Organic Chemistry I For Dummies, 2nd Edition (2014)
Part I. Getting Started with Organic Chemistry
Chapter 5. Reactivity Centers: Functional Groups
IN THIS CHAPTER
Recognizing functional groups
Understanding the properties of functional groups
Finding functional groups in nature
Consider this: Millions of reactions of organic molecules are currently known, and that number is getting bigger by the day. That’s probably a scary thought. Can you imagine trying to learn all of them? The good news is that you don’t have to learn all the reactions of specific molecules because organic molecules often react in predictable ways based on what kinds of groups a particular molecule contains.
Alkanes (molecules containing just singly bonded hydrogen and carbon atoms) are pretty much inert under most conditions. Carbon, though, is unique among the elements in that it has the capability of forming stable compounds that have multiple bonds to other carbons, in addition to stable bonds to other non-carbon atoms, forming reactive centers. These reactive centers are called functional groups and are the reactive portions in an organic molecule. Chemists organize organic compounds based on what functional groups are present in a particular molecule.
One reason learning the functional groups is important is that if you learn the general reactions of a particular functional group, you have essentially learned the reactions of thousands of specific molecules that contain that functional group. The naming of organic molecules is based on what functional groups are present in a molecule because the functional groups dictate how a molecule will react. So, the sooner you learn these functional groups, the better.
In this chapter, I present the most important functional groups that you encounter throughout organic chemistry. I give examples of natural sources in which these molecules are found to show their relevance to biological systems. Additionally, I discuss their commercial uses, general nomenclature points, and interesting properties. This overview, I hope, will help you remember these functional groups and will give you a feel for the uses of particular functional groups.
Hydrocarbons
Hydrocarbons, as the name suggests, are molecules that contain just hydrogen and carbon. Simple hydrocarbons are generally cheap and commercially available, because they’re found as components in crude oil. Hydrocarbons include alkanes (which contain only single bonds and are generally not considered a functional group), alkenes (molecules containing carbon-carbon double bonds), alkynes (molecules containing carbon-carbon triple bonds), and aromatics (double-bond-containing ring systems).
Double the fun: The alkenes
An alkene is a molecule that contains a carbon-carbon double bond (see Chapter 2 for more on double bonds). The general form of an alkene is shown in Figure 5-1. (See Chapter 3 for an overview of molecular drawings.)
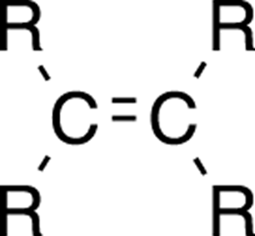
FIGURE 5-1: The general form of an alkene.
 The R is an abbreviation for the rest of the molecule. An R group most often implies a hydrogen atom or a hydrocarbon group. It’s used when a generality is being demonstrated or when the rest of the molecule isn’t very important in understanding what’s being discussed.
The R is an abbreviation for the rest of the molecule. An R group most often implies a hydrogen atom or a hydrocarbon group. It’s used when a generality is being demonstrated or when the rest of the molecule isn’t very important in understanding what’s being discussed.
Alkenes are often found in natural products, compounds isolated from living organisms. In the simplest alkene, ethylene, each R group is a hydrogen atom (see Figure 5-2). Ethylene is a gaseous plant hormone that is released when the plant reaches maturity, signaling that it’s time for the fruit to start ripening. Farmers often have special equipment that they use to spray ethylene on their crops when they want to force the fruit to ripen.
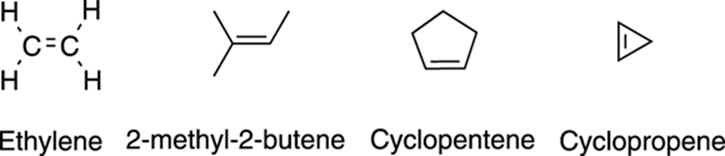
FIGURE 5-2: The structures of some common alkenes.
Alkenes are also used commercially. Ethylene polymerizes (combines many small units to make large molecules) to make polyethylene — a molecule formed from many ethylene molecules strung one after another to form a big chain. Polyethylene is a type of plastic used in containers such as grocery bags, milk bottles, and many different items that you see and use every day.
