March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 4. Stereochemistry and Conformation
4.P. Strain
Steric strain418 exists in a molecule when bonds are forced to make abnormal angles, usually due to repulsion of large atoms or groups attached to those bonds, but not always. This repulsion results in a higher energy than would be the case in the absence of the angle distortions. It has been shown that there is a good correlation between the 13C–H coupling constants in NMR and the bond angles and bond force angles in strained organic molecules.419 There are, in general, two kinds of structural features that result in sterically caused abnormal bond angles. One of these is found in small-ring compounds, where the angles must be less than those resulting from normal orbital overlap.420 Such strain is called small-angle strain or Baeyer strain. The other arises when nonbonded atoms are forced into close proximity by the geometry of the molecule. These are called nonbonded interactions. This latter type of strain is most often associated with the term steric strain.
Strained molecules possess strain energy. That is, their potential energies are higher than they would be if strain were absent.421 The strain energy for a particular molecule can be estimated from heat of atomization or heat of combustion data. A strained molecule has a lower heat of atomization than it would have if it were strain-free (Fig. 4.6). As in the similar case of resonance energies (Sec. 2.B), strain energies cannot be known exactly, because the energy of a real molecule can be measured, but not the energy of a hypothetical unstrained model. It is also possible to calculate strain energies by molecular mechanics, not only for real molecules, but also for those that cannot be made.422
Fig. 4.6 Strain energy calculation.
4.P.i Strain in Small Rings
Three-membered rings have a great deal of angle strain (also called Baeyer strain), since 60° angles represent a large departure from the “normal” tetrahedral angles. Calculations have been interpreted to say that Baeyer strain in small ring systems originates from a decrease in nucleus–electron attraction compared to acyclic compounds,423 but this has been challenged in later work.424 However, in sharp contrast to other ethers, ethylene oxide is quite reactive, the ring being opened by many reagents (see Sec. 10.G.iii). Ring opening, of course, relieves the strain.425 Cyclopropane,426 which is even more strained427 than ethylene oxide, is also cleaved more easily than would be expected for an alkane.428 Thus, pyrolysis at 450–500°C converts it to propene, bromination gives 1,3-dibromopropane,429 and it can be hydrogenated to propane (though at high pressure).430 Other three-membered rings are similarly reactive.431 Alkyl substituents influence the strain energy of small ring compounds,432 and carbonyl substitution also influences the strain energy.433 gem-Dimethyl substitution, for example, “lowers the strain energy of cyclopropanes, cyclobutanes, epoxides, and dimethyldioxirane by 6–10 kcal mol−1 (25–42 kJ mol−1) relative to an unbranched acyclic reference molecule.”432 The CH bond dissociation energy also tends to increase ring strain in small-ring alkenes.434 Computation of the ring strain energy of 1,1-dimethylcyclobutane, however, shows “no significant enthalpic component of the gem-dimethyl effect as measured by the ring strain energy.”435
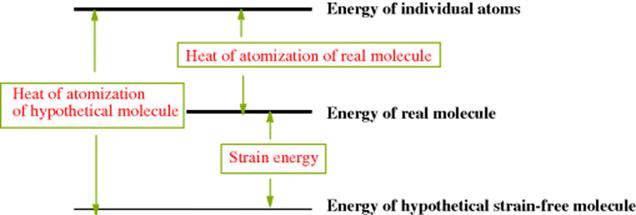
There is much evidence, chiefly derived from NMR coupling constants, that the bonding in cyclopropanes is not the same as in compounds that lack small-angle strain.436 For a normal carbon atom, one s and three p orbitals are hybridized to give four approximately equivalent sp3 orbitals, each containing ~25% s character. But for a cyclopropane carbon atom, the four hybrid orbitals are far from equivalent. The two orbitals directed to the outside bonds have more s character than a normal sp3 orbital, while the two orbitals involved in ring bonding have less, because the more p-like they are the more they resemble ordinary p orbitals, whose preferred bond angle is 90° rather than 109.5°. Since the small-angle strain in cyclopropanes is the difference between the preferred angle and the real angle of 60°, this additional p character relieves some of the strain. The external orbitals have ~33% s character, so that they are ~sp2 orbitals, while the internal orbitals have ~17% s character, so that they may be called ~sp5 orbitals.437 Each of the three carbon–carbon bonds of cyclopropane is therefore formed by overlap of two sp5 orbitals. Molecular-orbital calculations show that such bonds are not completely s in character. In normal C–C bonds, sp3 orbitals overlap in such a way that the straight line connecting the nuclei becomes an axis about which the electron density is symmetrical. But in cyclopropane, the electron density is directed away from the ring.438 Figure 4.7 shows the direction of orbital overlap.439 For cyclopropane, the angle (marked θ) is 21°. Cyclobutane exhibits the same phenomenon but to a lesser extent, θ being 7°.439 Molecular orbital calculations also show that the maximum electron densities of the C–C σ orbitals are bent away from the ring, with θ = 9.4° for cyclopropane and 3.4° for cyclobutane.440 The bonds in cyclopropane are called bent bonds (sometimes, banana bonds), and are intermediate in character between σ and π, so that cyclopropanes behave in some respects like double-bond compounds.441 For one thing, there is much evidence, chiefly from UV spectra,442 that a cyclopropane ring is conjugated with an adjacent double bond. The conjugation is greatest for the conformation shown in Fig. 4.8a and is least or absent for the conformation shown in 4.8b, since overlap of the double-bond π orbital with two of the p-like orbitals of the cyclopropane ring is greatest in conformation a. However, the conjugation between a cyclopropane ring and a double bond is less than that between two double bonds.443 For other examples of the similarities in behavior of a cyclopropane ring and a double bond (see Sec. 4.O.iv).
Fig. 4.7 Orbital overlap in cyclopropane. The arrows point toward the center of electron density.
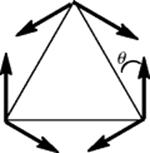
Fig. 4.8 Conformations of α-cyclopropylalkenes. Conformation (a) leads to maximum conjugation and conformation (b) to minimum conjugation
Four-membered rings also exhibit angle strain, but much less than three-membered rings, and for that reason are less easily opened. Cyclobutane is more resistant than cyclopropane to bromination, and although it can be hydrogenated to butane, more strenuous conditions are required. Nevertheless, pyrolysis at 420°C gives two molecules of ethylene. As mentioned earlier (Sec. 4.O.iv), cyclobutane is not planar.
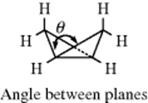
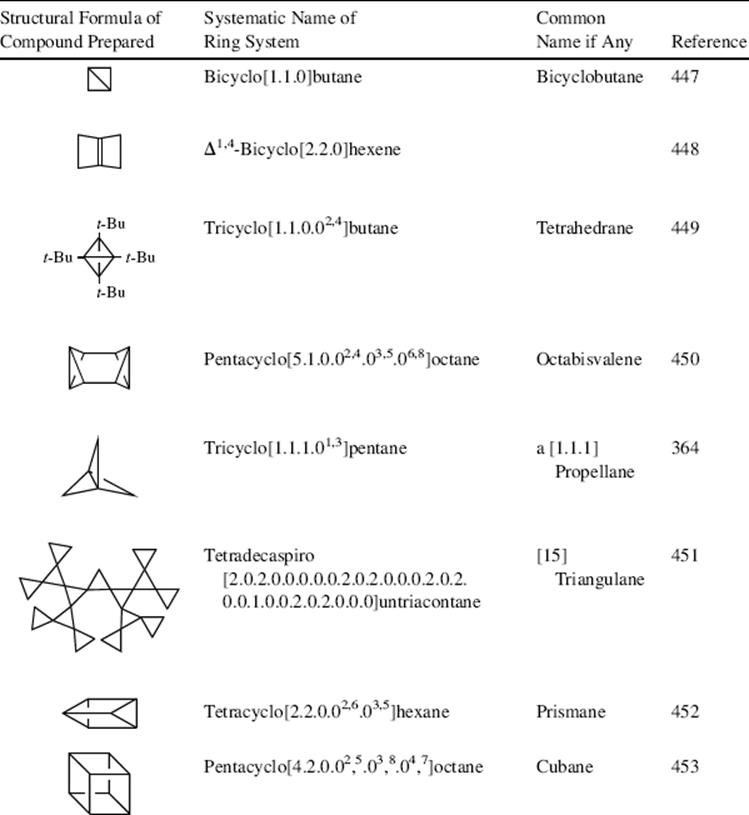
Many highly strained compounds containing small rings in fused systems have been prepared,444 showing that organic molecules can exhibit much more strain than simple cyclopropanes or cyclobutanes.445 Table 4.5 shows a few of these compounds.446 Perhaps the most interesting are cubane, prismane,460 and the substituted tetrahedrane, since preparation of these ring systems had been the object of much endeavor. Prismane is tetracyclo[2.2.0.02,6.03,5]hexane and many derivatives are known,461 including bis(homohexaprismane) derivatives.462 The bicyclobutane molecule is bent, with the angle θ between the planes equal to 126 ± 3°.463 The rehybridization effect, described above for cyclopropane, is even more extreme in this molecule. Calculations have shown that the central bond is essentially formed by overlap of two p orbitals with little or no
s character.464 Propellanes are compounds in which two carbons, directly connected, are also connected by three other bridges. [1.1.1]Propellane is in the table and it is the smallest possible propellane.465 It is in fact more stable than the larger [2.1.1]propellane and [2.2.1]propellane, which have been isolated only in solid matrixes at low temperature.466 The bicyclo[1.1.1]pentanes are related to the propellanes except that the central connecting bond is missing, and several derivatives are known.467 Even more complex systems are known.468
Table 4.5 Some Strained Small-Ring Compounds.
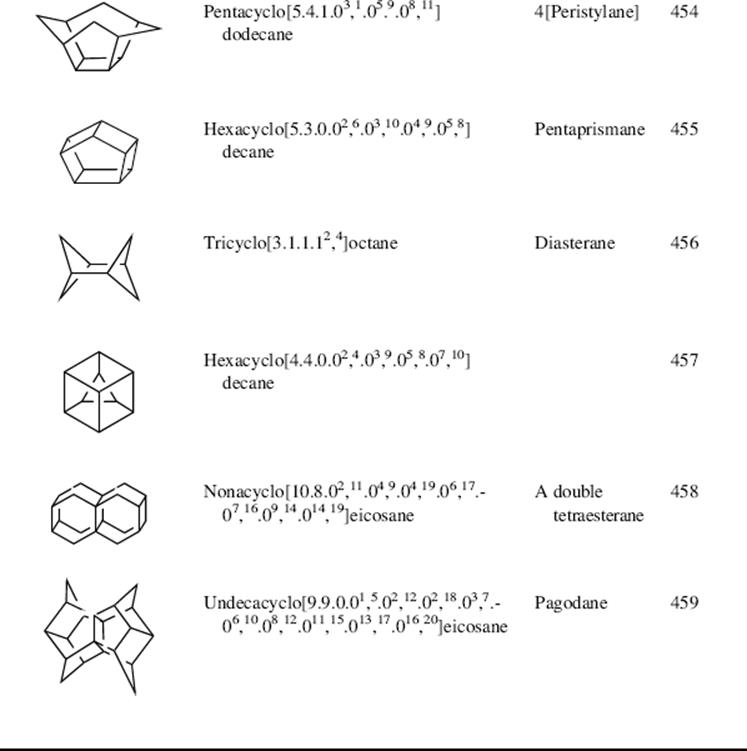
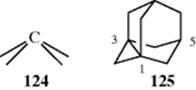
In certain small-ring systems, including small propellanes, the geometry of one or more carbon atoms is so constrained that all four of their valences are directed to the same side of a plane (inverted tetrahedron), as in 124.469 An example is 1,3-dehydroadamantane, 125, which is also a propellane.470 X-ray crystallography of the 5-cyano derivative of 125 shows that the four carbon valences at C-1 and C-3 are all directed “into” the molecule and none point outside.471 Compound 125 is quite reactive; it is unstable in air, readily adds hydrogen, water, bromine, or acetic acid to the C-1–C-3 bond, and is easily polymerized. When two such atoms are connected by a bond (as in 125), the bond is very long (the C-1–C-3 bond length in the 5-cyano derivative of 125 is 1.64 Å), as the atoms try to compensate in this way for their enforced angles. The high reactivity of the C-1–C-3 bond of 125 is not only caused by strain, but also by the fact that reagents find it easy to approach these atoms since there are no bonds (e.g., C–H bonds on C-1 or C-3) to get in the way.
4.P.ii. Strain in Other Rings472
In rings larger than four-membered, there is no strain due to small bond angles, but there are three other kinds of strain. In the chair form of cyclohexane, which does not exhibit any of the three kinds of strain, all six carbon–carbon bonds have the two attached carbons in the gauche conformation. However, in five-membered rings and in rings containing from 7 to 13 carbons, any conformation in which all the ring bonds are gauche contains transannular interactions, that is, interactions between the substituents on C-1 and C-3 or C-1 and C-4, and so on. These interactions occur because the internal space is not large enough for all the quasi-axial hydrogen atoms to fit without coming into conflict. The molecule can adopt other conformations in which this transannular strain is reduced, but then some of the carbon–carbon bonds must adopt eclipsed or partially eclipsed conformations. The strain resulting from eclipsed conformations is called Pitzer strain. For saturated rings from 3- to 13-membered (except for the chair form of cyclohexane) there is no escape from at least one of these two types of strain. In practice, each ring adopts conformations that minimize both sorts of strain as much as possible. For cyclopentane, as seen in Section 4.O.iv, this means that the molecule is not planar. In rings larger than nine-membered, Pitzer strain seems to disappear, but transannular strain is still present.473 For 9- and 10-membered rings, some of the transannular and Pitzer strain may be relieved by the adoption of a third type of strain, large-angle strain. Thus, C–C–C angles of 115–120° have been found in X-ray diffraction of cyclononylamine hydrobromide and 1,6-diaminocyclodecane dihydrochloride.474
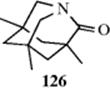
Strain can exert other influences on molecules. 1-Aza-2-adamantanone (126) is an extreme case of a twisted amide.475 The overlap of the lone-pair electrons on nitrogen with the π-system of the carbonyl is prevented.475 In chemical reactions, 126 reacts more or less like a ketone, giving a Wittig reaction (16-44) and it can form a ketal (16-7). A twisted biadamantylidene compound has been reported.476
The amount of strain in cycloalkanes is shown in Table 4.6,477 which lists heats of combustion per CH2 group. As can be seen, cycloalkanes > 13-membered are as strain-free as cyclohexane.
Table 4.6 Heats of Combustion in the Gas Phase for Cycloalkanes, per CH2 Groupa
[Reprinted with permission. Gol'dfarb, Ya.L.; Belen'kii, L.I. Russ. Chem. Rev. 1960, 29, 214, p. 218].
a. See Ref. 472.
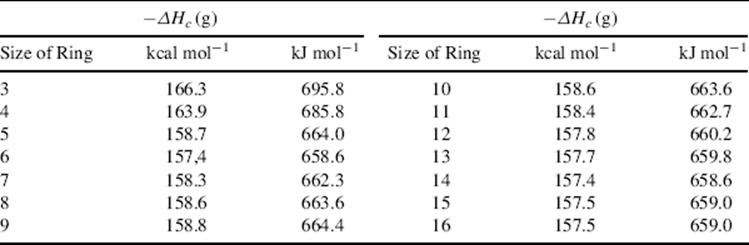
Transannular interactions can exist across rings from 8- to 11-membered and even larger.478 Such interactions can be detected by dipole and spectral measurements. For example, that the carbonyl group in 127a is affected by the nitrogen (127b is probably another canonical form) has been demonstrated by photoelectron spectroscopy, which shows that the ionization potentials of the nitrogen n and C=O π orbitals in 127 differ from those of the two comparison molecules 128 and 129.479 It is significant that when 127 donates electrons to a proton, it goes to the oxygen rather than to the nitrogen. Many examples of transannular reactions are known, including the following:
Ref. 480
Ref. 481
where DMF = N, N-dimethylformamide (Solvent)
In summary, saturated rings may be divided into four groups, of which the first and third are more strained than the other two.482
1. Small rings (three- and four-membered). Small-angle strain predominates.
2. Common rings (five-, six-, and seven-membered). Largely unstrained. The strain that is present is mostly Pitzer strain.
3. Medium rings (8- to 11-membered). Considerable strain; Pitzer, transannular, and large-angle strain.
4. Large rings (12-membered and larger). Little or no strain.483
4.P.iii. Unsaturated Rings484
Double bonds can exist in rings of any size. As expected, the most highly strained are the three-membered rings (e.g., cyclopropene). Small-angle strain, which is so important in cyclopropane, is even greater in cyclopropene485because the ideal angle is more distorted. In cyclopropane, the bond angle is forced to be 60°, ~50° smaller than the tetrahedral angle; but in cyclopropene, the angle, also ~60°, is now ~60° smaller than the ideal angle of 120° for an alkene Thus, the angle of cyclopropene is ~10° more strained than in cyclopropane. However, this additional strain is offset by a decrease in strain arising from another factor. Cyclopropene, lacking two hydrogens, has none of the eclipsing strain present in cyclopropane. Cyclopropene has been prepared486 and is stable at liquid-nitrogen temperatures, although on warming even to −80°C it rapidly polymerizes. Many other cyclopropenes are stable at room temperature and above.464 The highly strained benzocyclopropene,487 in which the cyclopropene ring is fused to a benzene ring, has been prepared488 and is stable for weeks at room temperature, although it decomposes on distillation at atmospheric pressure.

As previously mentioned, double bonds in relatively small rings must be cis. A stable trans double bond489 first appears in an eight-membered ring (trans-cyclooctene, Sec. 4.C, category 6), although the transient existence of trans-cyclohexene and cycloheptene has been demonstrated.490 Above ~11 members, the trans isomer is more stable than the cis.232 It has proved possible to prepare compounds in which a trans double bond is shared by two cycloalkene rings (e.g., 130). Such compounds have been called [m.n]betweenanenes, and several have been prepared with m and n values from 8 to 26.491 The double bonds of the smaller betweenanenes, as might be expected from the fact that they are deeply buried within the bridges, are much less reactive than those of the corresponding cis–cis isomers.
