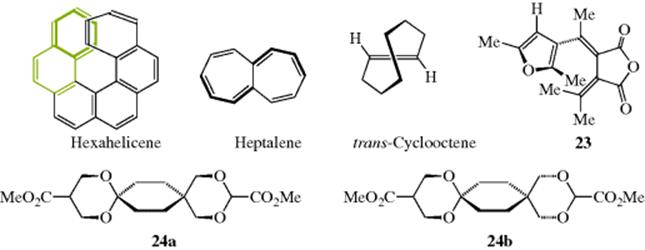
trans-Cyclooctene (see also, Sec. 4.K.i) also exhibits helical chirality because the carbon chain must lie above the double bond on one side and below it on the other.77 Similar helical chirality also appears in fulgide 2378 and dispiro-1,3-dioxane (24), shows two enantiomers (24a and 24b).79
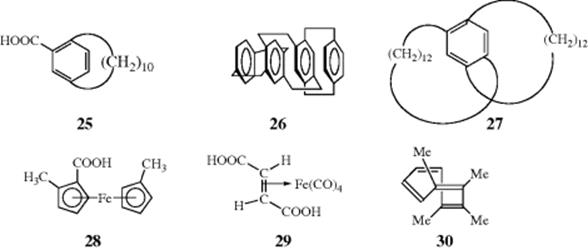
7. Optical Activity Caused by Restricted Rotation of Other Types. Substituted paracyclophanes may be optically active80 and 25, for example, has been resolved.81 In this case, chirality results because the benzene ring cannot rotate in such a way that the carboxyl group goes through the alicyclic ring. Many chiral layered cyclophanes (e.g., 26), have been prepared.82 Another cyclophane83 with a different type of chirality is [12][12]paracyclophane (27), where the chirality arises from the relative orientation of the two rings attached to the central benzene ring.84 An acetylenic cyclophane was shown to have helical chirality.85 Metallocenes (Sec. 2.I.ii) substituted with at least two different groups on one ring are also chiral.86 Several hundred such compounds have been resolved, one being 28. Chirality is also found in other metallic complexes of suitable geometry.87 Fumaric acid–iron tetracarbonyl (29) has been resolved,88 and 1,2,3,4-tetramethylcyclooctatetraene (30) is also chiral.89 This molecule, which exists in the tub form (Sec. 2.K), has
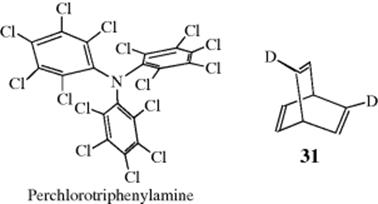
neither a plane nor an alternating axis of symmetry. Another compound that is chiral solely because of hindered rotation is the propeller-shaped perchlorotriphenylamine, which has been resolved.90 The 2,5-dideuterio derivative (31) of barrelene is chiral, though the parent hydrocarbon and the monodeuterio derivative are not. Compound 25 has been prepared in optically active form91 and is another case where chirality is due to isotopic substitution.
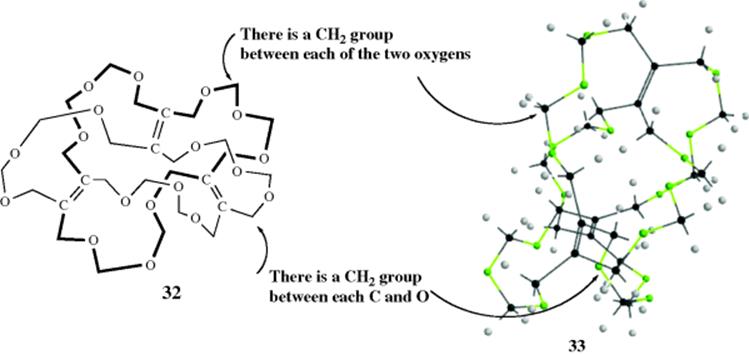
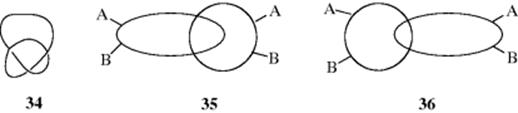
The main molecular chain in compound 32 has the form of a Möbius strip (see Fig. 15.7 and 3D model 33).92 This molecule has no stereogenic carbons, nor does it have a rigid shape, a plane, nor an alternating axis of symmetry. However, 32 has been synthesized and shown to be chiral.93 Rings containing 50 or more members should be able to exist as knots (34, and see 39 on Sec. 3.D). Such a knot would be nonsuperimposable on its mirror image. Calixarenes,94 crown ethers,95 catenanes, and rotaxanes (see Sec. 3.D) can also be chiral if suitably substituted.96 For example, 40 and 41 are nonsuperimposable mirror images.
A stereogenic center may be created from an achiral molecule via a chemical reaction. One example is the α-bromination of a carboxylic acid (Hell–Volhardt–Zelenskii reaction, 12-05) to form the α-bromo acid.
![]()
In this case, the α-carbon in the product is the stereogenic carbon. If there are no asymmetric components in the reaction, the product must be racemic. This means that no optically active material can be created if all starting materials and conditions are optically inactive.97 This statement also holds when one begins with a racemic mixture, unless there is kinetic resolution (Sec. 4.I). Thus racemic 2-butanol, treated with HBr, must give racemic 2-bromobutane.
Fig. 4.2 Perpendicular dissymmetric planes and a chiral molecule with no stereogenic center.