Alkenes can even be arranged in rings, like with cyclopropene, although they generally don’t like to be in very small rings like cyclopropene (refer to Figure 5-2). I talk about alkenes in Chapters 9 and 10.
Alkenes are particularly important to organic chemists because they can be transformed into many different functional groups. They’re easily made and converted into other things; this makes them particularly useful as go-betweens in the synthesis of complex molecules. In this book, I show how alkenes are converted into alkanes, cycloalkanes, cyclic ethers, alcohols, alkyl halides, aldehydes, and carboxylic acids. Talk about versatile!
The names of alkenes generally end with the suffix –ene. One particularly important alkene is vitamin A (also known as retinol). Vitamin A (see Figure 5-3) is an organic compound that contains five double bonds. It is important in vision, protects against sickness, and is used in skin-care products as an antioxidant that protects the skin from free radicals suspected of causing premature aging. (Read more about free radicals in Chapter 8.) I discuss the reactions, properties, and nomenclature of alkenes in detail in Chapters 9 and 10.
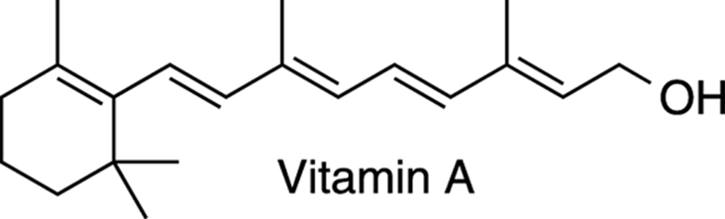
FIGURE 5-3: The structure of vitamin A (retinol).
Alkynes of fun
Alkynes are molecules that contain a carbon-carbon triple bond. See Figure 5-4. Many of their reactions and properties are similar to those of the alkenes, although the chemistry of alkenes and alkynes has some interesting differences.
![]()
FIGURE 5-4: The general form of an alkyne.
The simplest alkyne, acetylene, has the two R groups substituted with hydrogen (see Figure 5-5). Acetylene is a gas that is used as a welding fuel; it usually smells of garlic because of sulfurous impurities contained in it. Because acetylene burns cleanly and produces a very hot flame, acetylene torches reach temperatures of over 3,000°C, hot enough to weld metals, melt glass, and scorch off your pinkies if you aren’t careful. Other common alkynes include methyl acetylene (or propyne) and dimethyl acetylene (or 2-butyne); see Figure 5-5 for the structures of these alkynes.
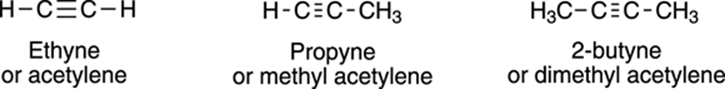
FIGURE 5-5: The structures of some common alkynes.
Alkynes are less common than alkenes in nature, but they do pop up occasionally. Calicheamicin (try saying that three times fast), for example, is a complicated organic molecule that is made by a bacterium that grows in a kind of sedimentary rock called caliche. Calicheamicin was recently found to selectively attack and kill the DNA of cancer cells and has, along with similar compounds, been used as a drug in anticancer therapies.
 The biologically active portion of the calicheamicin molecule contains two triple bonds (see Figure 5-6). This portion is called an enediyne. The “diyne” part of the name means “two ynes,” or “two triple bonds.” Can you guess where the “ene” portion of the name comes from?
The biologically active portion of the calicheamicin molecule contains two triple bonds (see Figure 5-6). This portion is called an enediyne. The “diyne” part of the name means “two ynes,” or “two triple bonds.” Can you guess where the “ene” portion of the name comes from?