The smallest unstrained cyclic triple bond is found in cyclononyne.492 Cyclooctyne has been isolated,493 but its heat of hydrogenation shows that it is considerably strained. There have been a few compounds isolated with triple bonds in seven-membered rings. 3,3,7,7-Tetramethylcycloheptyne (131) is known and dimerizes within 1 h at room temperature,494 but the thia derivative (132), in which the C–S bonds are longer than the corresponding C–C bonds in 131, is indefinitely stable even at 140°C.495 Cycloheptyne itself has not been isolated, although its transient existence has been shown.496 Cyclohexyne497 and its 3,3,6,6-tetramethyl derivative498 have been trapped at 77 K, and in an Ar π matrix at 12 K, respectively. Its IR spectra have also been
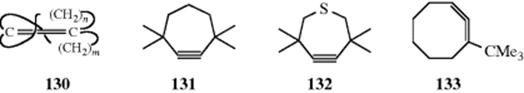
obtained. Transient six- and even five-membered rings containing triple bonds have also been demonstrated.499 A derivative of cyclopentyne has been trapped in a matrix.500 Although cycloheptyne and cyclohexyne have not been isolated at room temperatures, Pt(0) complexes of these compounds have been prepared and are stable.501 The smallest cyclic allene502 so far isolated is 1-tert-butyl-1,2-cyclooctadiene (133).503 The parent 1,2-cyclooctadiene has not been isolated. It has been shown to exist as a transient species, but rapidly dimerizes.504 Incorporation of the tert-butyl group apparently prevents this. The transient existence of 1,2-cycloheptadiene has also been shown,505 and both 1,2-cyclooctadiene and 1,2-cycloheptadiene have been isolated in Pt complexes.506 1,2-Cyclohexadiene has been trapped at low temperatures, and its structure has been proved by spectral studies.507 Cyclic allenes in general are less strained than their acetylenic isomers.508 The cyclic cumulene 1,2,3-cyclononatriene also has been synthesized and is reasonably stable in solution at room temperature in the absence of air.509
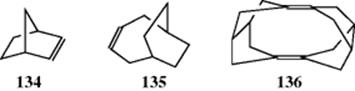
There are many examples of polycyclic molecules and bridged molecules that have one or more double bonds. There is flattening of the ring containing the C=C unit, and this can have a significant effect on the molecule. Norbornene (bicyclo[2.2.1]hept-2-ene, 134) is a simple example and it has been calculated that it contains a distorted π-face.510 The double bond can appear away from the bridgehead carbon atoms, as in bicyclo[4.2.2]dec-3-ene (135), which flattens that part of the molecule. The C=C units in pentacyclo[8.2.1.12,5.14,7.18,11]hexadeca-1,7-diene (136) are held in a position where there is significant π–π interactions across the molecule.511
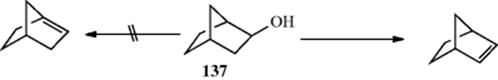
Double bonds at the bridgehead of bridged bicyclic compounds are impossible in small systems. This result is the basis of Bredt's rule,512 which states that elimination to give a double bond in a bridged bicyclic system (e.g., 137) always leads away from the bridgehead. This rule no longer applies when the rings are large enough. In
determining whether a bicyclic system is large enough to accommodate a bridgehead double bond, the most reliable criterion is the size of the ring in which the double bond is located.513 Bicyclo[3.3.1]non-1-ene514 (138) and bicyclo[4.2.1]non-1(8)ene515 (139) are stable compounds. Both can be looked upon as derivatives of trans-cyclooctene, which is of course a known compound. Compound 138 has been shown to have a strain
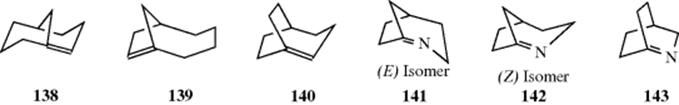
energy of the same order of magnitude as that of trans-cyclooctene.516 On the other hand, in bicyclo[3.2.2]non-1-ene (140), the largest ring that contains the double bond is a trans-cycloheptene, which is as yet unknown. Compound 140 has been prepared, but dimerized before it could be isolated.517 Even smaller systems ([3.2.1] and [2.2.2]), but with imine double bonds (141–143), have been obtained in matrixes at low temperatures.518 These compounds are destroyed on warming. Compounds 141 and 142 are the first reported example of (E–Z) isomerism at a strained bridgehead double bond.519
4.P.iv. Strain Due to Unavoidable Crowding520
In some molecules, large groups are so close to each other that they cannot fit into the available space in such a way that normal bond angles are maintained. It has proved possible to prepare compounds with a high degree of this type of strain. For example, success has been achieved in synthesizing benzene rings containing ortho tert-butyl groups. Two examples that have been prepared, of several, are 1,2,3-tri-tert-butyl compound 144521 and the 1,2,3,4-tetra-tert-butyl compound 145.522 That these molecules are strained is demonstrated by UV and IR spectra, which show that the ring is not planar in 1,2,4-tri-tert-butylbenzene, and by a comparison of the heats of reaction of this compound and its 1,3,5 isomer, which show that the 1,2,4 compound possesses ~22 kcal mol−1 (92 kJ mol−1) more strain energy than its isomer523 (see also Reaction 18-27). Although SiMe3 groups are larger
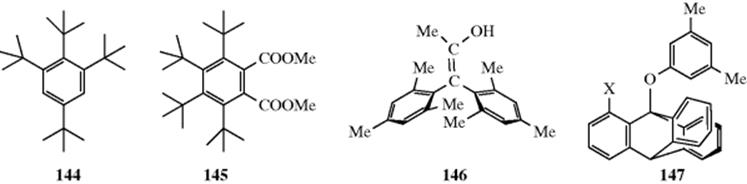
than CMe3 groups, it has proven possible to prepare C6(SiMe3)6. This compound has a chair-shaped ring in the solid state, and a mixture of chair and boat forms in solution.524 Even smaller groups can sterically interfere in ortho positions. In hexaisopropylbenzene, the six isopropyl groups are so crowded that they cannot rotate, but are lined up around the benzene ring, all pointed in the same direction.525 This compound is an example of a geared molecule.526 The isopropyl groups fit into each other in the same manner as interlocked
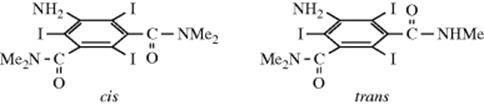
gears. Another example is 146, which is a stable enol.527 In this case, each ring can rotate about its C–aryl bond only by forcing the other to rotate as well. In the case of triptycene derivatives (e.g., 147), a complete 360° rotation of the aryl group around the O–aryl bond requires the aryl group to pass over three rotational barriers; one of which is the C–X bond and the other two the “top” C–H bonds of the other two rings. As expected, the C–X barrier is the highest, ranging from 10.3 kcal mol−1 (43.1 kJ mol−1) for X = F to 17.6 kcal mol−1 (73.6 kJ mol−1) for X = tert-butyl.528 In another instance, it has proved possible to prepare cis and trans isomers of 5-amino-2,4,6-triiodo-N,N,N′,N′-tetramethylisophthalamide because there is no room for the CONMe2 groups to rotate, caught as they are between two bulky iodine atoms.529 The trans isomer is chiral and has been resolved, while the cis isomer is a meso form. Another example of cis-trans isomerism resulting from restricted rotation about single bonds530 is found in 1,8-di-o-tolylnapthalene531 (see also, Sec. 4.K.i).
There are many other cases of intramolecular crowding that result in the distortion of bond angles. Hexahelicene (Sec. 4.C, category 6) and bent benzene rings (Sec. 2.G) have been mentioned previously. The compounds tri-tert-butylamine, and tetra-tert-butylmethane are as yet unknown. In the latter, there is no way for the strain to be relieved and it is questionable whether this compound can ever be made. In tri-tert-butylamine, the crowding can be eased somewhat if the three bulky groups assume a planar instead of the normal pyramidal configuration. In tri-tert-butylcarbinol, coplanarity of the three tert-butyl groups is prevented by the presence of the OH group, and yet this compound has been prepared.532 Tri-tert-butylamine should have less steric strain than tri-tert-butylcarbinol and it should be possible to prepare it.533 The tetra-tert-butylphosphonium cation (t-Bu)4P+ has been prepared.534 Although steric effects are nonadditive in crowded molecules, a quantitative measure has been proposed by DeTar, based on molecular mechanics calculations. This is called formal steric enthalpy (FSE), and values have been calculated for alkanes, alkenes, alcohols, ethers, and methyl esters535 For example, some FSE values for alkanes are butane 0.00; 2,2,3,3-tetramethylbutane 7.27; 2,2,4,4,5-pentamethylhexane 11.30; and tri-tert-butylmethane 38.53.
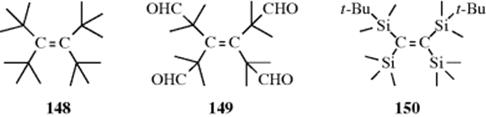
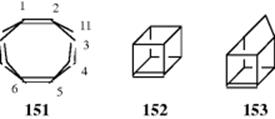
The two carbon atoms of a C=C double bond and the four groups attached to them are normally in a plane, but if the groups are large enough, significant deviation from planarity can result.536 The compound tetra-tert-butylethene (148) has not been prepared,537 but the tetraaldehyde (149), which should have about the same amount of strain, has been made. X-ray crystallography shows that 149 is twisted out of a planar shape by an angle of 28.6°.538 Also, the C=C double bond distance is 1.357 Å, significantly longer than normal C=C bond of 1.32 Å (Table 1.5). (Z)-1,2-Bis(tert-butyldimethylsilyl)-1,2-bis(trimethylsilyl)ethene (150) has an even greater twist, but could not be made to undergo conversion to the (E) isomer, probably because the groups are too large to slide past each other.539 A different kind of double-bond strain is found in tricyclo[4.2.2.22,5]dodeca-1,5-diene (151),540 cubene (152),541 and homocub-4(5)-ene (153).542 In these molecules, the four groups on the
double bond are all forced to be on one side of the double-bond plane.543 In 151, the angle between the line C1–C2 (extended) and the plane defined by C2, C3, and C11 is 27°. An additional source of strain in this molecule is the fact that the two double bonds are pushed into close proximity by the four bridges. In an effort to alleviate this sort of strain, the bridge bond distances (C-3–C-4) are 1.595 Å, which is considerably longer than the 1.53 Å expected for a normal sp3–sp3 C–C bond (Table 1.5). Compounds 152 and 153 have not been isolated, but have been generated as intermediates that were trapped by reaction with other compounds.541,542
Notes
1. See Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley-Interscience, NY, 1994; Sokolov, V.I. Introduction to Theoretical Stereochemistry, Gordon and Breach, NY, 1991; Nógrádi, M. Sterochemistry, Pergamon, Elmsford, NY, 1981; Kagan, H. Organic Sterochemistry, Wiley, NY, 1979; Testa, B. Principles of Organic Stereochemistry, Marcel Dekker, NY, 1979; Izumi, Y.; Tai, A. Stereo-Differentiating Reactions, Academic Press, NY, Kodansha Ltd., Tokyo, 1977; Natta, G.; Farina, M. Stereochemistry, Harper and Row, NY, 1972; Eliel, E.L. Elements of Stereochemistry, Wiley, NY, 1969; Mislow, K. Introduction to Stereochemistry, W.A. Benjamin, NY, 1965. For a historical treatment, see Ramsay, O.B. Stereochemistry, Heyden & Son, Ltd., London, 1981.
2. See Pure Appl. Chem. 1976, 45, 13 and in Nomenclature of Organic Chemistry, Pergamon, Elmsford, NY, 1979 (the Blue Book).
3. See Cintas, P. Angew. Chem. Int. Ed. 2007, 46, 4016.
4. For a discussion of the conditions for optical activity in liquids and crystals, see O'Loane, J.K. Chem. Rev. 1980, 80, 41. For a discussion of chirality as applied to molecules, see Quack, M. Angew. Chem. Int. Ed. 1989, 28, 571.
5. Avalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Palacios, J.C. Tetrahedron Asymm. 2000, 11, 2845.
6. Interactions among electrons, nucleons, and certain components of nucleons (e.g., bosons), called weak interactions, violate parity; that is, mirror image interactions do not have the same energy. It has been contended that interactions of this sort cause one of a pair of enantiomers to be (slightly) more stable than the other. See Tranter, G.E. J. Chem. Soc. Chem. Commun. 1986, 60, and references cited therein. See also, Barron, L.D. Chem. Soc. Rev.1986, 15, 189.
7. For a reported exception, see Hata, N. Chem. Lett. 1991, 155.
8. See Craig, D.P.; Mellor, D.P. Top. Curr. Chem. 1976, 63, 1.
9. Strictly speaking, the term racemic mixture applies only when the mixture of molecules is present as separate solid phases, but in this book this expression refers to any equimolar mixture of enantiomeric molecules, liquid, solid, gaseous, or in solution.
10. See Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions, Wiley, NY, 1981.
11. See Wynberg, H.; Lorand, J.P. J. Org. Chem. 1981, 46, 2538 and references cited therein.
12. A good example is found in Kumata, Y.; Furukawa, J.; Fueno, T. Bull. Chem. Soc. Jpn. 1970, 43, 3920.
13. For a review of polarimetry see Lyle, G.G.; Lyle, R.E. in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, pp. 13–27.
14. For examples, see Shriner, R.L.; Adams, R.; Marvel, C.S. in Gilman, H. Advanced Organic Chemistry, Vol. 1, 2nd ed. Wiley, NY, 1943, pp. 291–301.
15. Krow, G.; Hill, R.K. Chem. Commun. 1968, 430.
16. Wiberg, K. B.; Vaccaro, P. H.; Cheeseman, J.R. J. Am. Chem. Soc. 2003, 125, 1888.
17. See Barron L.D. Chem. Soc. Rev. 1986, 15, 189.
18. The definitions of plane, center, and alternating axis of symmetry are taken from Eliel, E.L. Elements of Stereochemistry, Wiley, NY, 1969, pp. 6,7. See also, Lemière, G.L.; Alderweireldt, F.C. J. Org. Chem. 1980, 45, 4175.
19. McCasland, G.E.; Proskow, S. J. Am. Chem. Soc. 1955, 77, 4688.
20. For discussions of the relationship between a chiral carbon and chirality, see Mislow, K.; Siegel, J. J. Am. Chem. Soc. 1984, 106, 3319; Brand, D.J.; Fisher, J. J. Chem. Educ. 1987, 64, 1035.
21. For a review of such molecules, see Nakazaki, M. Top. Stereochem. 1984, 15, 199.
22. See Barth, G.; Djerassi, C. Tetrahedron 1981, 24, 4123; Verbit, L. Prog. Phys. Org. Chem. 1970, 7, 51; Floss, H.G.; Tsai, M.; Woodard, R.W. Top. Stereochem. 1984, 15, 253.
23. Streitwieser, Jr., A.; Schaeffer, W.D. J. Am. Chem. Soc. 1956, 78, 5597.
24. For a discussion of optical activity in paraffins, see Brewster, J.H. Tetrahedron 1974, 30, 1807.
25. Ten Hoeve, W.; Wynberg, H. J. Org. Chem. 1980, 45, 2754.
26. For compounds with asymmetric atoms other than carbon, see Aylett, B.J. Prog. Stereochem. 1969, 4, 213; Belloli, R. J. Chem. Educ. 1969, 46, 640; Sokolov, V.I.; Reutov, O.A. Russ. Chem. Rev. 1965, 34, 1.
27. See Corriu, R.J.P.; Guérin, C.; Moreau, J.J.E. in Patai, S.; Rappoport, Z. The Chemistry of Organic Silicon Compounds, pt. 1, Wiley, NY, 1989, pp. 305–370; Top. Stereochem. 1984, 15, 43; Maryanoff, C.A.; Maryanoff, B.E. in Morrison, J.D. Asymmetric Synthesis, Vol. 4, Academic Press, NY, 1984, pp. 355–374.
28. See Gielen, M. Top. Curr. Chem. 1982, 104, 57; Top. Stereochem. 1981, 12, 217.
29. See Davis, F.A.; Jenkins Jr., R.H. in Morrison, J.D. Asymmetric Synthesis, Vol. 4, Academic Press, NY, 1984, pp. 313–353; Pope, W, J.; Peachey, S.J. J. Chem. Soc. 1899, 75, 1127.
30. Stirling, C.J.M. J. Chem. Soc. 1963, 5741; Sabol, M.A.; Andersen, K.K. J. Am. Chem. Soc. 1969, 91, 3603; Annunziata, R.; Cinquini, M.; Colonna, S. J. Chem. Soc. Perkin Trans. 1 1972, 2057.
31. Lowe, G.; Parratt, M.J. J. Chem. Soc. Chem. Commun. 1985, 1075.
32. Abbott, S.J.; Jones, S.R.; Weinman, S.A.; Knowles, J.R. J. Am. Chem. Soc. 1978, 100, 2558; Cullis, P.M.; Lowe, G. J. Chem. Soc. Chem. Commun. 1978, 512. See Lowe, G. Acc. Chem. Res. 1983, 16, 244.
33. For a review of the stereochemistry at trivalent nitrogen, see Raban, M.; Greenblatt, J. in Patai, S. The Chemistry of Functional Groups, Supplement F, pt. 1, Wiley, NY, 1982, pp. 53–83.
34. See Lambert, J.B. Top. Stereochem. 1971, 6, 19; Rauk, A.; Allen, L.C.; Mislow, K. Angew. Chem. Int. Ed. 1970, 9, 400; Lehn, J.M. Fortschr. Chem. Forsch. 1970, 15, 311.
35. For example, see Stackhouse, J.; Baechler, R.D.; Mislow, K. Tetrahedron Lett. 1971, 3437, 3441.
36. Forsyth, D.A.; Zhang, W.; Hanley, J.A. J. Org. Chem. 1996, 61, 1284. Also see, Adams, D.B. J. Chem. Soc. Perkin Trans. 2 1993, 567.
37. Brois, S.J. J. Am. Chem. Soc. 1968, 90, 506, 508. See also, Shustov, G.V.; Kadorkina, G.K.; Kostyanovsky, R.G.; Rauk, A. J. Am. Chem. Soc. 1988, 110, 1719; Lehn, J.M.; Wagner, J. Chem. Commun. 1968, 148; Felix, D.; Eschenmoser, A. Angew. Chem. Int. Ed. 1968, 7, 224. For a review, see Brois, S.J. Trans. N.Y. Acad. Sci. 1969, 31, 931.
38. Schurig, V.; Leyrer, U. Tetrahedron Asymm. 1990, 1, 865.
39. Bucciarelli, M.; Forni, A.; Moretti, I.; Torre, G.; Brückner, S.; Malpezzi, L. J. Chem. Soc. Perkin Trans. 2 1988, 1595. See also, Forni, A.; Moretti, I.; Torre, G.; Brückner, S.; Malpezzi, L.; Di Silvestro, G.D. J. Chem. Soc. Perkin Trans. 2 1984, 791. See Schmitz, E. Adv. Heterocycl. Chem. 1979, 24, 63.
40. Shustov, G.V.; Denisenko, S.N.; Chervin, I.I.; Asfandiarov, N.L.; Kostyanovsky, R.G. Tetrahedron 1985, 41, 5719 and cited references. See also, Mannschreck, A.; Radeglia, R.; Gründemann, E.; Ohme, R. Chem. Ber. 1967, 100, 1778.
41. Hilpert, H.; Hoesch, L.; Dreiding, A.S. Helv. Chim. Acta 1985, 68, 1691; 1987, 70, 381.
42. See Wu, G.; Huang, M. Chem. Rev. 2006, 106 , 2596.
43. Kostyanovsky, R.G.; Rudchenko, V.F.; Shtamburg, V.G.; Chervin, I.I.; Nasibov, S.S. Tetrahedron 1981, 37, 4245; Kostyanovsky, R.G.; Rudchenko, V.F. Doklad. Chem. 1982, 263, 121. See also, Rudchenko, V.F.; Ignatov, S.M.; Chervin, I.I.; Kostyanovsky, R.G. Tetrahedron 1988, 44, 2233.
44. Prelog, V.; Wieland, P. Helv. Chim. Acta 1944, 27, 1127.
45. For reviews, see Yambushev, F.D.; Savin, V.I. Russ. Chem. Rev. 1979, 48, 582; Gallagher, M.J.; Jenkins, I.D. Top. Stereochem. 1968, 3, 1; Kamai, G.; Usacheva, G.M. Russ. Chem. Rev. 1966, 35, 601.
