March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 10. Aliphatic Substitution, Nucleophilic and Organometallic
10.B. SET Mechanisms
In certain reactions, where nucleophilic substitutions would seem obviously indicated, there is evidence that radicals and/or radical ions are actually involved.100 The first step in such a process is transfer of an electron from the nucleophile to the substrate to form a radical anion:
![]()
Mechanisms that begin this way are called SET mechanisms.101 Once formed, the radical ion cleaves:
![]()
The radicals formed in this way can go on to product by reacting with the Y• produced in Step 1 or with the original nucleophilic ion Y−, in which case an additional step is necessary:
![]()
In the latter case, the radical ion R–X• is formed by Step 4, as well as by Step 1, so that a chain reaction (Sec. 14.A.i) can take place.
One type of evidence for an SET mechanism is the finding of some racemization. A free radical would likely result in a completely racemized product RY, but it has been suggested102 that inversion can also take place in some SET processes. The suggestion is that in Step 1 the Y− still approaches from the back side, even though an ordinary SN2 mechanism will not follow, and that the radical R•, once formed, remains in a solvent cage with Y• still opposite X−, so that Steps 1–3 can lead to inversion.
![]()
Reactions with SET mechanisms typically show predominant, although not 100%, inversion.
Other evidence cited103 for SET mechanisms has been detection of radical or radical-ion intermediates by ESR104 or CIDNP; the finding that such reactions can take place at 1-norbornyl bridgeheads;105 and the formation of cyclic side products when the substrate has a double bond in the 5,6-position (such substrates are called radical probes).
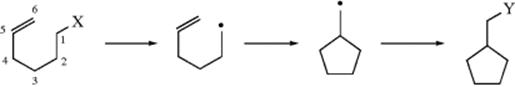
Free radicals with double bonds in this position are known to cyclize readily (Sec. 15.A.iii).106
The SET mechanism is chiefly found where X = I or NO2 (see Reaction 10-67). A closely related mechanism, the SRN1, takes place with aromatic substrates (Chap 13).107 In that mechanism, the initial attack is by an electron donor, rather than a nucleophile. The SRN1 mechanism has also been invoked for reactions of enolate anions with 2-iodobicyclo[4.1.0]heptane.108 An example is the reaction of 1-iodobicyclo[2.2.1]heptane (20) with NaSnMe3 or LiPPh2, and some other nucleophiles, to give the substitution product.109 Another is the reaction of bromo 4-bromoacetophenone (21) with Bu4NBr in cumene.110 The two mechanisms, SN2 versus SET, have been compared and contrasted.111 There are also reactions where it is reported that radical, carbanion, and carbene pathways occur simultaneously.112
The mechanisms so far considered can, in theory at least, operate on any type of saturated (or for that matter unsaturated) substrate. There are other mechanisms that are more limited in scope.