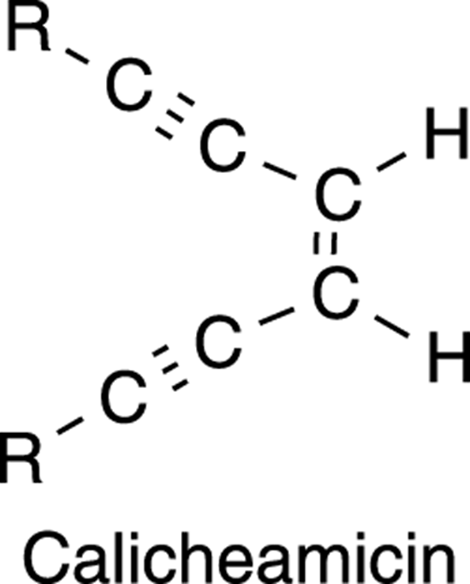
FIGURE 5-6: The structure of calicheamicin.
Alkynes prefer to have bond angles of 180 degrees, where the triple bond and the two R groups on either side lie in a straight line. Triple bonds aren’t particularly stretchy, and because of this, molecules with triple bonds are generally unstable in rings of fewer than eight carbons.
Alkyne names end with the suffix –yne. Sometimes, they’re called by the common name that derives from the simplest alkyne, acetylene. I cover the specifics of alkyne properties and reactions in Chapter 11.
Smelly compounds: The aromatics
The aromatics (or arenes, as they’re often called) consist of rings containing alternating double bonds. The principal aromatic compound is benzene, a six-carbon ring containing three alternating double bonds (see Figure 5-7). You might think that benzene would behave like a ring containing three alkenes, but this turns out not to be the case. Aromatic compounds have a special property that makes them significantly more stable, and less reactive, than alkenes. In Chapter 15, I discuss why benzene and other aromatics are so stable, and also talk about aromatic compounds other than benzene.
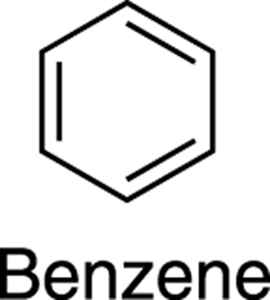
FIGURE 5-7: The structure of benzene.
These rings are called aromatics because the first of these compounds to be discovered (even before their structures were determined) had funky smells. Benzene itself is found as a component in crude oil; it has even better burning properties than does the octane fuel that you use to top off your gas tank. Unfortunately, it is a carcinogen and has other undesirable properties, so it isn’t used as a fuel. However, it is often used by organic chemists (with careful handling) as a solvent and as a cheap starting material in multistep syntheses.
Many compounds made by living organisms contain aromatic rings. (See Figure 5-8 for the structures of some aromatic compounds; see Chapter 6 for more on 3-D structure.) Morphine, for example, contains a benzene ring that is vital to its pain-relieving properties. Auto exhaust, soot, and tobacco smoke contain fused rings of benzene, like benzopyrene, where the rings are squished together to make very large aromatic compounds. These compounds have been found to be carcinogenic. In fact, chimney sweeps (the guys who used to clean chimneys in the days when coal was used for home heating) had a remarkably high incidence of testicular cancer because of their high exposure to these aromatic compounds in the chimney soot.
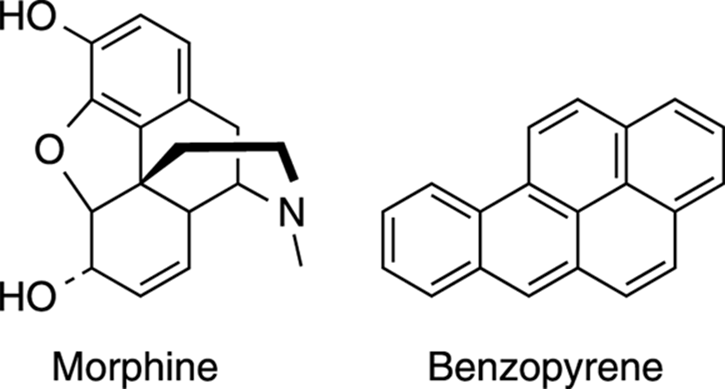
FIGURE 5-8: The structures of some aromatic compounds.
Singly Bonded Heteroatoms
Heteroatoms are atoms other than carbon or hydrogen. They include such important atoms as the halogens, oxygen, and sulfur, and are the components of the halide, alcohol, ether, and thiol functional groups. Each of these functional groups is described in this section.