46. See Valentine, Jr., D.J. in Morrison, J.D. Asymmetric Synthesis, Vol. 4, Academic Press, NY, 1984, pp. 263–312.
47. Horner, L.; Fuchs, H. Tetrahedron Lett. 1962, 203.
48. See Andersen, K.K. in Patai, S.; Rappoport, Z.; Stirling, C. The Chemistry of Sulphones and Sulphoxides, Wiley, NY, 1988, pp. 55–94; and in Stirling, C.J.M. The Chemistry of the Sulphonium Group, pt. 1, Wiley, NY, 1981, pp. 229–312; Barbachyn, M.R.; Johnson, C.R. in Morrison, J.D. Asymmetric Synthesis, Vol. 4, Academic Press, NY, 1984, pp. 227–261; Cinquini, M.; Cozzi, F.; Montanari, F. in Bernardi, F.; Csizmadia, I.G.; Mangini, A. Organic Sulfur Chemistry, Elsevier, NY, 1985, pp. 355–407; Miko![]() /ajczyk, M.; Drabowicz, J. Top. Stereochem. 1982, 13, 333.
/ajczyk, M.; Drabowicz, J. Top. Stereochem. 1982, 13, 333.
49. Andersen, K.K.; Colonna, S.; Stirling, C.J.M. J. Chem. Soc. Chem. Commun. 1973, 645.
50. Balcells, D.; Maseras, F.; Khiar, N. Org. Lett. 2004, 6, 2197.
51. Hamill, H.; McKervey, M.A. Chem. Commun. 1969, 864; Applequist, J.; Rivers, P.; Applequist, D.E. J. Am. Chem. Soc. 1969, 91, 5705.
52. When the two rings of a biphenyl are connected by a bridge, rotation is of course impossible. For a review of such compounds, see Hall, D.M. Prog. Stereochem. 1969, 4, 1.
53. Ceccacci, F.; Mancini, G.; Mencarelli, P.; Villani, C. Tetrahedron Asymm. 2003, 14, 3117.
54. For a review, see Öki, M. Top. Stereochem. 1983, 14, 1. Also see Miljani![]() , O.S.; Han, S.; Holmes, D.; Schaller, G.R.; Vollhardt, K.P.C. Chem. Commun. 2005, 2606.
, O.S.; Han, S.; Holmes, D.; Schaller, G.R.; Vollhardt, K.P.C. Chem. Commun. 2005, 2606.
55. Becker, H.-D.; Langer, V.; Sieler, J.; Becker, H.-C. J. Org. Chem. 1992, 57, 1883.
56. Lunazzi, L.; Mazzanti, A.; Minzoni, M. J. Org. Chem. 2005, 70, 10062.
57. Casarini, D.; Coluccini, C.; Lunazzi, L.; Mazzanti, A. J. Org. Chem. 2005, 70, 5098.
58. Patterson, W.I.; Adams, R. J. Am. Chem. Soc. 1935, 57, 762.
59. Stoughton, R.W.; Adams, R. J. Am. Chem. Soc. 1932, 54, 4426.
60. See Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985.
61. Boiadjiev, S.E.; Lightner, S.A. Tetrahedron Asymm. 2002, 13, 1721.
62. Casarini, D.; Foresti, E.; Gasparrini, F.; Lunazzi, L.; Macciantelli, D.; Misiti, D.; Villani, C. J. Org. Chem. 1993, 58, 5674.
63. See Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Chem. Rev. 2005, 105, 1801. For a review of BINOL, see Brunel, J.M. Chem. Rev. 2005, 105, 857.
64. Alcock, N.W.; Brown, J.M.; Pérez-Torrente, J.J. Tetrahedron Lett. 1992, 33, 389. See also, Mikami, K.; Aikawa, K.; Yusa, Y.; Jodry, J.J.; Yamanaka, M. Synlett 2002, 1561.
65. Dolbier Jr., W.R.; Palmer, K.W. Tetrahedron Lett. 1992, 33, 1547.
66. Cavazza, M.; Zandomeneghi, M.; Pietra, F. Tetrahedron Lett. 2000, 41, 9129.
67. For reviews of allene chirality, see Runge, W. in Landor, S.R. The Chemistry of the Allenes, Vol. 3, Academic Press, NY, 1982, pp. 579–678, and in Patai, S. The Chemistry of Ketenes, Allenes, and Related Compounds, pt. 1, Wiley, NY, 1980, pp. 99–154; Rossi, R.; Diversi, P. Synthesis 1973, 25.
68. Nakagawa, M.; Shing![]() , K.; Naemura, K. Tetrahedron Lett. 1961, 802.
, K.; Naemura, K. Tetrahedron Lett. 1961, 802.
69. Consoli, G.M.L.; Cunsolo, F.; Geraci, C.; Gavuzzo, E.; Neri, P. Org. Lett. 2002, 4, 2649.
70. For a review, see Meurer, K.P.; Vögtle, F. Top. Curr. Chem. 1985, 127, 1. See also, Laarhoven, W.H.; Prinsen, W.J.C. Top. Curr. Chem. 1984, 125, 63; Martin, R.H. Angew. Chem. Int. Ed. 1974, 13, 649.
71. Martin, R.H.; Baes, M. Tetrahedron 1975, 31, 2135; Bernstein, W.J.; Calvin, M.; Buchardt, O. J. Am. Chem. Soc. 1973, 95, 527; Defay, N.; Martin, R.H. Bull. Soc. Chim. Belg. 1984, 93, 313; Bestmann, H.J.; Roth, W. Chem. Ber. 1974, 107, 2923.
72. For reviews of the helicenes, see Laarhoven, W.H.; Prinsen, W.J.C. Top. Curr. Chem. 1984, 125, 63; Martin, R.H. Angew. Chem. Int. Ed. 1974, 13, 649.
73. Janke, R.H.; Haufe, G.; Würthwein, E.-U.; Borkent, J.H. J. Am. Chem. Soc. 1996, 118, 6031.
74. Frim, R.; Goldblum, A.; Rabinovitz, M. J. Chem. Soc. Perkin Trans. 2 1992, 267.
75. Murguly, E.; McDonald, R.; Branda, N.R. Org. Lett. 2000, 2, 3169.
76. Staab, H.A.; Diehm, M.; Krieger, C. Tetrahedron Lett. 1994, 35, 8357.
77. Cope, A.C.; Ganellin, C.R.; Johnson, Jr., H.W.; Van Auken, T.V.; Winkler, H.J.S. J. Am. Chem. Soc. 1963, 85, 3276. Also see, Levin, C.C.; Hoffmann, R. J. Am. Chem. Soc. 1972, 94, 3446.
78. Yokoyama, Y.; Iwai, T.; Yokoyama, Y.; Kurita, Y. Chem. Lett. 1994, 225.
79. Grosu, I.; Mager, S.; Plé, G.; Mesaros, E. Tetrahedron 1996, 52, 12783.
80. For an example, see Rajakumar, P.; Srisailas, M. Tetrahedron 2001, 57, 9749.
81. Blomquist, A.T.; Stahl, R.E.; Meinwald, Y.C.; Smith, B.H. J. Org. Chem. 1961, 26, 1687. For a review of chiral cyclophanes and related molecules, see Schlögl, K. Top. Curr. Chem. 1984, 125, 27.
82. Nakazaki, M.; Yamamoto, K.; Tanaka, S.; Kametani, H. J. Org. Chem. 1977, 42, 287. Also see, Pelter, A.; Crump, R.A.N.C.; Kidwell, H. Tetrahedron Lett. 1996, 37, 1273. For an example of a chiral [2.2]-paracyclophane.
83. For a treatise on the quantitative chirality of helicenes, see Katzenelson, O.; Edelstein, J.; Avnir, D. Tetrahedron Asymm. 2000, 11, 2695.
84. Chan, T.-L.; Hung, C.-W.; Man, T.-O.; Leung, M.-k. J. Chem. Soc. Chem. Commun. 1994, 1971.
85. Collins, S.K.; Yap, G.P.A.; Fallis, A.G. Org. Lett. 2000, 2, 3189.
86. For reviews on the stereochemistry of metallocenes, see Schlögl, K. J. Organomet. Chem. 1986, 300, 219; Top. Stereochem. 1967, 1, 39; Pure Appl. Chem. 1970, 23, 413.
87. For reviews of such complexes, see Paiaro, G. Organomet. Chem. Rev. Sect. A 1970, 6, 319.
88. Paiaro, G.; Palumbo, R.; Musco, A.; Panunzi, A. Tetrahedron Lett. 1965, 1067. Also see, Paiaro, G.; Panunzi, A. J. Am. Chem. Soc. 1964, 86, 5148.
89. Paquette, L.A.; Gardlik, J.M.; Johnson, L.K.; McCullough, K.J. J. Am. Chem. Soc. 1980, 102, 5026.
90. Okamoto, Y.; Yashima, E.; Hatada, K.; Mislow, K. J. Org. Chem. 1984, 49, 557. See Grilli, S.; Lunazzi, L.; Mazzanti, A.; Casarini, D.; Femoni, C. J. Org. Chem. 2001, 66, 488.
91. Lightner, D.A.; Paquette, L.A.; Chayangkoon, P.; Lin, H.; Peterson, J.R.J. Org. Chem. 1988, 53, 1969.
92. See Walba, D.M. Tetrahedron 1985, 41, 3161.
93. Walba, D.M.; Richards, R.M.; Haltiwanger, R.C. J. Am. Chem. Soc. 1982, 104, 3219.
94. Iwanek, W.; Wolff, C.; Mattay, J. Tetrahedron Lett. 1995, 36, 8969.
95. de Vries, E.F.J.; Steenwinkel, P.; Brussee, J.; Kruse, C.G.; van der Gen, A. J. Org. Chem. 1993, 58, 4315; Pappalardo, S.; Palrisi, M.F. Tetrahedron Lett. 1996, 37, 1493; Geraci, C.; Piattelli, M.; Neri, P. Tetrahedron Lett. 1996, 37, 7627.
96. See Schill, G. Catenanes, Rotaxanes, and Knots, Academic Press, NY, 1971, pp. 11–18.
97. There is one exception to this statement. In a very few cases, racemic mixtures may crystalize from solution in such a way that all the (+) molecules go into one crystal and the (−) molecules into another. If one of the crystals crystallizes before the other, a rapid filtration results in optically active material. For a discussion, see Pincock, R.E.; Wilson, K.R. J. Chem. Educ. 1973, 50, 455.
98. The use of small d and l is now discouraged, since some authors used it for rotation, and some for configuration. However, a racemic mixture is still a dl mixture, since there is no ambiguity here.
99. For lists of absolute configurations of thousands of compounds, with references, mostly expressed as (R) or (S) rather than d or l, see Klyne, W.; Buckingham, J. Atlas of Stereochemistry, 2nd ed., 2 Vols., Oxford University Press, Oxford, 1978; Jacques, J.; Gros, C.; Bourcier, S.; Brienne, M.J.; Toullec, J. Absolute Configurations (Vol. 4 of Kagan, H. Stereochemistry), Georg Thieme Publishers, Stuttgart, 1977.
100. Bijvoet, J.M.; Peerdeman, A.F.; van Bommel, A.J. Nature (London) 1951, 168, 271. For a list of organic structures whose absolute configurations have been determined by this method, see Neidle, S.; Rogers, D.; Allen, F.H. J. Chem. Soc. C 1970, 2340.
101. For descriptions of the system and sets of sequence rules, see Pure Appl. Chem. 19767, 45, 13; Nomenclature of Organic Chemistry, Pergamon, Elmsford, NY, 1979 (the Blue Book); Cahn, R.S.; Ingold, C.K.; Prelog, V. Angew. Chem. Int. Ed. 1966, 5, 385; Cahn, R.S. J. Chem. Educ. 1964, 41, 116; Fernelius, W.C.; Loening, K.; Adams, R.M. J. Chem. Educ. 1974, 51, 735. See also, Prelog, V.; Helmchen, G. Angew. Chem. Int. Ed. 1982, 21, 567. Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley-Interscience, NY, 1994, pp. 101–147. Also see, Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010, pp. 15–23.
102. Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley, NY, 1994, pp. 1119–1190. See Krow, G. Top. Stereochem. 1970, 5, 31.
103. Mata, P.; Lobo, A.M.; Marshall, C.; Johnson, A.P. Tetrahedron Asymm. 1993, 4, 657; Perdih, M.; Razinger, M. Tetrahedron Asymm. 1994, 5, 835.
104. See Kagan, H.B. Determination of Configuration by Chemical Methods (Vol. 3 of Kagan, H.B. Stereochemistry), Georg Thieme Publishers, Stuttgart, 1977; Brewster, J.H. in Bentley, K.W.; Kirby, G.W. Elucidation of Organic Structures by Physical and Chemical Methods, 2nd ed. (Vol. 4 of Weissberger, A. Techniques of Chemistry), pt. 3, Wiley, NY, 1972, pp. 1–249; Klyne, W.; Scopes, P.M. Prog. Stereochem.1969, 4, 97; Schlenk, Jr., W. Angew. Chem. Int. Ed. 1965, 4, 139. Also see Addadi, L.; Berkovitch-Yellin, Z.; Weissbuch, I.; Lahav, M.; Leiserowitz, L. Top. Stereochem. 1986, 16, 1.
105. Except the X-ray method of Bijvoet.
106. Parker, D. Chem. Rev. 1991, 91, 1441.
107. Dale, J. A.; Dull, D.L.; Mosher, H. S. J. Org. Chem. 1969, 34, 2543; Dale, J.A.; Mosher, H.S. J. Am. Chem. Soc. 1973, 95, 512.
108. See Mori, K.; Akao, H. Tetrahedron Lett. 1978, 4127; Plummer, E.L.; Stewart, T.E.; Byrne, K.; Pearce, G.T.; Silverstein, R.M. J. Chem. Ecol. 1976, 2, 307. See also, Seco, J.M.; Quiñoá, E.; Riguera, R. Tetrahedron Asymm.2000, 11, 2695.
109. Yamaguchi, S.; Yasuhara, F.; Kabuto, K. Tetrahedron 1976, 32, 1363; Yasuhara, F.; Yamaguchi, S. Tetrahedron Lett. 1980, 21, 2827; Yamaguchi, S.; Yasuhara, F. Tetrahedron Lett. 1977, 89.
110. Latypov, S.K.; Ferreiro, M.J.; Quiñoá, E.; Riguera, R. J. Am. Chem. Soc. 1998, 120, 4741; Latypov, S.K.; Seco, J.M.; Quiñoá, E.; Riguera, R. J. Org. Chem. 1995, 60, 1538.
111. Seco, J.M.; Quiñoá, E.; Riguera, R. Chem. Rev. 2004, 104, 17.
112. Smith, M.B.; Dembofsky, B.T.; Son, Y.C. J. Org. Chem. 1994, 59, 1719; Latypov, S.K.; Riguera, R.; Smith, M.B.; Polivkova, J. J. Org. Chem. 1998, 63, 8682. For a chiral compound used to determine the enantiomeric purity of primary amines, see Pérez-Fuertes, Y.; Kelly, A.M.; Johnson, A.L.; Arimori, S.; Bull, S.D.; James, T.D. Org. Lett. 2006, 8, 609.
113. Alexakis, A.; Mutti, S.; Mangeney, P. J. Org. Chem. 1992, 57, 1224.
114. See Ref. 277 for books and reviews on optical rotatory dispersion and CD. For predictions about anomalous ORD, see Polavarapu, P.L.; Zhao, C. J. Am. Chem. Soc. 1999, 121, 246.
115. Gawronski, J.; Grajewski, J. Org. Lett. 2003, 5, 3301. See Ref. 277; Stephens, P.J.; Aamouche, A.; Devlin, F.J.; Superchi, S.; Donnoli, M.I.; Rosini, C. J. Org. Chem. 2001, 66, 3671; McCann, D.M.; Stephens, P.J. J. Org. Chem. 2006, 71, 6074.
116. Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley, NY, 1994, pp. 1203, 999–1003.
117. Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley, NY, 1994, pp. 1195, 1003–1007.
118. Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley, NY, 1994, pp. 1007–1071; Nakanishi, K.; Berova, N.; Woody, R.W. Circular Dichroism: Principles and Applications, VCH, NY, 1994; Purdie, N.; Brittain, H.G. Analytical Applications of Circular Dichroism, Elsevier, Amsterdam, The Netherlands, 1994.
119. Devlin, F.J.; Stephens, P.J.; Osterle, C.; Wiberg, K.B.; Cheeseman, J.R.; Frisch, M.J. J. Org. Chem. 2002, 67, 8090.
120. Frelek, J.; Geiger, M.; Voelter, W. Curr. Org. Chem. 1999, 3, 117–146 and references cited therein; Snatzke, G.; Wagner, U.; Wolff, H. P. Tetrahedron 1981, 37, 349; Pakulski, Z.; Zamojski, A. Tetrahedron Asymm. 1996, 7,1363; Frelek, J.; Ikekawa, N.; Takatsuto, S.; Snatzke, G. Chirality 1997, 9, 578.
121. Di Bari, L.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. J. Org. Chem. 2001, 66, 4819.
122. Kobayashi, Y.; Hayashi, N.; Tan, C.-H.; Kishi, Y. Org. Lett. 2001, 3, 2245; Hayashi, N.; Kobayashi, Y.; Kishi, Y. Org. Lett. 2001, 3, 2249; Kobayashi, Y.; Hayashi, N.; Kishi, Y. Org. Lett. 2001, 3, 2253.
123. For another protocol, see Dambruoso, P.; Bassarello, C.; Bifulco, G.; Appendino, G.; Battaglia, A.; Fontana, G.; Gomez-Paloma, L. Org. Lett. 2005, 7, 983.
124. Kobayashi, Y.; Tan, C.-H.; Kishi, Y. J. Am. Chem. Soc. 2001, 123, 2076.
125. Kobayashi, Y.; Hayashi, N.; Tan, C.-H.; Kishi, Y. Org. Lett. 2001, 3, 2245.
126. Roush, W.R.; Bannister, T.D.; Wendt, M.D.; VanNieuwenhze, M.S.; Gustin, D.J.; Dilley, G.J.; Lane, G.C.; Scheidt, K.A.; Smith, III, W.J. J. Org. Chem. 2002, 67, 4284.
127. Hong, S.-p.; McIntosh, M.C. Tetrahedron 2002, 57, 5055.
128. See Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 93–94, 992–999; Wheland, G.W. Advanced Organic Chemistry, 3rd ed., Wiley, NY, 1960, pp. 204–211; Caldwell, D.J.; Eyring, H. The Theory of Optical Activity Wiley, NY, 1971; Buckingham, A.D.; Stiles, P.J. Acc. Chem. Res. 1974, 7, 258; Mason, S.F. Q. Rev. Chem. Soc. 1963, 17, 20.
129. Brewster, J.H. Top. Stereochem. 1967, 2, 1, J. Am. Chem. Soc. 1959, 81, 5475, 5483, 5493; Sathyanarayana, B.K.; Stevens, E.S. J. Org. Chem. 1987, 52, 3170; Wroblewski, A.E.; Applequist, J.; Takaya, A.; Honzatko, R.; Kim, S.; Jacobson, R.A.; Reitsma, B.H.; Yeung, E.S.; Verkade, J.G. J. Am. Chem. Soc. 1988, 110, 4144.
130. Fidler, J.; Rodger, P.M.; Rodger, A. J. Chem. Soc. Perkin Trans. 2 1993, 235.
131. For a method of generating all stereoisomers consistent with a given empirical formula, suitable for computer use, see Nourse, J.G.; Carhart, R.E.; Smith, D.H.; Djerassi, C. J. Am. Chem. Soc. 1979, 101, 1216; 1980, 102, 6289.
132. Available at http://old.iupac.org/reports/provisional/abstract04/favre_310305.html, Preferred IUPAC Names, Chapter 9, September, 2004, p. 6.
133. A method has been developed for the determination of stereochemistry in six-membered chair-like rings using residual dipolar couplings. See Yan, J.; Kline, A. D.; Mo, H.; Shapiro, M. J.; Zartler, E. R. J. Org. Chem. 2003, 68, 1786.
134. See Carey, F.A.; Kuehne, M.E. J. Org. Chem. 1982, 47, 3811; Boguslavskaya, L.S. J. Org. Chem. USSR 1986, 22, 1412; Seebach, D.; Prelog, V. Angew. Chem. Int. Ed. 1982, 21, 654; Brewster, J.H. J. Org. Chem. 1986, 51, 4751. See also, Tavernier, D. J. Chem. Educ. 1986, 63, 511; Brook, M.A. J. Chem. Educ. 1987, 64, 218.
135. For still another system, see Seebach, D.; Prelog, V. Angew. Chem. Int. Ed. 1982, 21, 654.
136. Masamune, S.; Kaiho, T.; Garvey, D.S. J. Am. Chem. Soc. 1982, 104, 5521.
137. See Morrison, J.D.; Scott, J.W. Asymmetric Synthesis, Vol. 4; Academic Press, NY, 1984; Williams, R.M. Synthesis of Optically Active α-Amino Acids, Pergamon, Elmsford, NY, 1989; Crosby, J. Tetrahedron 1991, 47, 4789; Mori, K. Tetrahedron 1989, 45, 3233.
138. See Coppola, G.M.; Schuster, H.F. Asymmetric Synthesis, Wiley, NY, 1987; Hanessian, S. Total Synthesis of Natural Products: The Chiron Approach, Pergamon, Elmsford, NY, 1983; Hanessian, S. Aldrichim. Acta 1989, 22, 3; Jurczak, J.; Gotebiowski, A. Chem. Rev. 1989, 89, 149.
139. See Morrison, J.D. Asymmetric Synthesis 5 Vols. [Vol. 4 coedited by Scott, J.W.], Academic Press, NY, 1983–1985; Nógrádi, M. Stereoselective Synthesis, VCH, NY, 1986; Eliel, E.L.; Otsuka, S. Asymmetric Reactions and Processes in Chemistry, American Chemical Society, Washington, 1982; Morrison, J.D.; Mosher, H.S. Asymmetric Organic Reactions, Prentice-Hall, Englewood Cliffs, NJ, 1971, paperback reprint, American Chemical Society, Washington, 1976. For reviews, see Ward, R.S. Chem. Soc. Rev. 1990, 19, 1; Whitesell, J.K. Chem. Rev. 1989, 89, 1581; Fujita, E.; Nagao, Y. Adv. Heterocycl. Chem. 1989, 45, 1; Kochetkov, K.A.; Belikov, V.M. Russ. Chem. Rev.1987, 56, 1045; Oppolzer, W. Tetrahedron 1987, 43, 1969; Seebach, D.; Imwinkelried, R.; Weber, T. Mod. Synth. Methods, 1986, 4, 125; ApSimon, J.W.; Collier, T.L. Tetrahedron 1986, 42, 5157.
140. Leitereg, T.J.; Cram, D.J. J. Am. Chem. Soc. 1968, 90, 4019. For discussions, see Anh, N.T. Top. Curr. Chem, 1980, 88, 145, pp. 151–161; Eliel, E.L. in Morrison, J.D. Asymmetric Synthesis, Vol. 2, Academic Press, NY, 1983, pp. 125–155. See Smith, R.J.; Trzoss, M; Bühl, M.; Bienz, S. Eur. J. Org. Chem. 2002, 2770.
141. See Eliel, E.L. The Stereochemistry of Carbon Compounds, McGraw-Hill, NY, 1962, pp. 68–74; Bartlett, P.A. Tetrahedron 1980, 36, 2, pp. 22–28; Ashby, E.C.; Laemmle, J.T. Chem. Rev. 1975, 75, 521; Goller, E.J. J. Chem. Educ. 1974, 51, 182; Toromanoff, E. Top. Stereochem. 1967, 2, 157.
142. Chérest, M.; Felkin, H.; Prudent, N. Tetrahedron Lett. 1968, 2199; Chérest, M.; Felkin, H. Tetrahedron Lett. 1968, 2205; Anh, N.T.; Eisenstein, O. Nov. J. Chem. 1977, 1, 61. For experiments that show explanations for certain systems based on the Felkin–Anh model to be weak, see Yadav, V.K.; Gupta, A.; Balamurugan, R.; Sriramurthy, V.; Kumar, N.V. J. Org. Chem. 2006, 71, 4178.
143. Cornforth, J.W.; Cornforth, R.H.; Mathew, K.K. J. Chem. Soc. 1959, 112; Evans, D.A.; Siska, S.J.; Cee, V.J. Angew. Chem. Int. Ed. 2003, 42, 1761.
144. See Eliel, E.L. in Morrison, J.D. Asymmetric Synthesis, Vol. 2, Academic Press, NY, 1983, pp. 125–155; Eliel, E.L.; Koskimies, J.K.; Lohri, B. J. Am. Chem. Soc. 1978, 100, 1614; Still, W.C.; McDonald, J.H. Tetrahedron Lett. 1980, 21, 1031; Still, W.C.; Schneider, J.A. Tetrahedron Lett. 1980, 21, 1035.
145. Cee, V.J.; Cramer, C.J.; Evans, D.A. J. Am. Chem. Soc. 2006, 128, 2920.
146. Evans, S.V.; Garcia-Garibay, M.; Omkaram, N.; Scheffer, J.R.; Trotter, J.; Wireko, F. J. Am. Chem. Soc. 1986, 108, 5648; Garcia-Garibay, M.; Scheffer, J.R.; Trotter, J.; Wireko, F. Tetrahedron Lett. 1987, 28, 4789. For an earlier example, see Penzien, K.; Schmidt, G.M.J. Angew. Chem. Int. Ed. 1969, 8, 608.
147. Enders, D.; Eichenauer, H.; Baus, U.; Schubert, H.; Kremer, K.A.M. Tetrahedron 1984, 40, 1345.
148. See Brockmann, Jr., H.; Risch, N. Angew. Chem. Int. Ed. 1974, 13, 664; Potapov, V.M.; Gracheva, R.A.; Okulova, V.F. J. Org. Chem. USSR 1989, 25, 311.
149. Meyers, A.I.; Oppenlaender, T. J. Am. Chem. Soc. 1986, 108, 1989. For reviews of asymmetric reduction, see Morrison, J.D. Surv. Prog. Chem. 1966, 3, 147; Yamada, S.; Koga, K. Sel. Org. Transform., 1970, 1, 1. See also, Morrison, J.D. Asymmetric Synthesis, Vol. 2, Academic Press, NY, 1983.
150. See, in Morrison, J.D. Asymmetric Synthesis, Vol. 5, Academic Press, NY, 1985, the reviews by Halpern, J. pp. 41–69, Koenig, K.E. pp. 71–101, Harada, K. pp. 345–383; Ojima, I.; Clos, N.; Bastos, C. Tetrahedron 1989, 45, 6901, pp. 6902–6916; Jardine, F.H. in Hartley, F.R. The Chemistry of the Metal–Carbon Bond, Vol. 4, Wiley, NY, 1987, pp. 751–775; Nógrádi, M. Stereoselective Synthesis, VCH, NY, 1986, pp. 53–87; Knowles, W.S. Acc. Chem. Res. 1983, 16, 106; Brunner, H. Angew. Chem. Int. Ed. 1983, 22, 897; Sathyanarayana, B.K.; Stevens, E.S. J. Org. Chem. 1987, 52, 3170; Wroblewski, A.E.; Applequist, J.; Takaya, A.; Honzatko, R.; Kim, S.; Jacobson, R.A.; Reitsma, B.H.; Yeung, E.S.; Verkade, J.G. J. Am. Chem. Soc. 1988, 110, 4144.
151. Goering, H.L.; Kantner, S.S.; Tseng, C.C. J. Org. Chem. 1983, 48, 715.
152. For a review, see Masamune, S.; Choy, W.; Petersen, J.S.; Sita, L.R. Angew. Chem. Int. Ed. 1985, 24, 1.
153. For a monograph, see Morrison, J.D. Asymmetric Synthesis, Vol. 5, Academic Press, NY, 1985. For reviews, see Tomioka, K. Synthesis 1990, 541; Consiglio, G.; Waymouth, R.M. Chem. Rev. 1989, 89, 257; Brunner, H. in Hartley, F.R. The Chemistry of the Metal–Carbon Bond, Vol. 5, Wiley, NY, 1989, pp. 109–146; Noyori, R.; Kitamura, M. Mod. Synth. Methods 1989, 5, 115; Pfaltz, A. Mod. Synth. Methods 1989, 5, 199; Kagan, H.B. Bull. Soc. Chim. Fr. 1988, 846; Brunner, H. Synthesis 1988, 645; Wynberg, H. Top. Stereochem. 1986, 16, 87.
154. For reviews of these and related topics, see Zief, M.; Crane, L.J. Chromatographic Separations, Marcel Dekker, NY, 1988; Brunner, H. J. Organomet. Chem. 1986, 300, 39; Bosnich, B.; Fryzuk, M.D. Top. Stereochem. 1981, 12, 119.
155. See Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994. Also see, Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010. For random examples, see Wu, Q.-F.; He, H.; Liu, W.-B.; You, S.-L. J. Am. Chem. Soc. 2010, 132, 11418; Berhal, F.; Wu, Z.; Genet, J.-P.; Ayad, T.; Ratovelomanana-Vidal, V. J. Org. Chem. 2011, 76, 6320; He, P.; Liu, X.; Shi, J.; Lin, L.; Feng, X. Org. Lett. 2011, 13, 936; Yang, H.-M.; Li, L.; Li, F.; Jiang, K.-Z.; Shang, J.-Y.; Lai, G.-Q.; Xu, L.-W. Org. Lett. 2011, 13, 6508.
156. Findeis, M.A.; Whitesides, G.M. J. Org. Chem. 1987, 52, 2838; Réty, J.; Robinson, J.A. Stereospecificity in Organic Chemistry and Enzymology, Verlag Chemie, Deerfield Beach, FL, 1982. For reviews, see Klibanov, A.M. Acc. Chem. Res. 1990, 23, 114; Jones, J.B. Tetrahedron 1986, 42, 3351; Jones, J.B. in Morrison, J.D. Asymmetric Synthesis, Vol. 5, Academic Press, NY, 1985, pp. 309–344.
157. Heathcock, C.H.; White, C.T. J. Am. Chem. Soc. 1979, 101, 7076.
158. For a review, See Buchardt, O. Angew. Chem. Int. Ed. 1974, 13, 179. For a discussion, see Barron L.D. J. Am. Chem. Soc. 1986, 108, 5539.
159. See Bernstein, W.J.; Calvin, M.; Buchardt, O. J. Am. Chem. Soc. 1973, 95, 527; Nicoud, J.F.; Kagan, J.F. Isr. J. Chem. 1977, 15, 78. See also, Zandomeneghi, M.; Cavazza, M.; Pietra, F. J. Am. Chem. Soc. 1984, 106, 7261.
160. Faigl, F.; Fogassy, E.; Nógrádi, M.; Pálovics, E.; Schindler, J. Tetrahedron Asymm. 2008, 19, 519. See Wilen, S.H.; Collet, A.; Jacques, J. Tetrahedron 1977, 33, 2725; Boyle, P.H. Q. Rev. Chem. Soc. 1971, 25, 323; Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 297–424; Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, aand Resolutions, Wiley, NY, 1981.
161. Schiffers, I.; Rantanen, T.; Schmidt, F.; Bergmans, W.; Zani, L.; Bolm, C. J. Org. Chem. 2006, 71, 2320.
162. See Boyle, P.H. Q. Rev. Chem. Soc. 1971, 25, 323; Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 322–424.
163. For an extensive list of reagents that have been used for this purpose and of compounds resolved, see Wilen, S.H. Tables of Resolving Agents and Optical Resolutions, University of Notre Dame Press, Notre Dame, IN, 1972.
164. See Klyashchitskii, B.A.; Shvets, V.I. Russ. Chem. Rev. 1972, 41, 592.
165. Periasamy, M.; Kumar, N.S.; Sivakumar, S.; Rao, V.D.; Ramanathan, C.R.; Venkatraman, L. J. Org. Chem. 2001, 66, 3828.
166. Andersen, N.G.; Ramsden, P.D.; Che, D.; Parvez, M.; Keay, B.A. J. Org. Chem. 2001, 66, 7478.
167. Caccamese, S.; Bottino, A.; Cunsolo, F.; Parlato, S.; Neri, P. Tetrahedron Asymm. 2000, 11, 3103.
168. See Slingenfelter, D.S.; Helgeson, R.C.; Cram, D.J. J. Org. Chem. 1981, 46, 393; Davidson, R.B.; Bradshaw, J.S.; Jones, B.A.; Dalley, N.K.; Christensen, J.J.; Izatt, R.M.; Morin, F.G.; Grant, D.M. J. Org. Chem. 1984, 49, 353.
169. See Prelog, V.; Kova![]() evi
evi![]() , M.; Egli, M. Angew. Chem. Int. Ed. 1989, 28, 1147; Worsch, D.; Vögtle, F. Top. Curr. Chem. 1987, 140, 21; Toda, F. Top. Curr. Chem. 1987, 140, 43; Stoddart, J.F. Top. Stereochem. 1987, 17, 207; Arad-Yellin, R.; Green, B.S.; Knossow, M.; Tsoucaris, G. in Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D. Inclusion Compounds, Vol. 3, Academic Press, NY, 1984, pp. 263–295.
, M.; Egli, M. Angew. Chem. Int. Ed. 1989, 28, 1147; Worsch, D.; Vögtle, F. Top. Curr. Chem. 1987, 140, 21; Toda, F. Top. Curr. Chem. 1987, 140, 43; Stoddart, J.F. Top. Stereochem. 1987, 17, 207; Arad-Yellin, R.; Green, B.S.; Knossow, M.; Tsoucaris, G. in Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D. Inclusion Compounds, Vol. 3, Academic Press, NY, 1984, pp. 263–295.
170. See Schlenk, Jr., W. Liebigs Ann. Chem. 1973, 1145, 1156, 1179, 1195. See Arad-Yellin, R.; Green, B.S.; Knossow, M.; Tsoucaris, G. J. Am. Chem. Soc. 1983, 105, 4561.
171. Miyamoto, H.; Sakamoto, M.; Yoskioka, K.; Takaoka, R.; Toda, F. Tetrahedron Asymm. 2000, 11, 3045.
172. For a review, see Tsuji, J. Adv. Org. Chem. 1969, 6, 109, see p. 220.
173. Amos, R.D.; Handy, N.C.; Jones, P.G.; Kirby, A.J.; Parker, J.K.; Percy, J.M.; Su, M.D. J. Chem. Soc. Perkin Trans. 2 1992, 549.
174. See Westley, J.W.; Halpern, B.; Karger, B.L. Anal. Chem. 1968, 40, 2046; Kawa, H.; Yamaguchi, F.; Ishikawa, N. Chem. Lett. 1982, 745.
175. See Meyers, A.I.; Slade, J.; Smith, R.K.; Mihelich, E.D.; Hershenson, F.M.; Liang, C.D. J. Org. Chem. 1979, 44, 2247; Goldman, M.; Kustanovich, Z.; Weinstein, S.; Tishbee, A.; Gil-Av, E. J. Am. Chem. Soc. 1982, 104, 1093.
176. See Lough, W.J. Chiral Liquid Chromatography; Blackie and Sons: London, 1989; Krstulovi![]() , A.M. Chiral Separations by HPLC, Ellis Horwood, Chichester, 1989; Zief, M.; Crane, L.J. Chromatographic Separations, Marcel Dekker, NY, 1988. For a review, see Karger, B.L. Anal. Chem. 1967, 39(8), 24A.
, A.M. Chiral Separations by HPLC, Ellis Horwood, Chichester, 1989; Zief, M.; Crane, L.J. Chromatographic Separations, Marcel Dekker, NY, 1988. For a review, see Karger, B.L. Anal. Chem. 1967, 39(8), 24A.
177. Weinstein, S. Tetrahedron Lett. 1984, 25, 985.
178. See Allenmark, S.G. Chromatographic Enantioseparation, Ellis Horwood, Chichester, 1988; König, W.A. The Practice of Enantiomer Separation by Capillary Gas Chromatography, Hüthig, Heidelberg, 1987. For reviews, see Schurig, V.; Nowotny, H. Angew. Chem. Int. Ed. 1990, 29, 939; Pirkle, W.H.; Pochapsky, T.C. Chem. Rev. 1989, 89, 347; Blaschke, G. Angew. Chem. Int. Ed. 1980, 19, 13; Rogozhin, S.V.; Davankov, V.A. Russ. Chem. Rev.1968, 37, 565. See also, many articles in the journal Chirality.
179. Ohara, M.; Ohta, K.; Kwan, T. Bull. Chem. Soc. Jpn. 1964, 37, 76. See also, Blaschke, G.; Donow, F. Chem. Ber. 1975, 108, 2792; Hess, H.; Burger, G.; Musso, H. Angew. Chem. Int. Ed. 1978, 17, 612.
180. See Schurig, V.; Nowotny, H.; Schmalzing, D. Angew. Chem. Int. Ed. 1989, 28, 736; Ôi, S.; Shijo, M.; Miyano, S. Chem. Lett. 1990, 59; Erlandsson, P.; Marle, I.; Hansson, L.; Isaksson, R.; Pettersson, C.; Pettersson, G. J. Am. Chem. Soc. 1990, 112, 4573.
181. See, for example, Pirkle, W.H.; Welch, C.J. J. Org. Chem. 1984, 49, 138.
182. Kanoh, S.; Hongoh, Y.; Katoh, S.; Motoi, M.; Suda, H. J. Chem. Soc. Chem. Commun. 1988, 405; Bradshaw, J.S.; Huszthy, P.; McDaniel, C.W.; Zhu, C.Y.; Dalley, N.K.; Izatt, R.M.; Lifson, S. J. Org. Chem. 1990, 55, 3129.