Happy halides
The halides are organic compounds that contain one or more halogens. (Halogens are those elements found in column 7A of the periodic table.) The four halogens that you frequently see in organic compounds are fluorine, chlorine, bromine, and iodine. The general form of a halide is shown in Figure 5-9.
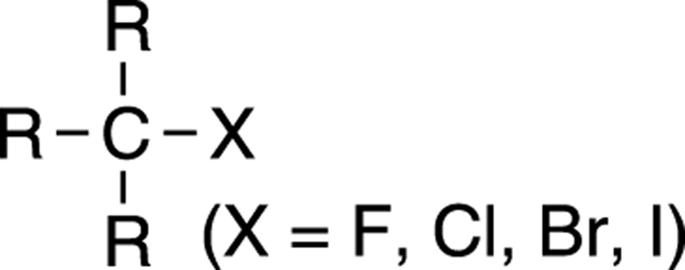
FIGURE 5-9: The structure of a simple halide.
Halides, like alkynes, are seldom found in natural products, and when they are found, they’re often in compounds that are toxins. Commercially, halides are used as propellants in aerosol cans such as hairspray and spray paint, solvents such as chloroform, and as refrigerants. Evidence that certain alkyl halides (that is, halides attached to alkanes) persist in the atmosphere and that these compounds contribute to depleting the ozone layer has led many countries to limit or outlaw their use as propellants. An example of a refrigerant halide is shown in Figure 5-10.
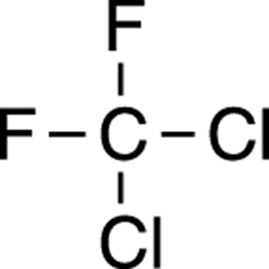
FIGURE 5-10: The structure of a refrigerant.
Halides are also used as insecticides. For example, DDT (dichlorodiphenyl-trichloroethane; see Figure 5-11) was widely used to protect crops from insects, until evidence suggested that this compound accumulated in the environment and caused unwanted side effects in wildlife, like the thinning of the eggshells of wild birds, and the death of bats and rodents. In large enough doses, DDT was even found to cause death to humans. Not surprisingly, this compound is now banned in the United States.
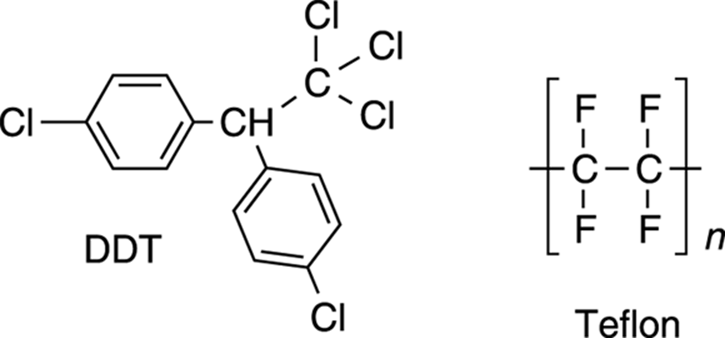
FIGURE 5-11: The structures of some common halides.
Other halides are still used in homes and businesses. For example, Teflon is a fluorine-containing polymer used in nonstick surfaces like pots and pans (see Figure 5-11). The brackets with the n subscript in the structure of Teflon indicate that that portion of the molecule is a repeating unit.
Many other halides are important to organic chemists because they’re good throughways to other molecules; they’re easily made and converted into other things. I talk more about halides and their reactions in Chapter 12.
For rubbing and drinking: Alcohols
Alcohols are also a very common and important group of organic compounds. Alcohols consist of the general formula R-OH, and have names that end with the suffix –ol. Probably the alcohol you’re most familiar with is ethanol (or ethyl alcohol) because it’s the alcohol found in beer, wine, and liquor. Ethanol is toxic in large enough doses. Some of its effects include a decrease in your body’s motor coordination, lowered inhibition, loud and off-key karaoke singing, and crank calls to your mother-in-law. Simple alcohols, such as isopropanol (rubbing alcohol), are used in cleaning solutions and as disinfectants, and an alcohol with two OH groups, ethylene glycol (which is also toxic, so don’t let your dog drink it), is used in antifreeze. Figure 5-12 illustrates some common alcohols.