183. See, for example, Hamilton, J.A.; Chen, L. J. Am. Chem. Soc. 1988, 110, 5833.
184. See Miyata, M.; Shibakana, M.; Takemoto, K. J. Chem. Soc. Chem. Commun. 1988, 655.
185. For a review, see Sih, C.J.; Wu, S. Top. Stereochem. 1989, 19, 63.
186. See Nakamura, K.; Inoue, Y.; Ohno, A. Tetrahedron Lett. 1994, 35, 4375; Kazlauskas, R.J. J. Am. Chem. Soc. 1989, 111, 4953; Schwartz, A.; Madan, P.; Whitesell, J.K.; Lawrence, R.M. Org. Synth. 69, 1. For resolution with Subtilisin, see Savile, C.K.; Magloire, V.P.; Kazlauskas, R.J. J. Am. Chem. Soc. 2005, 127, 2104. For the chemoenzymatic kinetic resolution of primary amines, see Paetzold, J.; Bäckvall, J.E. J. Am. Chem. Soc. 2005, 127, 17620.
187. For an example, see Gais, H.-J.; Jungen, M.; Jadhav, V. J. Org. Chem. 2001, 66, 3384.
188. For an example of the resolution of acyloins, see Ödman, P.; Wessjohann, L.A.; Bornscheuer, U.T. J. Org. Chem. 2005, 70, 9551.
189. For reviews, see Collet, A.; Brienne, M.; Jacques, J. Chem. Rev. 1980, 80, 215; Bull. Soc. Chim. Fr. 1972, 127; 1977, 494. For a discussion, see Curtin, D.Y.; Paul, I.C. Chem. Rev. 1981, 81, 525 pp. 535–536.
190. Besides discovering this method of resolution, Pasteur also discovered the method of conversion to diastereomers and separation by fractional crystallization and the method of biochemical separation (and, by extension, kinetic resolution).
191. This is a case of optically active materials arising from inactive materials. However, it may be argued that an optically active investigator is required to use the tweezers. Perhaps a hypothetical human being constructed entirely of inactive molecules would be unable to tell the difference between left- and right-handed crystals.
192. For a review of the seeding method, see Secor, R.M. Chem. Rev. 1963, 63, 297.
193. Martin, R.H; Baes, M. Tetrahedron 1975, 31, 2135. See also, Wynberg, H.; Groen, M.B. J. Am. Chem. Soc. 1968, 90, 5339; McBride, J.M.; Carter, R.L. Angew. Chem. Int. Ed. 1991, 30, 293.
194. Kress, R.B.; Duesler, E.N.; Etter, M.C.; Paul, I.C.; Curtin, D.Y. J. Am. Chem. Soc. 1980, 102, 7709. See also, Gottarelli, G.; Spada, G.P. J. Org. Chem. 1991, 56, 2096. For a discussion and other examples, see Agranat, I.; Perlmutter-Hayman, B.; Tapuhi, Y. Nouv. J. Chem. 1978, 2, 183.
195. Addadi, L.; Weinstein, S.; Gati, E.; Weissbuch, I.; Lahav, M. J. Am. Chem. Soc. 1982, 104, 4610. See also, Weissbuch, I.; Addadi, L.; Berkovitch-Yellin, Z.; Gati, E.; Weinstein, S.; Lahav, M.; Leiserowitz, L. J. Am. Chem. Soc. 1983, 105, 6615.
196. Paquette, L.A.; Lau, C.J. J. Org. Chem. 1987, 52, 1634.
197. For reviews, see Pellissier, H. Tetrahedron 2008, 64, 1563; Ward, R.S. Tetrahedron Asymm. 1995, 6, 1475; Pellissier, H. Tetrahedron 2003, 59, 8291.
198. Lu, Y.; Zhao, X.; Chen, Z.-N. Tetrahedron Asymm. 1995, 6, 1093.
199. Brown, H.C.; Ayyangar, N.R.; Zweifel, G. J. Am. Chem. Soc. 1964, 86, 397.
200. Carlier, P.R.; Mungall, W.S.; Schröder, G.; Sharpless, K.B. J. Am. Chem. Soc. 1988, 110, 2978; Discordia, R.P.; Dittmer, D.C. J. Org. Chem. 1990, 55, 1414. For other examples, see Katamura, M.; Ohkuma, T.; Tokunaga, M.; Noyori, R. Tetrahedron Asymm. 1990, 1, 1; Hayashi, M.; Miwata, H.; Oguni, N. J. Chem. Soc. Perkin Trans. 2 1991, 1167.
201. Lohray, B.B.; Bhushan, V. Tetrahedron Lett. 1993, 34, 3911.
202. Kubota, H.; Kubo, A.; Nunami, K. Tetrahedron Lett. 1994, 35, 3107.
203. Tomooka, K.; Komine, N.; Fujiki, D.; Nakai, T.; Yanagitsuru, S. J. Am. Chem. Soc. 2005, 127, 12182.
204. Pirkle, W.H.; Reno, D.S. J. Am. Chem. Soc. 1987, 109, 7189. For another example, see Reider, P.J.; Davis, P.; Hughes, D.L.; Grabowski, E.J.J. J. Org. Chem. 1987, 52, 955.
205. Stecher, H.; Faber, K. Synthesis 1997, 1.
206. Allan, G. R.; Carnell, A. J. J. Org. Chem. 2001, 66, 6495.
207. For a review, see Raban, M.; Mislow, K. Top. Stereochem. 1967, 2, 199.
208. If a sample contains 80% (+) and 20% (−) isomer, the (−) isomer cancels an equal amount of (+) isomer and the mixture behaves as if 60% of it were (+) and the other 40% inactive. Therefore the rotation is 60% of 80° or 48°. This type of calculation, however, is not valid for cases in which [α] is dependent on concentration (Sec. 4.B); see Horeau, A. Tetrahedron Lett. 1969, 3121.
209. For a method to measure %ee using electrooptics, see Walba, D.M.; Eshdat, L.; Korblova, E.; Shao, R.; Clark, N.A. Angew. Chem. Int. Ed. 2007, 46, 1473.
210. Raban, M.; Mislow, K. Tetrahedron Lett. 1965, 4249, 1966, 3961; Jacobus, J.; Raban, M. J. Chem. Educ. 1969, 46, 351; Tokles, M.; Snyder, J.K. Tetrahedron Lett. 1988, 29, 6063. For a review, see Yamaguchi, S. in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, pp. 125–152. See also, Raban, M.; Mislow, K. Top. Stereochem. 1967, 2, 199.
211. Though enantiomers give identical NMR spectra, the spectrum of a single enantiomer may be different from that of the racemic mixture, even in solution. See Williams, T.; Pitcher, R.G.; Bommer, P.; Gutzwiller, J.; Uskokovi![]() , M. J. Am. Chem. Soc. 1969, 91, 1871.
, M. J. Am. Chem. Soc. 1969, 91, 1871.
212. Raban, M.; Mislow, K. Top. Stereochem. 1967, 2, 199, see pp. 216–218.
213. For a method that relies on diastereomer formation without a chiral reagent, see Feringa, B.L.; Strijtveen, B.; Kellogg, R.M. J. Org. Chem. 1986, 51, 5484. See also, Pasquier, M.L.; Marty, W. Angew. Chem. Int. Ed. 1985, 24, 315; Luchinat, C.; Roelens, S. J. Am. Chem. Soc. 1986, 108, 4873.
214. See Trost, B.M.; Belletire, J.L.; Godleski, S.; McDougal, P.G.; Balkovec, J.M.; Baldwin, J.J.; Christy, M.E.; Ponticello, G.S.; Varga, S.L.; Springer, J.P. J. Org. Chem. 1986, 51, 2370.
215. For reviews of NMR chiral solvating agents, see Weisman, G.R. in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, pp. 153–171; Pirkle, W.H.; Hoover, D.J. Top. Stereochem. 1982, 13, 263. Sweeting, L.M.; Anet, F.A.L. Org. Magn. Reson. 1984, 22, 539. See also, Pirkle, W.H.; Tsipouras, A. Tetrahedron Lett. 1985, 26, 2989; Parker, D.; Taylor, R.J. Tetrahedron 1987, 43, 5451.
216. Sweeting, L.M.; Crans, D.C.; Whitesides, G.M. J. Org. Chem. 1987, 52, 2273; Morrill, T.C. Lanthanide Shift Reagents in Stereochemical Analysis, VCH, NY, 1986; Fraser, R.R. in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, pp. 173–196; Sullivan, G.R. Top. Stereochem. 1978, 10, 287.
217. See Westley, J.W.; Halpern, B. J. Org. Chem. 1968, 33, 3978.
218. For a review, see Pirkle, W.H.; Finn, J. in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, pp. 87–124.
219. For reviews, see in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, the articles by Schurig, V. pp. 59–86 and Pirkle, W.H.; Finn, J. pp. 87–124.
220. See Hill, H.W.; Zens, A.P.; Jacobus, J. J. Am. Chem. Soc. 1979, 101, 7090; Matsumoto, M.; Yajima, H.; Endo, R. Bull. Chem. Soc. Jpn. 1987, 60, 4139.
221. Berson, J.A.; Ben-Efraim, D.A. J. Am. Chem. Soc. 1959, 81, 4083; Andersen, K.K.; Gash, D.M.; Robertson, J.D. in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, pp. 45–57.
222. Horeau, A.; Guetté, J.; Weidmann, R. Bull. Soc. Chim. Fr. 1966, 3513. For a review, see Schoofs, A.R.; Guetté, J. in Morrison, J.D. Asymmetric Synthesis, Vol. 1, Academic Press, NY, 1983, pp. 29–44.
223. Hofer, E.; Keuper, R. Tetrahedron Lett. 1984, 25, 5631.
224. Schippers, P.H.; Dekkers, H.P.J.M. Tetrahedron 1982, 38, 2089.
225. cis-trans isomerism was formerly called geometrical isomerism.
226. For a complete description of the system, see Pure Appl. Chem. 1976, 45, 13; Nomenclature of Organic Chemistry, Pergamon, Elmsford, NY, 1979 (the Blue Book).
227. See in Patai, S. The Chemistry of the Carbon–Nitrogen Double Bond, Wiley, NY, 1970, the articles by McCarty, C.G. pp. 363–464 (pp. 364–408), and Wettermark, G. pp. 565–596 (pp. 574–582).
228. Wang, Y.-N.; Bohle, D.S.; Bonifant, C.L.; Chmurny, G.N.; Collins, J.R.; Davies, K.M.; Deschamps, J.; Flippen-Anderson, J.L.; Keefer, L.K.; Klose, J.R.; Saavedra, J.E.; Waterhouse, D.J.; Ivanic, J. J. Am. Chem. Soc. 2005, 127, 5388.
229. King, J.F.; Durst, T. Can. J. Chem. 1966, 44, 819.
230. A mechanism has been reported for the acid-catalyzed Z/E isomerization of imines. See Johnson, J.E.; Morales, N.M.; Gorczyca, A.M.; Dolliver, D.D.; McAllister, M.A. J. Org. Chem. 2001, 66, 7979.
231. This rule does not apply to allenes, which do not show cis--trans isomerism (see Sec. 4.C, category 5).
232. Cope, A.C.; Moore, P.T.; Moore, W.R. J. Am. Chem. Soc. 1959, 81, 3153.
233. Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985, pp. 41–71.
234. Mannschreck, A. Angew. Chem. Int. Ed. 1965, 4, 985. See also, Völter, H.; Helmchen, G. Tetrahedron Lett. 1978, 1251; Walter, W.; Hühnerfuss, H. Tetrahedron Lett. 1981, 22, 2147.
235. This is another example of atropisomerism (Sec. 4.C, category 5).
236. For reviews, see Sandström, J. Top. Stereochem. 1983, 14, 83; Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985, pp. 111–125.
237. Sandström, J.; Wennerbeck, I. Acta Chem. Scand. Ser. B, 1978, 32, 421.
238. Yamamoto, T.; Tomoda, S. Chem. Lett. 1997, 1069.
239. See Leonard, J.E.; Hammond, G.S.; Simmons, H.E. J. Am. Chem. Soc. 1975, 97, 5052.
240. Sorensen, T.S.; Sun, F. J. Chem. Soc. Perkin Trans. 2 1998, 1053.
241. Meinwald, J.; Tufariello, J.J.; Hurst, J.J. J. Org. Chem. 1964, 29, 2914.
242. Paukstelis, J.V.; Kao, J. J. Am. Chem. Soc. 1972, 94, 4783. For references to other examples, see Dixon, D.A.; Gassman, P.G. J. Am. Chem. Soc. 1988, 110, 2309.
243. Corbally, R.P.; Perkins, M.J.; Carson, A.S.; Laye, P.G.; Steele, W.V. J. Chem. Soc. Chem. Commun. 1978, 778.
244. Winkler, J.D.; Hey, J.P.; Williard, P.G. Tetrahedron Lett. 1988, 29, 4691.
245. See Alder, R.W. Acc. Chem. Res. 1983, 16, 321.
246. Alder, R.W.; East, S.P. Chem. Rev. 1996, 96, 2097.
247. Alder, R.W. Tetrahedron 1990, 46, 683.
248. Alder, R.W.; Orpen, A.G.; Sessions, R.B. J. Chem. Soc., Chem. Commun. 1983, 999.
249. Alder, R.W.; Goode, N.C.; King, T.J.; Mellor, J.M.; Miller, B.W. J. Chem. Soc., Chem. Commun. 1976, 173; Alder, R.W.; Arrowsmith, R.J.; Casson, A.; Sessions, R.B.; Heilbronner, E.; Kovac, B.; Huber, H.; Taagepera, M. J. Am. Chem. Soc. 1981, 103, 6137.
250. Alder, R.W.; Heilbronner, E.; Honegger, E.; McEwen, A.B.; Moss, R.E.; Olefirowicz, E.; Petillo, P.A.; Sessions, R.B.; Weisman, G.R.; White, J.M.; Yang, Z.-Z. J. Am. Chem. Soc. 1993, 115, 6580.
251. Simmons, H.E.; Park, C.H. J. Am. Chem. Soc. 1968, 90, 2428; Park, C.H.; Simmons, H.E. J. Am. Chem. Soc. 1968, 90, 2429, 2431; Simmons, H.E.; Park, C.H.; Uyeda, R.T.; Habibi, M.F. Trans. N.Y. Acad. Sci. 1970, 32, 521. See also, Dietrich, B.; Lehn, J.M.; Sauvage, J.P. Tetrahedron 1973, 29, 1647; Dietrich, B.; Lehn, J.M.; Sauvage, J.P.; Blanzat, J. Tetrahedron 1973, 29, 1629.
252. See Schmidtchen, F.P.; Gleich, A.; Schummer, A. Pure. Appl. Chem. 1989, 61, 1535; Pierre, J.; Baret, P. Bull. Soc. Chim. Fr. 1983, II-367. See also, Hosseini, M.W.; Lehn, J. Helv. Chim. Acta 1988, 71, 749.
253. Dietrich, B.; Lehn, J.M.; Guilhem, J.; Pascard, C. Tetrahedron Lett. 1989, 30, 4125; Wallon, A.; Peter-Katalini![]() , J.; Werner, U.; Müller, W.M.; Vögtle, F. Chem. Ber. 1990, 123, 375.
, J.; Werner, U.; Müller, W.M.; Vögtle, F. Chem. Ber. 1990, 123, 375.
254. Schmidtchen, F.P.; Müller, G. J. Chem. Soc. Chem. Commun. 1984, 1115. See also, Schmidtchen, F.P. J. Am. Chem. Soc. 1986, 108, 8249, Top. Curr. Chem. 1986, 132, 101.
255. McMurry, J.E.; Hodge, C.N. J. Am. Chem. Soc. 1984, 106, 6450; Winkler, J.D.; Hey, J.P.; Williard, P.G. J. Am. Chem. Soc. 1986, 108, 6425.
256. See Baechler, R.D.; Mislow, K. J. Am. Chem. Soc. 1970, 92, 3090; Rauk, A.; Allen, L.C.; Mislow, K. Angew. Chem. Int. Ed. 1970, 9, 400.
257. Alder, R.W.; Read, D. Angew. Chem. Int. Ed. 2000, 39, 2879.
258. Alder, R.W.; Ellis, D.D.; Gleiter, R.; Harris, C.J.; Lange, H.; Orpen, A.G.; Read, D.; Taylor, P.N. J. Chem. Soc., Perkin Trans. I 1998, 1657.
259. These terms were coined by Mislow. See Eliel, E.L. Top. Curr. Chem. 1982, 105, 1; Mislow, K.; Raban, M. Top. Stereochem. 1967, 1, 1. See also, Jennings, W.B. Chem. Rev. 1975, 75, 307.
260. In the case where Y is itself a chiral group, this statement is only true when the two Y groups have the same configuration.
261. For a review, see Benner, S.A.; Glasfeld, A.; Piccirilli, J.A. Top. Stereochem. 1989, 19, 127. For a nonenzymatic example, see Job, R.C.; Bruice, T.C. J. Am. Chem. Soc. 1974, 96, 809.
262. The experiments were carried out by Evans Jr., E.A.; Slotin, L. J. Biol. Chem. 1941, 141, 439; Wood, H.G.; Werkman, C.H.; Hemingway, A.; Nier, A.O. J. Biol. Chem. 1942, 142, 31. The correct interpretation was given by Ogston, A.G. Nature (London) 1948, 162, 963. For discussion, see Eliel, E.L. Top. Curr. Chem. 1982, 105, 1, pp. 5–7, 45–70.
263. Hirschmann, H.; Hanson, K.R. Tetrahedron 1974, 30, 3649.
264. Pirkle, W.H. J. Am. Chem. Soc. 1966, 88, 1837; Burlingame, T.G.; Pirkle, W.H. J. Am. Chem. Soc. 1966, 88, 4294; Pirkle, W.H.; Burlingame, T.G. Tetrahedron Lett. 1967, 4039.
265. For a review of isochronous and nonisochronous nuclei in NMR, see van Gorkom, M.; Hall, G.E. Q. Rev. Chem. Soc. 1968, 22, 14. For a discussion, see Silverstein, R.M.; LaLonde, R.T. J. Chem. Educ. 1980, 57, 343.
266. Fujita, S. J. Org. Chem. 2002, 67, 6055.
267. Fujita, S. J. Am. Chem. Soc. 1990, 112, 3390.
268. For a further discussion of these terms and of stereoselective reactions in general, see Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 835–990.
269. See Bonchev, D.; Rouvray, D.H. Chemical Topology, Gordon and Breach, Australia, 1999.
270. See Dale, J. Stereochemistry and Conformational Analysis, Verlag Chemie, Deerfield Beach, FL, 1978; Chiurdoglu, G. Conformational Analysis, Academic Press, NY, 1971; Eliel, E.L.; Allinger, N.L.; Angyal, S.J.; Morrison, G.A. Conformational Analysis, Wiley, NY, 1965; Hanack, M. Conformation Theory, Academic Press, NY, 1965. For reviews, see Dale, J. Top. Stereochem. 1976, 9, 199; Truax, D.R.; Wieser, H. Chem. Soc. Rev. 1976, 5, 411; Eliel, E.L. J. Chem. Educ. 1975, 52, 762; Bastiansen, O.; Bushweller, C.H.; Gianni, M.H. in Patai, S. The Chemistry of Functional Groups, Supplement E, Wiley, NY, 1980, pp. 215–278.
271. Öki, M. The Chemistry of Rotational Isomers, Springer–Verlag, Berlin, 1993.
272. For a review, see Eliel, E.L.; Allinger, N.L.; Angyal, S.J.; Morrison, G.A. Conformational Analysis, Wiley, NY, 1965, pp. 129–188.
273. See Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985; Marshall, J.L. Carbon–Carbon and Carbon–Proton NMR Couplings, VCH, NY, 1983. For reviews, see Anet, F.A.L.; Anet, R. in Nachod, F.C.; Zuckerman, J.J. Determination of Organic Structures by Physical Methods, Vol. 3, Academic Press, NY, 1971, pp. 343–420; Kessler, H. Angew. Chem. Int. Ed. 1970, 9, 219; Ivanova, T.M.; Kugatova-Shemyakina, G.P. Russ. Chem. Rev. 1970, 39, 510; See also, Whitesell, J.K.; Minton, M. Stereochemical Analysis of Alicyclic Compounds by C-13 NMR Spectroscopy, Chapman and Hall, NY, 1987.
274. For a review see Wilson, E.B. Chem. Soc. Rev. 1972, 1, 293.
275. For a review, see Klessinger, M.; Rademacher, P. Angew. Chem. Int. Ed. 1979, 18, 826.
276. Breen, P.J.; Warren, J.A.; Bernstein, E.R.; Seeman, J.I. J. Am. Chem. Soc. 1987, 109, 3453.
277. See Kagan, H.B. Determination of Configurations by Dipole Moments, CD, or ORD (Vol. 2 of Kagan, H.B. Stereochemistry), Georg Thieme Publishers, Stuttgart, 1977; Crabbé, P. ORD and CD in Chemistry and Biochemistry, Academic Press, NY, 1972; Snatzke, G. Optical Rotatory Dispersion and Circular Dichroism in Organic Chemistry, Sadtler Research Laboratories, Philadelphia, 1967; Velluz, L.; Legrand, M.; Grosjean, M. Optical Circular Dichroism, Academic Press, NY, 1965. For reviews, see Smith, H.E. Chem. Rev. 1983, 83, 359; Håkansson, R. in Patai, S. The Chemistry of Acid Derivatives, pt. 1, Wiley, NY, 1979, pp. 67–120; Hudec, J.; Kirk, D.N. Tetrahedron 1976, 32, 2475; Schellman, J.A. Chem. Rev. 1975, 75, 323.
278. Chen, J.; Cammers-Goodwin, A. Eur. J. Org. Chem. 2003, 3861.
279. Iwamoto, H.; Yang, Y.; Usui, S.; Fukazawa, Y. Tetrahedron Lett. 2001, 42, 49.
280. See Kessler, H.; Zimmermann, G.; Förster, H.; Engel, J.; Oepen, G.; Sheldrick, W.S. Angew. Chem. Int. Ed. 1981, 20, 1053.
281. Bérces, A.; Whitfield, D.M.; Nukada, T. Tetrahedron 2001, 57, 477.
282. Öki, M.; Toyota, S. Eur. J. Org. Chem. 2004, 255.
283. See Berg, U.; Sandström, J. Adv. Phys. Org. Chem. 1989, 25, 1. Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 597–664. Also see, Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010, pp. 35–47.
284. Goto, H.; Osawa, E.; Yamato, M. Tetrahedron 1993, 49, 387.
285. Lide Jr., D.R. J. Chem. Phys. 1958, 29, 1426; Weiss, S.; Leroi, G.E. J. Chem. Phys. 1968, 48, 962; Hirota, E.; Saito, S.; Endo, Y. J. Chem. Phys. 1979, 71, 1183.
286. Mo, Y.; Gao, J. Acc. Chem. Res. 2007, 40, 113.
287. See Lowe, J.P. Prog. Phys. Org. Chem. 1968, 6, 1; Oosterhoff, L.J. Pure Appl. Chem. 1971, 25, 563; Wyn-Jones, E.; Pethrick, R.A. Top. Stereochem. 1970, 5, 205; Pethrick, R.A.; Wyn-Jones, E. Q. Rev. Chem. Soc. 1969, 23, 301; Brier, P.N. J. Mol. Struct. 1970, 6, 23; Lowe, J.P. Science, 1973, 179, 527.
288. See Pitzer, R.M. Acc. Chem. Res. 1983, 16, 207. See, however, Bader, R.F.W.; Cheeseman, J.R.; Laidig, K.E.; Wiberg, K.B.; Breneman, C. J. Am. Chem. Soc. 1990, 112, 6530.
289. See Bader, W.; Cortés-Guzmán, F. Can. J. Chem. 2009, 87, 1583.
290. See Wiberg, K.B.; Murcko, M.A. J. Am. Chem. Soc. 1988, 110, 8029; Allinger, N.L.; Grev, R.S.; Yates, B.F.; Schaefer, III, H.F. J. Am. Chem. Soc. 1990, 112, 114.
291. Cormanich, R.A.; Freitas, M.P. J. Org. Chem. 2009, 74, 8384; Mo, Y. J. Org. Chem. 2010, 75, 2733.
292. Le Fèvre, R.J.W.; Orr, B.J. Aust. J. Chem. 1964, 17, 1098.
293. See Schrumpf, G. Angew. Chem. Int. Ed. 1982, 21, 146.
294. See Davenport, D.; Schwartz, M. J. Mol. Struct. 1978, 50, 259; Huang, J.; Hedberg, K. J. Am. Chem. Soc. 1989, 111, 6909.
295. See Friesen, D.; Hedberg, K. J. Am. Chem. Soc. 1980, 102, 3987; Fernholt, L.; Kveseth, K. Acta Chem. Scand. Ser. A 1980, 34, 163.
296. Abraham, R.J.; Monasterios, J.R. Org. Magn. Reson. 1973, 5, 305.
297. See Wolfe, S. Acc. Chem. Res. 1972, 5, 102. See also, Phillips, L.; Wray, V. J. Chem. Soc. Chem. Commun. 1973, 90; Radom, L.; Hehre, W.J.; Pople, J.A. J. Am. Chem. Soc. 1972, 94, 2371; Zefirov, N.S. J. Org. Chem. USSR1974, 10, 1147; Juaristi, E. J. Chem. Educ. 1979, 56, 438.
298. Griffith, R.C.; Roberts, J.D. Tetrahedron Lett. 1974, 3499.
299. Amos, R.D.; Handy, N.C.; Jones, P.G.; Kirby, A.J.; Parker, J.K.; Percy, J.M.; Su, M.D. J. Chem. Soc. Perkin Trans. 2 1992, 549.
300. Kagarise, R.E. J. Chem. Phys. 1956, 24, 300.
301. Brown, D.E.; Beagley, B. J. Mol. Struct. 1977, 38, 167.
302. Ritter, W.; Hull, W.; Cantow, H. Tetrahedron Lett. 1978, 3093.
303. Lunazzi, L.; Macciantelli, D.; Bernardi, F.; Ingold, K.U. J. Am. Chem. Soc. 1977, 99, 4573.
304. Tan, B.; Chia, L.H.L.; Huang, H.; Kuok, M.; Tang, S. J. Chem. Soc. Perkin Trans. 2 1984, 1407.
305. Smith, G.D.; Jaffe, R.L.; Yoon, D.Y. J. Am. Chem. Soc. 1995, 117, 530. For an analysis of N,N-dimethylacetamide, see Mack, H.-G.; Oberhammer, H. J. Am. Chem. Soc. 1997, 119, 3567.
306. Flamm-ter Meer; Beckhaus, H.; Peters, K.; von Schnering, H.; Fritz, H.; Rüchardt, C. Chem. Ber. 1986, 119, 1492; Rüchardt, C.; Beckhaus, H. Angew. Chem. Int. Ed. 1985, 24, 529.
307. See Sinegovskaya, L.M.; Keiko, V.V.; Trofimov, B.A. Sulfur Rep. 1987, 7, 337 (for enol ethers and thioethers); Karabatsos, G.J.; Fenoglio, D.J. Top. Stereochem. 1970, 5, 167; Jones, G.I.L.; Owen, N.L. J. Mol. Struct. 1973, 18, 1 (for carboxylic esters). See also, Cossé-Barbi, A.; Massat, A.; Dubois, J.E. Bull. Soc. Chim. Belg. 1985, 94, 919; Dorigo, A.E.; Pratt, D.W.; Houk, K.N. J. Am. Chem. Soc. 1987, 109, 6591.
308. Allinger, N.L.; Hickey, M.J. J. Mol. Struct. 1973, 17, 233; Gupta, V.P. Can. J. Chem. 1985, 63, 984.
309. Macoas, E.M.S.; Khriachtchev, L.; Pettersson, M.; Fausto, R.; Rasanen, M. J. Am. Chem. Soc. 2003, 125, 16188.
310. Davidson, R.B.; Allen, L.C. J. Chem. Phys. 1971, 54, 2828.
311. Ratajczyk, T.; Pecul, M.; Sadlej, J. Tetrahedron 2004, 60, 179.
312. Avalos, M.; Babiano, R.; Barneto, J.L.; Bravo, J.L.; Cintas, P.; Jiménez, J.L.; Palcios, J.C. J. Org. Chem. 2001, 66, 7275. Also see Modarresi-Alam, A.R.; Najafi, P.; Rostamizadeh, M.; Keykha, H.; Bijanzadeh, H.-R.; Kleinpeter, E. J. Org. Chem. 2007, 72, 2208.
313. Saito, S.; Toriumi, Y.; Tomioka, A.; Itai, A. J. Org. Chem. 1995, 60, 4715.
314. Axe, F.U.; Renugopalakrishnan, V.; Hagler, A.T. J. Chem. Res. 1998, 1. For an analysis of DMF see Wiberg, K.B.; Rablen, P.R.; Rush, D.J.; Keith, T.A. J. Am. Chem. Soc. 1995, 117, 4261.
315. Avalos, M.; Babiano, R.; Barneto, J.L.; Cintas, P.; Clemente, F.R.; Jiménez, J.L.; Palcios, J.C. J. Org. Chem. 2003, 68, 1834.
316. Kakkar, R.; Grover, R.; Chadha, P. Org. Biomol. Chem. 2003, 1, 2200.
317. Wiberg, K.B.; Rablen, P.R. J. Am. Chem. Soc. 1995, 117, 2201.
318. Bach, R.D.; Mintcheva, I.; Kronenberg, W.J.; Schlegel, H.B. J. Org. Chem. 1993, 58, 6135.
319. Ilieva, S.; Hadjieva, B.; Galabov, B. J. Org. Chem. 2002, 67, 6210.
320. Wiberg, K. B.; Rush, D. J. J. Am. Chem. Soc. 2001, 123, 2038; J. Org. Chem. 2002, 67, 826.
321. Rablen, P.R.; Miller, D.A.; Bullock, V.R.; Hutchinson, P.H.; Gorman, J.A. J. Am. Chem. Soc. 1999, 121, 218.
322. Deetz, M.J.; Forbes, C.C.; Jonas, M.; Malerich, J.P.; Smith, B.D.; Wiest, O. J. Org. Chem. 2002, 67, 3949.
323. Kim, Y.-J.; Streitwieser, A.; Chow, A.; Fraenkel, G. Org. Lett. 1999, 1, 2069.
324. Smith, B.D.; Goodenough-Lashua, D.M.; D'Souza, C.J.E.; Norton, K.J.; Schmidt, L.M.; Tung, J.C. Tetrahedron Lett. 2004, 45, 2747.
325. For an analysis of barriers to rotation in such compounds, see Mazzanti, A.; Lunazzi, L.; Minzoni, M.; Anderson, J.E. J. Org. Chem. 2006, 71, 5474.
326. Abraham, R.J.; Angioloni, S.; Edgar, M.; Sancassan, F. J. Chem. Soc. Perkin Trans. 2 1997, 41.
327. Casarini, D.; Lunazzi, L.; Mazzanti, A. J. Org. Chem. 1997, 62, 7592.
328. See Jensen, F.R.; Bushweller, C.H. Adv. Alicyclic Chem. 1971, 3, 139; Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 686–753. Also see, Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010, pp. 54–67.
329. See Geise, H.J.; Buys, H.R.; Mijlhoff, F.C. J. Mol. Struct. 1971, 9, 447; Bastiansen, O.; Fernholt, L.; Seip, H.M.; Kambara, H.; Kuchitsu, K. J. Mol. Struct. 1973, 18, 163.
330. See Dunitz, J.D. J. Chem. Educ. 1970, 47, 488.
331. For a review of nonchair forms, see Kellie, G.M.; Riddell, F.G. Top. Stereochem. 1974, 8, 225.
332. Squillacote, M.; Sheridan, R.S.; Chapman, O.L.; Anet, F.A.L. J. Am. Chem. Soc. 1975, 97, 3244.
333. See Wiberg, K.B.; Castejon, H.; Bailey, W.F.; Ochterski, J. J. Org. Chem. 2000, 65, 1181.
334. Gill, G.; Pawar, D.M.; Noe, E.A. J. Org. Chem. 2005, 70, 10726.
335. See Wehle, D.; Fitjer, L. Tetrahedron Lett. 1986, 27, 5843.
336. See Anet, F.A.L.; Bourn, A.J.R. J. Am. Chem. Soc. 1967, 89, 760. See also, Strauss, H.L. J. Chem. Educ. 1971, 48, 221.
337. See Oki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985, pp. 287–307; Anderson, J.E. Top. Curr. Chem. 1974, 45, 139.
338. See Jensen, F.R.; Bushweller, C.H.; J. Chem. Soc. 1969, 91, 3223.
339. Weiser, J.; Golan, O.; Fitjer, L.; Biali, S.E. J. Org. Chem. 1996, 61, 8277.
340. For a study of thioether, sulfoxide and sulfone substituents, see Juaristi, E.; Labastida, V.; Antúnez, S. J. Org. Chem., 2000, 65, 969.
341. Jensen, F.R.; Gale, L.H. J. Am. Chem. Soc. 1959, 81, 6337.
342. Anet, F.A.L.; Krane, J.; Kitching, W.; Dodderel, D.; Praeger, D. Tetrahedron Lett. 1974, 3255.
343. These values are from Corey, E.J.; Feiner, N.F. J. Org. Chem. 1980, 45, 765. Also see Jensen, F.R.; Bushweller, C.H. Adv. Alicyclic Chem. 1971, 3, 139. See also, Schneider, H.; Hoppen, V. Tetrahedron Lett. 1974, 579 and see Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010, pp. 54–67.
344. See Ford, R.A.; Allinger, N.L. J. Org. Chem. 1970, 35, 3178. For a critical review of the methods used to obtain these values, see Jensen, F.R.; Bushweller, C.H. Adv. Alicyclic Chem. 1971, 3, 139.
345. Anet, F.A.L.; O'Leary, D.J. Tetrahedron Lett. 1989, 30, 1059.
346. Buchanan, G.W.; Webb, V.L. Tetrahedron Lett. 1983, 24, 4519.
347. Eliel, E.L.; Manoharan, M. J. Org. Chem. 1981, 46, 1959.
348. Booth, H.; Everett, J.R. J. Chem. Soc. Chem. Commun. 1976, 278.
349. Hirsch, J.A. Top. Stereochem. 1967, 1, 199.
350. Kitching, W.; Olszowy, H.A.; Drew, G.M.; Adcock, W. J. Org. Chem. 1982, 47, 5153.
351. Schneider, H.; Hoppen, V. Tetrahedron Lett. 1974, 579.
352. Squillacote, M.E.; Neth, J.M. J. Am. Chem. Soc. 1987, 109, 198. Values of 2.59–2.92 kcal mol−1(10.84–12.23 kJ mol−1) were determined for 4-X-C6H4– substituents (X = NO2, Cl, MeO): see Kirby, A.J.; Williams, N.H. J. Chem. Soc. Chem. Commun. 1992, 1285, 1286.
353. Manoharan, M.; Eliel, E.L. Tetrahedron Lett. 1984, 25, 3267.
354. Abraham, R.J.; Rossetti, Z.L. J. Chem. Soc. Perkin Trans. 2 1973, 582. See also, Hammarström, L.; Berg, U.; Liljefors, T. Tetrahedron Lett. 1987, 28, 4883.
355. Abraham, M.H.; Xodo, L.E.; Cook, M.J.; Cruz, R. J. Chem. Soc. Perkin Trans. 2 1982, 1503; Samoshin, V.V.; Svyatkin, V.A.; Zefirov, N.S. J. Org. Chem. USSR 1988, 24, 1080, and references cited therein. See Zefirov, N.S.; Samoshin, V.V.; Subbotin, O.A.; Sergeev, N.M. J. Org. Chem. USSR 1981, 17, 1301.
356. For a case of a preferential diaxial conformation in 1,3 isomers, see Ochiai, M.; Iwaki, S.; Ukita, T.; Matsuura, Y.; Shiro, M.; Nagao, Y. J. Am. Chem. Soc. 1988, 110, 4606.
357. Golan, O.; Goren, Z.; Biali, S.E. J. Am. Chem. Soc. 1990, 112, 9300.
358. Abraham, R.J.; Chambers, E.J.; Thomas, W.A. J. Chem. Soc. Perkin Trans. 2 1993, 1061.
359. Wiberg, K.B. J. Org. Chem. 1999, 64, 6387.
360. This idea was suggested by Winstein, S.; Holness, N.J. J. Am. Chem. Soc. 1955, 77, 5561. See Saunders, M.; Wolfsberg, M.; Anet, F.A.L.; Kronja, O. J. Am. Chem. Soc. 2007, 129, 10276.
361. Marzabadi, C. H.; Anderson, J. E.; Gonzalez-Outeirino, J.; Gaffney, P. R. J.; White, C. G. H.; Tocher, D. A.; Todaro, L. J. J. Am. Chem. Soc. 2003, 125, 15163.
362. See Rabideau, P.W. The Conformational Analysis of Cyclohexenes, Cyclohexadienes, and Related Hydroaromatic Compounds, VCH, NY, 1989; Vereshchagin, A.N. Russ. Chem. Rev. 1983, 52, 1081; Johnson, F. Chem. Rev. 1968, 68, 375. See also, Lambert, J.B.; Clikeman, R.R.; Taba, K.M.; Marko, D.E.; Bosch, R.J.; Xue, L. Acc. Chem. Res. 1987, 20, 454.
363. See Dale, J. Stereochemistry and Conformational Analysis, Verlag Chemie, Deerfield Beach, FL, 1978; Chiurdoglu, G. Conformational Analysis, Academic Press, NY, 1971; Eliel, E.L.; Allinger, N.L.; Angyal, S.J.; Morrison, G.A. Conformational Analysis, Wiley, NY, 1965; Dale, J. Top. Stereochem. 1976, 9, 199; Truax, D.R.; Wieser, H. Chem. Soc. Rev. 1976, 5, 411; Eliel, E.L. J. Chem. Educ. 1975, 52, 762; Bushweller, C.H.; Gianni, M.H. in Patai, S. The Chemistry of Functional Groups, Supplement E, Wiley, NY, 1980, pp. 215–278.
364. See Jensen, F.R.; Bushweller, C.H. Adv. Alicyclic Chem. 1971, 3, 139; Robinson, D.L.; Theobald, D.W. Q. Rev. Chem. Soc. 1967, 21, 314; Eliel, E.L. Angew. Chem. Int. Ed. 1965, 4, 761; Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley-Interscience, NY, 1994, pp. 686–753. Also see, Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010, pp. 61–65.
365. Laane, J.; Choo, J. J. Am. Chem. Soc. 1994, 116, 3889.
366. Basso, E.A.; Kaiser, C.; Rittner, R.; Lambert, J.B. J. Org. Chem. 1993, 58, 7865.
367. See Glass, R.S. Conformational Analysis of Medium-Sized Heterocycle, VCH, NY, 1988; Riddell, F.G. The Conformational Analysis of Heterocyclic Compounds, Academic Press, NY, 1980; Juaristi, E. Acc. Chem. Res. 1989, 22, 357; Crabb, T.A.; Katritzky, A.R. Adv. Heterocycl. Chem. 1984, 36, 1; Eliel, E.L. Angew. Chem. Int. Ed. 1972, 11, 739; Pure Appl. Chem. 1971, 25, 509; Acc. Chem. Res. 1970, 3, 1; Lambert, J.B. Acc. Chem. Res. 1971, 4, 87.
368. Bachrach, S.M.; Liu, M. Tetrahedron Lett. 1992, 33, 6771.
369. These factors are discussed by Eliel, E.L. Angew. Chem. Int. Ed. 1972, 11, 739.
370. Riddell, F.G.; Robinson, M.J.T. Tetrahedron 1967, 23, 3417; Eliel, E.L.; Knoeber, M.C. J. Am. Chem. Soc. 1968, 90, 3444. See also, Eliel, E.L.; Alcudia, F. J. Am. Chem. Soc. 1974, 96, 1939. See Cieplak, P.; Howard, A.E.; Powers, J.P.; Rychnovsky, S.D.; Kollman, P.A. J. Org. Chem. 1996, 61, 3662 for conformational energy differences in 2,2,6-trimethyl-4-alkyl-1,3-dioxane.
371. Cai, J.; Davies, A.G.; Schiesser, C.H. J. Chem. Soc. Perkin Trans. 2 1994, 1151.
372. Hutchins, R.O.; Eliel, E.L. J. Am. Chem. Soc. 1969, 91, 2703. See also, Juaristi, E.; Cuevas, G. Tetrahedron 1999, 55, 359.
373. Strelenko, Y.A.; Samoshin, V.V.; Troyansky, E.I.; Demchuk, D.V.; Dmitriev, D.E.; Nikishin, G.I.; Zefirov, N.S. Tetrahedron 1994, 50, 10107.
374. Gordillo, B.; Juaristi, E.; Matínez, R.; Toscano, R.A.; White, P.S.; Eliel, E.L. J. Am. Chem. Soc. 1992, 114, 2157.
375. Kaloustian, M.K.; Dennis, N.; Mager, S.; Evans, S.A.; Alcudia, F.; Eliel, E.L. J. Am. Chem. Soc. 1976, 98, 956. See also, Eliel, E.L.; Kandasamy, D.; Sechrest, R.C. J. Org. Chem. 1977, 42, 1533.
376. See Kirby, A.J. The Anomeric Effect and Related Stereoelectronic Effects at Oxygen, Springer, NY, 1983; Szarek, W.A.; Horton, D. Anomeric Effect, American Chemical Society, Washington, 1979; Deslongchamps, P. Stereoelectronic Effects in Organic Chemistry, Pergamon, Elmsford, NY, 1983, pp. 4–26; Zefirov, N.S. Tetrahedron 1977, 33, 3193; Lemieux, R.U. Pure Appl. Chem. 1971, 27, 527.
377. Juaristi, E.; Cuevas, G. Tetrahedron 1992, 48, 5019.
378. See Romers, C.; Altona, C.; Buys, H.R.; Havinga, E. Top. Stereochem. 1969, 4, 39, pp. 73–77; Wolfe, S.; Whangbo, M.; Mitchell, D.J. Carbohydr. Res. 1979, 69, 1.
379. See Praly, J.; Lemieux, R.U. Can. J. Chem. 1987, 65, 213; Booth, H.; Khedhair, K.A.; Readshaw, S.A. Tetrahedron 1987, 43, 4699. For evidence against it, see Box, V.G.S. Heterocycles 1990, 31, 1157.
380. Cramer, C.J. J. Org. Chem. 1992, 57, 7034; Booth, H.; Dixon, J.M.; Readshaw, S.A. Tetrahedron 1992, 48, 6151.
381. Anderson, J.E. J. Org. Chem. 2000, 65, 748.
382. Stolow, R.D. J. Am. Chem. Soc. 1964, 86, 2170; Stolow, R.D.; McDonagh, P.M.; Bonaventura, M.M. J. Am. Chem. Soc. 1964, 86, 2165. Also see Fitjer, L.; Scheuermann, H.; Klages, U.; Wehle, D.; Stephenson, D.S.; Binsch, G. Chem. Ber. 1986, 119, 1144.
383. Rychnovsky, S.D.; Yang, G.; Powers, J.P. J. Org. Chem. 1993, 58, 5251.
384. Arnason, I.; Kvaran, A.; Jonsdottir, S.; Gudnason, P. I.; Oberhammer, H. J. Org. Chem. 2002, 67, 3827.
385. Salzner, U.; Schleyer, P.v.R. J. Am. Chem. Soc. 1993, 115, 10231; Aggarwal, V.K.; Worrall, J.M.; Adams, H.; Alexander, R.; Taylor, B.F. J. Chem. Soc. Perkin Trans. 1 1997, 21.
386. Salzner, U.; Schleyer, P.v.R. J. Org. Chem. 1994, 59, 2138.
387. Perrin, C.L. Tetrahedron 1995, 51, 11901.
388. Anderson, J.E.; Cai, J.; Davies, A.G. J. Chem. Soc. Perkin Trans. 2 1997, 2633. For some controversy concerning the anomeric effect a related system, see Perrin, C.L.; Armstrong, K.B.; Fabian, M.A. J. Am. Chem.Soc. 1994, 116, 715; Salzner, U. J. Org. Chem. 1995, 60, 986.
389. Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 675–685 and 754–770.
390. For reviews of the stereochemistry of four-membered rings, see Legon, A.C. Chem. Rev. 1980, 80, 231; Moriarty, R.M. Top. Stereochem. 1974, 8, 271; Cotton, F.A.; Frenz, B.A. Tetrahedron 1974, 30, 1587.
391. Miller, F.A.; Capwell, R.J.; Lord, R.C.; Rea, D.G. Spectrochim. Acta Part A, 1972, 28, 603. However, see Margulis, T.N. J. Am. Chem. Soc. 1971, 93, 2193.
392. Luger, P.; Buschmann, J. J. Am. Chem. Soc. 1984, 106, 7118.
393. See Fuchs, B. Top. Stereochem. 1978, 10, 1; Legon, A.C. Chem. Rev. 1980, 80, 231.
394. Willy, W.E.; Binsch, G.; Eliel, E.L. J. Am. Chem. Soc. 1970, 92, 5394; Lipnick, R.L. J. Mol. Struct. 1974, 21, 423.
395. Lipnick, R.L. J. Mol. Struct. 1974, 21, 411; Poupko, R.; Luz, Z.; Zimmermann, H. J. Am. Chem. Soc. 1982, 104, 5307; Riddell, F.G.; Cameron K.S.; Holmes, S.A.; Strange, J.H. J. Am. Chem. Soc. 1997, 119, 7555.
396. Matallana, A.; Kruger, A.W.; Kingsbury, C.A. J. Org. Chem. 1994, 59, 3020.
397. Abraham, R.J.; Pollock, L.; Sancassan, F. J. Chem. Soc. Perkin Trans. 2 1994, 2329.
398. Carreira, L.A.; Jiang, G.J.; Person, W.B.; Willis, Jr., J.N. J. Chem. Phys. 1972, 56, 1440.
399. Verevkin, S.P.; Kümmerlin, M.; Beckhaus, H.-D.; Galli, C.; Rüchardt, C. Eur. J. Org. Chem. 1998, 579.
400. See Arshinova, R.P. Russ. Chem. Rev. 1988, 57, 1142; Ounsworth, J.P.; Weiler, L. J. Chem. Educ. 1987, 64, 568; Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985, pp. 307–321; Casanova, J.; Waegell, B. Bull. Soc. Chim. Fr. 1975, 911; Anet, F.A.L. Top. Curr. Chem. 1974, 45, 169; Dunitz, J.D. Pure Appl. Chem. 1971, 25, 495. See Glass, R.S. Conformational Analysis of Medium-Sized Heterocycles VCH, NY, 1988.
401. Wiberg, K.B. J. Org. Chem. 2003, 68, 9322.
402. Meyer, W.L.; Taylor, P.W.; Reed, S.A.; Leister, M.C.; Schneider, H.-J.; Schmidt, G.; Evans, F.E.; Levine, R.A. J. Org. Chem. 1992, 57, 291.
403. Pawar, D.M.; Brown II, J.; Chen, K.-H.; Allinger, N.L.; Noe, E.A. J. Org. Chem. 2006, 71, 6512.
404. Spracklin, D.K.; Weiler, L. J. Chem. Soc. Chem. Commun. 1992, 1347; Keller, T.H.; Neeland, E.G.; Rettig, S.; Trotter, J.; Weiler, L. J. Am. Chem. Soc. 1988, 110, 7858.
405. Pawar, D.M.; Smith, S.V.; Moody, E.M.; Noe, E.A. J. Am. Chem. Soc. 1998, 120, 8241.
406. Borgen, G.; Dale, J.; Gundersen, L.-L.; Krivokapic, A.; Rise, F.; Øverås, A.T. Acta Chem. Scand. B, 1998, 52, 1110.
407. Pawar, D.M.; Davids, K.L.; Brown, B.L.; Smith, S.V.; Noe, E.A. J. Org. Chem. 1999, 64, 4580; Pawar, D.M.; Moody, E.M.; Noe, E.A. J. Org. Chem. 1999, 64, 4586.
408. Kleinpeter, E.; Koch, A.; Pihlaja, K. Tetrahedron 2005, 61, 7349.
409. Daoust, K.J.; Hernandez, S.M.; Konrad, K.M.; Mackie, I.D.; Winstanley, Jr., J.; Johnson, R.P. J. Org. Chem. 2006, 71, 5708.
410. For definitions of axial, equatorial, and related terms for rings of any size, see Anet, F.A.L. Tetrahedron Lett. 1990, 31, 2125.
411. For a discussion of the angles of the ring positions, see Cremer, D. Isr. J. Chem. 1980, 20, 12.
412. Thanks to Dr. Warren Hehre, Wavefunction, Inc., Irvine, CA. Personal communication. See Hehre, W.J. A Guide to Molecular Mechanics and Quantum Chemical Calculations, Wavefunction, Inc., Irvine, CA, 2003, pp. 56–57.
413. For a review, see Rappe, A.K.; Casewit, C.J. Molecular Mechanics Across Chemistry, University Science Books, Sausalito, CA, 1997.
414. Clark, M.; Cramer, III, R.D.; van Opdenbosch, N. J. Computational Chem. 1989, 10, 982.
415. Allinger, N.L.; Li, F.; Yun, Y.H. J. Computational Chem. 1990, 11, 855, and later papers in this series.
416. Allinger, N.L.; Chen, K.; Lii, J.-H. J. Computational Chem. 1996, 17, 642, and later papers in this series.
417. Halgren, T.A. J. Computational Chem 1996, 17, 490, and later papers in this series.
418. See Greenberg, A.; Liebman, J.F. Strained Organic Molecules, Academic Press, NY, 1978; Wiberg, K.B. Angew. Chem. Int. Ed. 1986, 25, 312; Greenberg, A.; Stevenson, T.A. Mol. Struct. Energ. 1986, 3, 193; Liebman, J.F.; Greenberg, A. Chem. Rev. 1976, 76, 311; Cremer, D.; Kraka, E. Mol. Struct. Energ. 1988, 7, 65.
419. Zhao, C.-Y.; Duan, W.-S.; Zhang, Y.; You, X.-Z. J. Chem. Res. (S) 1998, 156.
420. Wiberg, K.B. Accts. Chem. Res. 1996, 29, 229.
421. For discussions, see Wiberg, K.B.; Bader, R.F.W.; Lau, C.D.H. J. Am. Chem. Soc. 1987, 109, 985, 1001.
422. For a review, see Rüchardt, C.; Beckhaus, K. Angew. Chem. Int. Ed. 1985, 24, 529. See also, Burkert, U.; Allinger, N.L. Molecular Mechanisms, American Chemical Society, Washington, 1982, pp. 169–194; Allinger, N.L. Adv. Phys. Org. Chem. 1976, 13, 1, 45–47.
423. Bari![]() , D.; Maksi
, D.; Maksi![]() , Z.B. Theor. Chem. Acc.2005, 114, 222.
, Z.B. Theor. Chem. Acc.2005, 114, 222.
424. Hohlneicher, G.; Packschies, L. Tetrahedron Lett. 2007, 48, 6429. However, see Bari![]() , D.; Maksi
, D.; Maksi![]() , Z.B. Tetrahedron Lett. 2008, 49, 1428.
, Z.B. Tetrahedron Lett. 2008, 49, 1428.
425. For reviews of reactions of cyclopropanes and cyclobutanes, see Trost, B.M. Top. Curr. Chem. 1986, 133, 3; Wong, H.N.C.; Lau, C.D.H.; Tam, K. Top. Curr. Chem. 1986, 133, 83.
426. For a treatise, see Rappoport, Z. The Chemistry of the Cyclopropyl Group, 2 pts.; Wiley, NY, 1987.
427. See in Rappoport, Z. The Chemistry of the Cyclopropyl Group, 2 pts, Wiley, NY, 1987, the papers by Wiberg, K.B. pt. 1., pp. 1–26; Liebman, J.F.; Greenberg, A. pt. 2, pp. 1083–1119; Liebman, J.F.; Greenberg, A. Chem. Rev.1989, 89, 1225.
428. See Wong, H.N.C.; Hon, M.; Ts, C.e; Yip, Y.; Tanko, J.; Hudlicky, T. Chem. Rev. 1989, 89, 165; Reissig, H. in Rappoport, Z. The Chemistry of the Cyclopropyl Group, pt. 1, Wiley, NY, 1987, pp. 375–443.
429. Ogg, Jr., R.A.; Priest, W.J. J. Am. Chem. Soc. 1938, 60, 217.
430. Shortridge, R.W.; Craig, R.A.; Greenlee, K.W.; Derfer, J.M.; Boord, C.E. J. Am. Chem. Soc. 1948, 70, 946.
431. See Frey, H.M. Adv. Phys. Org. Chem. 1966, 4, 147.
432. Bach, R. D.; Dmitrenko, O. J. Org. Chem. 2002, 67, 2588.
433. Bach, R.D.; Dmitrenko, O. J. Am. Chem. Soc. 2006, 128, 4598.
434. Bach, R. D.; Dmitrenko, O. J. Am. Chem. Soc. 2004, 126, 4444; Tian, Z.; Fattahi, A.; Lis, L.; Kass, S.R. J. Am. Chem. Soc. 2006, 128, 17087.
435. Bachrach, S.M. J. Org. Chem. 2008, 73, 2466. Also see Ringer, A.L.; Magers, D.H. J. Org. Chem. 2007, 72, 2533.
436. See Cremer, D.; Kraka, E. J. Am. Chem. Soc. 1985, 107, 3800, 3811; Slee, T.S. Mol. Struct. Energ. 1988, 5, 63; Casaarini, D.; Lunazzi, L.; Mazzanti, A. J. Org. Chem. 1997, 62, 7592.
437. Randi![]() , M.; Maksi
, M.; Maksi![]() , Z. Theor. Chim. Acta 1965, 3, 59; Weigert, F.J.; Roberts, J.D. J. Am. Chem. Soc. 1967, 89, 5962.
, Z. Theor. Chim. Acta 1965, 3, 59; Weigert, F.J.; Roberts, J.D. J. Am. Chem. Soc. 1967, 89, 5962.
438. Wiberg, K.B. Accts. Chem. Res. 1996, 29, 229.
439. See Hoffmann, R.; Davidson, R.B. J. Am. Chem. Soc. 1971, 93, 5699. See also Ref. 438
440. Wiberg, K.B.; Bader, R.F.W.; Lau, C.D.H. J. Am. Chem. Soc. 1987, 109, 985, 1001.
441. See Tidwell, T.T. in Rappoport, Z. The Chemistry of the Cyclopropyl Groups, pt. 1, Wiley, NY, 1987, pp. 565–632; Charton, M. in Zabicky, J. The Chemistry of Alkenes, Vol. 2, pp. 511–610, Wiley, NY, 1970.
442. See Tsuji, T.; Shibata, T.; Hienuki, Y.; Nishida, S. J. Am. Chem. Soc. 1978, 100, 1806; Drumright, R.E.; Mas, R.H.; Merola, J.S.; Tanko, J.M. J. Org. Chem. 1990, 55, 4098.
443. Staley, S.W. J. Am. Chem. Soc. 1967, 89, 1532; Pews, R.G.; Ojha, N.D. J. Am. Chem. Soc. 1969, 91, 5769. See, however, Noe, E.A.; Young, R.M. J. Am. Chem. Soc. 1982, 104, 6218.
444. See the reviews in Chem. Rev. 1989, 89, 975, and the following: Jefford, C.W. J. Chem. Educ. 1976, 53, 477; Seebach, D. Angew. Chem. Int. Ed. 1965, 4, 121; Greenberg, A.; Liebman, J.F. Strained Organic Molecules, Academic Press, NY, 1978, pp. 210–220; Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley–Interscience, NY, 1994, pp. 771–811.
445. For a useful classification of strained polycyclic systems, see Gund, P.; Gund, T.M. J. Am. Chem. Soc. 1981, 103, 4458.
446. For a computer program that generates IUPAC names for complex bridged systems, see Rücker, G.; Rücker, C. Chimia 1990, 44, 116.
460. Gribanova, T.N.; Minyaev, R.M.; Minkin, V.I. Russ. J. Org. Chem. 2007, 43, 1144.
461. Gleiter, R.; Treptow, B.; Irngartinger, H.; Oeser, T. J. Org. Chem. 1994, 59, 2787.
462. Golobish, T.D.; Dailey, W.P. Tetrahedron Lett. 1996, 37, 3239.
463. Haller, I.; Srinivasan, R. J. Chem. Phys. 1964, 41, 2745.
464. Newton, M.D.; Schulman, J.M. J. Am. Chem. Soc. 1972, 94, 767.
465. Wiberg, K.B.; Waddell, S.T. J. Am. Chem. Soc. 1990, 112, 2194; Seiler, S.T. Helv. Chim. Acta 1990, 73, 1574; Bothe, H.; Schlüter, A. Chem. Ber. 1991, 124, 587; Lynch, K.M.; Dailey, W.P. J. Org. Chem. 1995, 60, 4666. See Wiberg, K.B. Chem. Rev. 1989, 89, 975; Ginsburg, D. in Rappoport, Z The Chemistry of the Cyclopropyl Group, pt. 2, Wiley, NY, 1987, pp. 1193–1221; Ginsburg, D. Top. Curr. Chem. 1987, 137, 1. For a discussion of charge density and bonding, see Coppens, P. Angew. Chem. Int. Ed. 2005, 44, 6810.
466. Wiberg, K.B.; Walker, F.H.; Pratt, W.E.; Michl, J. J. Am. Chem. Soc. 1983, 105, 3638.
467. Della, E.W.; Taylor, D.K. J. Org. Chem. 1994, 59, 2986.
468. See Kuck, D.; Krause, R.A.; Gestmann, D.; Posteher, F.; Schuster, A. Tetrahedron 1998, 54, 5247.
447. Lemal, D.M.; Menger, F.M.; Clark, G.W. J. Am. Chem. Soc. 1963, 85, 2529; Wiberg, K.B.; Lampman, G.M. Tetrahedron Lett. 1963, 2173; Hoz, S. in Rappoport, Z The Chemistry of the Cyclopropyl Group, pt. 2, Wiley, NY, 1987, pp. 1121–1192; Wiberg, K.B. Adv. Alicyclic Chem. 1968, 2, 185. For a review of [n.1.1] systems, see Meinwald, J.; Meinwald, Y.C. Adv. Alicyclic Chem. 1966, 1, 1.
448. Casanova, J.; Bragin, J.; Cottrell, F.D. J. Am. Chem. Soc. 1978, 100, 2264.
449. Irngartinger, H.; Goldmann, A.; Jahn, R.; Nixdorf, M.; Rodewald, H.; Maier, G.; Malsch, K.; Emrich, R. Angew. Chem. Int. Ed. 1984, 23, 993; Maier, G.; Fleischer, F. Tetrahedron Lett. 1991, 32, 57. Also see Maier, G. Angew. Chem. Int. Ed. 1988, 27, 309; Maier, G.; Rang, H.; Born, D. in Olah, G.A. Cage Hydrocarbons, Wiley, NY, 1990, pp. 219–259; Maier, G.; Born, D. Angew. Chem. Int. Ed. 1989, 28, 1050.
450. Rücker, C.; Trupp, B. J. Am. Chem. Soc. 1988, 110, 4828.
451. Von Seebach, M.; Kozhushkov, S.I.; Boese, R.; Benet-Buchholz, J.; Yufit, D.S.; Howard, J.A.K.; de Meijere, A. Angew. Chem. Int. Ed. 2000, 39, 2495.
452. Katz, T.J.; Acton, N. J. Am. Chem. Soc. 1973, 95, 2738. See also, Wilzbach, K.E.; Kaplan, L. J. Am. Chem. Soc. 1965, 87, 4004.
453. Hedberg, L.; Hedberg, K.; Eaton, P.E.; Nodari, N.; Robiette, A.G. J. Am. Chem. Soc. 1991, 113, 1514. For a review of cubanes, see Griffin, G.W.; Marchand, A.P. Chem. Rev. 1989, 89, 997.
454. Paquette, L.A.; Fischer, J.W.; Browne, A.R.; Doecke, C.W. J. Am. Chem. Soc. 1985, 105, 686.
455. Eaton, P.E.; Or, Y.S.; Branca, S.J.; Shankar, B.K.R. Tetrahedron 1986, 42, 1621. See also, Dauben, W.G.; Cunningham Jr., A.F. J. Org. Chem. 1983, 48, 2842.
456. Otterbach, A.; Musso, H. Angew. Chem. Int. Ed. 1987, 26, 554.
457. Allred, E.L.; Beck, B.R. J. Am. Chem. Soc. 1973, 95, 2393.
458. Hoffmann, V.T.; Musso, H. Angew. Chem. Int. Ed. 1987, 26, 1006.
459. Rihs, G. Tetrahedron Lett. 1983, 24, 5857. See Mathew, T.; Keller, M.; Hunkler, D.; Prinzbach, H. Tetrahedron Lett. 1996, 37, 4491 for the synthesis of azapagodanes (also called azadodecahedranes).
469. For a review, see Wiberg, K.B. Acc. Chem. Res. 1984, 17, 379.
470. Scott, W.B.; Pincock, R.E. J. Am. Chem. Soc. 1973, 95, 2040.
471. Gibbons, C.S.; Trotter, J. Can. J. Chem. 1973, 51, 87.
472. See Raphael, R.A. Proc. Chem. Soc. 1962, 97; Sicher, J. Prog. Stereochem. 1962, 3, 202.
473. Huber-Buser, E.; Dunitz, J.D. Helv. Chim. Acta 1960, 43, 760.
474. Dunitz, J.D.; Venkatesan, K. Helv. Chim. Acta 1961, 44, 2033.
475. Kirby, A.J.; Komarov, I.V.; Wothers, P.D.; Feeder, N. Angew. Chem. Int. Ed., 1998, 37, 785. Also see Madder, R.D.; Kim, C.-Y.; Chandra, P.P.; Doyon, J.B.; Barid, Jr., T.A.; Fierke, C.A.; Christianson, D.W.; Voet, J.G.; Jain, A. J. Org. Chem. 2002, 67, 582.
476. Okazaki, T.; Ogawa, K.; Kitagawa, T.; Takeuchi, K. J. Org. Chem. 2002, 67, 5981.
477. Gol'dfarb, Ya.L.; Belen'kii, L.I. Russ. Chem. Rev. 1960, 29, 214, p. 218.
478. For a review, see Cope, A.C.; Martin, M.M.; McKervey, M.A. Q. Rev. Chem. Soc. 1966, 20, 119.
479. Spanka, G.; Rademacher, P. J. Org. Chem. 1986, 51, 592. See also, Spanka, G.; Rademacher, P.; Duddeck, H. J. Chem. Soc. Perkin Trans. 2 1988, 2119.
480. Uemura, S.; Fukuzawa, S.; Toshimitsu, A.; Okano, M.; Tezuka, H.; Sawada, S. J. Org. Chem. 1983, 48, 270.
481. Schläpfer-Dähler, M.; Prewo, R.; Bieri, J.H.; Germain, G.; Heimgartner, H. Chimia 1988, 42, 25.
482. See Granik, V.G. Russ. Chem. Rev. 1982, 51, 119.
483. An example is the calculated strain of 1.4–3.2 kcal mol−1(5.9–13.4 kJ mol−1) in cyclotetradecane. See Chickos, J.S.; Hesse, D.G.; Panshin, S.Y.; Rogers, D.W.; Saunders, M.; Uffer, P.M.; Liebman, J.F. J. Org. Chem. 1992, 57, 1897.
484. For a review of strained double bonds, see Zefirov, N.S.; Sokolov, V.I. Russ. Chem. Rev. 1967, 36, 87. For a review of double and triple bonds in rings, see Johnson, R.P. Mol. Struct. Energ. 1986, 3, 85.
485. See Baird, M.S. Top. Curr. Chem. 1988, 144, 137; Halton, B.; Banwell, M.G. in Rappoport, Z. The Chemistry of the Cyclopropyl Group, pt. 2, Wiley, NY, 1987, pp. 1223–1339; Closs, G.L. Adv. Alicyclic Chem. 1966, 1, 53; For a discussion of the bonding and hybridization, see Allen, F.H. Tetrahedron 1982, 38, 645.
486. Dem'yanov, N.Ya.; Doyarenko, M.N. Ber. 1923, 56, 2200; Schlatter, M.J. J. Am. Chem. Soc. 1941, 63, 1733; Stigliani, W.M.; Laurie, V.W.; Li, J.C. J. Chem. Phys. 1975, 62, 1890.
487. See Halton, B. Chem. Rev. 1989, 89, 1161; 1973, 73, 113; Billups, W.E.; Rodin, W.A.; Haley, M.M. Tetrahedron 1988, 44, 1305; Billups, W.E. Acc. Chem. Res. 1978, 11, 245.
488. Vogel, E.; Grimme, W.; Korte, S. Tetrahedron Lett. 1965, 3625. Also see Müller, P.; Bernardinelli, G.; Thi, H.C.G. Chimia 1988, 42, 261; Neidlein, R.; Christen, D.; Poignée, V.; Boese, R.; Bläser, D.; Gieren, A.; Ruiz-Pérez, C.; Hübner, T. Angew. Chem. Int. Ed. 1988, 27, 294.
489. For reviews of trans cycloalkenes, see Nakazaki, M.; Yamamoto, K.; Naemura, K. Top. Curr. Chem. 1984, 125, 1; Marshall, J.A. Acc. Chem. Res. 1980, 13, 213.
490. Wallraff, G.M.; Michl, J. J. Org. Chem. 1986, 51, 1794; Squillacote, M.; Bergman, A.; De Felippis, J. Tetrahedron Lett. 1989, 30, 6805.
491. Marshall, J.A.; Flynn, K.E. J. Am. Chem. Soc. 1983, 105, 3360. For reviews, see Nakazaki, M.; Yamamoto, K.; Naemura, K. Top. Curr. Chem. 1984, 125, 1; Marshall, J.A. Acc. Chem. Res. 1980, 13, 213. For a review of these and similar compounds, see Borden, W.T. Chem. Rev. 1989, 89, 1095.
492. See Meier, H. Adv. Strain Org. Chem. 1991, 1, 215; Krebs, A.; Wilke, J. Top. Curr. Chem. 1983, 109, 189; Nakagawa, M. in Patai, S. The Chemistry of the C![]() C Triple Bond, pt. 2, Wiley, NY, 1978, pp. 635–712; Krebs, A. in Viehe, H.G. Acetylenes, Marcel Dekker, NY, 1969, pp. 987–1062. See Meier, H.; Hanold, N.; Molz, T.; Bissinger, H.J.; Kolshorn, H.; Zountsas, J. Tetrahedron 1986, 42, 1711.
C Triple Bond, pt. 2, Wiley, NY, 1978, pp. 635–712; Krebs, A. in Viehe, H.G. Acetylenes, Marcel Dekker, NY, 1969, pp. 987–1062. See Meier, H.; Hanold, N.; Molz, T.; Bissinger, H.J.; Kolshorn, H.; Zountsas, J. Tetrahedron 1986, 42, 1711.
493. Blomquist, A.T.; Liu, L.H. J. Am. Chem. Soc. 1953, 75, 2153. See also, Bühl, H.; Gugel, H.; Kolshorn, H.; Meier, H. Synthesis 1978, 536.
494. Schmidt, H.; Schweig, A.; Krebs, A. Tetrahedron Lett. 1974, 1471.
495. Krebs, A.; Kimling, H. Tetrahedron Lett. 1970, 761.
496. Bottini, A.T.; Frost II, K.A.; Anderson, B.R.; Dev, V. Tetrahedron 1973, 29, 1975.
497. Wentrup, C.; Blanch, R.; Briehl, H.; Gross, G. J. Am. Chem. Soc. 1988, 110, 1874.
498. See Sander, W.; Chapman, O.L. Angew. Chem. Int. Ed. 1988, 27, 398.
499. See Bolster, J.M.; Kellogg, R.M. J. Am. Chem. Soc. 1981, 103, 2868; Gilbert, J.C.; Baze, M.E. J. Am. Chem. Soc. 1983, 105, 664.
500. Chapman, O.L.; Gano, J.; West, P.R.; Regitz, M.; Maas, G. J. Am. Chem. Soc. 1981, 103, 7033.
501. Bennett, M.A.; Robertson, G.B.; Whimp, P.O.; Yoshida, T. J. Am. Chem. Soc. 1971, 93, 3797.
502. See Johnson, R.P. Chem. Rev. 1989, 89, 1111; Thies, R.W. Isr. J. Chem. 1985, 26, 191; Schuster, H.F.; Coppola, G.M. Allenes in Organic Synthesis; Wiley, NY, 1984, pp. 38–56.
503. Price, J.D.; Johnson, R.P. Tetrahedron Lett. 1986, 27, 4679.
504. See Marquis, E.T.; Gardner, P.D. Tetrahedron Lett. 1966, 2793.
505. Wittig, G.; Dorsch, H.; Meske-Schüller, J. Liebigs Ann. Chem. 1968, 711, 55.
506. Visser, J.P.; Ramakers, J.E. J. Chem. Soc. Chem. Commun. 1972, 178.
507. Wentrup, C.; Gross, G.; Maquestiau, A.; Flammang, R. Angew. Chem. Int. Ed. 1983, 22, 542. 1,2,3-Cyclohexatriene has also been trapped: Shakespeare, W.C.; Johnson, R.P. J. Am. Chem. Soc. 1990, 112, 8578.
508. Moore, W.R.; Ward, H.R. J. Am. Chem. Soc. 1963, 85, 86.
509. Angus Jr., R.O.; Johnson, R.P. J. Org. Chem. 1984, 49, 2880.
510. Ohwada, T. Tetrahedron 1993, 49, 7649.
511. Lange, H.; Schäfer, W.; Gleiter, R.; Camps, P.; Vázquez, S. J. Org. Chem. 1998, 63, 3478.
512. See Shea, K.J. Tetrahedron 1980, 36, 1683; Buchanan, G.L. Chem. Soc. Rev. 1974, 3, 41; Köbrich, G. Angew. Chem. Int. Ed. 1973, 12, 464. See Billups, W.E.; Haley, M.M.; Lee, G. Chem. Rev. 1989, 89, 1147; Warner, P.M. Chem. Rev. 1989, 89, 1067; Keese, R. Angew. Chem. Int. Ed. 1975, 14, 528. Also see, Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010, pp. 553–555.
513. See Maier, W.F.; Schleyer, P.v.R. J. Am. Chem. Soc. 1981, 103, 1891.
514. Kim, M.; White, J.D. J. Am. Chem. Soc. 1975, 97, 451; Becker, K.B. Helv. Chim. Acta 1977, 60, 81. See Nakazaki, M.; Naemura, K.; Nakahara, S. J. Org. Chem. 1979, 44, 2438.
515. Wiseman, J.R.; Chan, H.; Ahola, C.J. J. Am. Chem. Soc. 1969, 91, 2812; Carruthers, W.; Qureshi, M.I. Chem. Commun. 1969, 832; Becker, K.B. Tetrahedron Lett. 1975, 2207.
516. Lesko, P.M.; Turner, R.B. J. Am. Chem. Soc. 1968, 90, 6888; Burkert, U. Chem. Ber. 1977, 110, 773.
517. Wiseman, J.R.; Chong, J.A. J. Am. Chem. Soc. 1969, 91, 7775.
518. Sheridan, R.S.; Ganzer, G.A. J. Am. Chem. Soc. 1983, 105, 6158; Radziszewski, J.G.; Downing, J.W.; Wentrup, C.; Kaszynski, P.; Jawdosiuk, M.; Kovacic, P.; Michl, J. J. Am. Chem. Soc. 1985, 107, 2799.
519. Radziszewski, J.G.; Downing, J.W.; Wentrup, C.; Kaszynski, P.; Jawdosiuk, M.; Kovacic, P.; Michl, J. J. Am. Chem. Soc. 1985, 107, 2799.
520. See Tidwell, T.T. Tetrahedron 1978, 34, 1855; Mosher, H.S.; Tidwell, T.T. J. Chem. Educ. 1990, 67, 9. For a review of van der Waals radii, see Zefirov, Yu.V.; Zorkii, P.M. Russ. Chem. Rev. 1989, 58, 421.
521. Arnett, E.M.; Bollinger, J.M. Tetrahedron Lett. 1964, 3803.
522. Maier, G.; Schneider, K. Angew. Chem. Int. Ed. 1980, 19, 1022. For another example, see Krebs, A.; Franken, E.; Müller, S. Tetrahedron Lett. 1981, 22, 1675.
523. Arnett, E.M.; Sanda, J.C.; Bollinger, J.M.; Barber, M. J. Am. Chem. Soc. 1967, 89, 5389;. See also, Barclay, L.R.C.; Brownstein, S.; Gabe, E.J.; Lee, F.L. Can. J. Chem. 1984, 62, 1358.
524. Sakurai, H.; Ebata, K.; Kabuto, C.; Sekiguchi, A. J. Am. Chem. Soc. 1990, 112, 1799.
525. Siegel, J.; Gutiérrez, A.; Schweizer, W.B.; Ermer, O.; Mislow, K. J. Am. Chem. Soc. 1986, 108, 1569. Also see Kahr, B.; Biali, S.E.; Schaefer, W.; Buda, A.B.; Mislow, K. J. Org. Chem. 1987, 52, 3713.
526. See Iwamura, H.; Mislow, K. Acc. Chem. Res. 1988, 21, 175; Mislow, K. Chemtracts: Org. Chem. 1989, 2, 151; Berg, U.; Liljefors, T.; Roussel, C.; Sandström, J. Acc. Chem. Res. 1985, 18, 80.
527. Nugiel, D.A.; Biali, S.E.; Rappoport, Z. J. Am. Chem. Soc. 1984, 106, 3357.
528. Yamamoto, G.; Öki, M. Bull. Chem. Soc. Jpn. 1986, 59, 3597. See Yamamoto, G. Pure Appl. Chem. 1990, 62, 569; Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985, pp. 269–284.
529. Ackerman, J.H.; Laidlaw, G.M.; Snyder, G.A. Tetrahedron Lett. 1969, 3879; Ackerman, J.H.; Laidlaw, G.M. Tetrahedron Lett. 1969, 4487. See also, Cuyegkeng, M.A.; Mannschreck, A. Chem. Ber. 1987, 120, 803.
530. See Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985; Förster, H.; Vögtle, F. Angew. Chem. Int. Ed. 1977, 16, 429; Öki, M. Angew. Chem. Int. Ed. 1976, 15, 87.
531. Clough, R.L.; Roberts, J.D. J. Am. Chem. Soc. 1976, 98, 1018. For a study of rotational barriers in this system, see Cosmo, R.; Sternhell, S. Aust. J. Chem. 1987, 40, 1107.
532. Bartlett, P.D.; Tidwell, T.T. J. Am. Chem. Soc. 1968, 90, 4421.
533. See Back, T.G.; Barton, D.H.R. J. Chem. Soc. Perkin Trans 1, 1977, 924; Kopka, I.E.; Fataftah, Z.A.; Rathke, M.W. J. Org. Chem. 1980, 45, 4616.
534. Schmidbaur, H.; Blaschke, G.; Zimmer-Gasser, B.; Schubert, U. Chem. Ber. 1980, 113, 1612.
535. DeTar, D.F.; Binzet, S.; Darba, P. J. Org. Chem. 1985, 50, 2826, 5298, 5304.
536. For reviews, see Luef, W.; Keese, R. Top. Stereochem. 1991, 20, 231; Sandström, J. Top. Stereochem. 1983, 14, 83, pp. 160–169.
537. For a list of crowded alkenes that have been made, see Drake, C.A.; Rabjohn, N.; Tempesta, M.S.; Taylor, R.B. J. Org. Chem. 1988, 53, 4555. See also, Garratt, P.J.; Payne, D.; Tocher, D.A. J. Org. Chem. 1990, 55, 1909.
538. Krebs, A.; Nickel, W.; Tikwe, L.; Kopf, J. Tetrahedron Lett. 1985, 26, 1639.
539. Sakurai, H.; Ebata, K.; Kabuto, C.; Nakadaira, Y. Chem. Lett. 1987, 301.
540. Wiberg, K.B.; Matturo, M.G.; Okarma, P.J.; Jason, M.E. J. Am. Chem. Soc. 1984, 106, 2194; Wiberg, K.B.; Adams, R.D.; Okarma, P.J.; Matturo, M.G.; Segmuller, B. J. Am. Chem. Soc. 1984, 106, 2200.
541. Eaton, P.E.; Maggini, M. J. Am. Chem. Soc. 1988, 110, 7230.
542. Hrovat, D.A.; Borden, W.T. J. Am. Chem. Soc. 1988, 110, 7229.
543. For a review of such molecules, see Borden, W.T. Chem. Rev. 1989, 89, 1095. See also, Hrovat, D.A.; Borden, W.T. J. Am. Chem. Soc. 1988, 110, 4710.