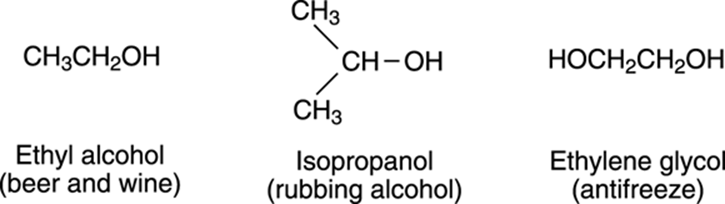
FIGURE 5-12: The structures of some common alcohols.
Alcohols are also commonly found in natural products. Sugars, for example, like the table sugar sucrose (shown in Figure 5-13), contain many OH groups.
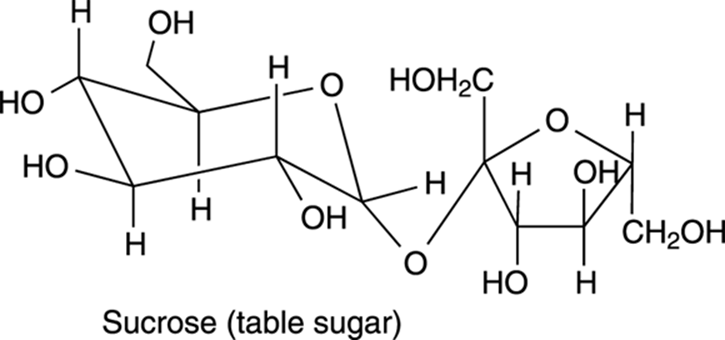
FIGURE 5-13: The structure of table sugar (sucrose), showing the fructose and glucose components.
What stinks? Thiols
Very few organic chemists have a burning desire to work with thiols. That’s because thiols are foul-smelling compounds, of the general formula R-SH. Thiols are the sulfur analog of alcohols and are often hideously unpleasant compounds. Certain thiols, for example, are found in skunk spray — that stinky stuff skunks use to fend off predators. Other thiols are responsible for the odors in flatulence, garlic, sewage, skunked beer, and rotten eggs. Commercially, very small amounts of thiols are added to methane gas so you can detect when a gas line has sprung a leak (methane itself is odorless).
Not all thiols are associated with such unpleasantries, however. Cysteine, for example, is one of the amino acids that the human body uses to produce proteins, and this amino acid plays a particularly important role in keratin, a protein found in hair. These cysteine amino acids (see Figure 5-14) are important because they can couple with each other and make disulfide bonds (S-S bonds) that hold the shape of your hair. If you’ve ever had a perm, you experienced thiol chemistry. A perm involves treating the hair with chemicals and heat to break the disulfide bonds, reshaping the hair in the desired way, and then resetting the disulfide bonds.
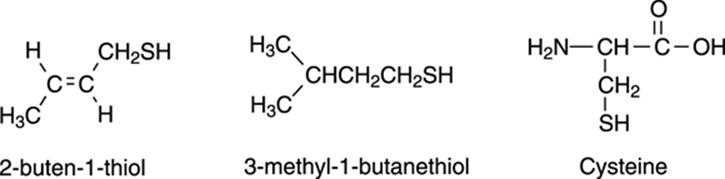
FIGURE 5-14: Cysteine and skunk-spray thiols.
Generally, names of thiols end with the suffix –thiol, as is the case with two of the thiols found in skunk spray — 2,3-dimethyl-2-buten-1-thiol and 3-methyl-1-butanethiol, both of which are shown in Figure 5-14.
How ethereal
Ethers are molecules containing an oxygen sandwiched between two carbons (see Figure 5-15). Molecules containing ether functional groups are widely used as solvents in organic reactions. Additionally, before it was replaced by modern anesthetics that make recovery less painful, diethyl ether (commonly known as “ether”) was used as an anesthetic to knock patients unconscious for surgery. Ethers found in three-membered rings are given the specific name of epoxides (the simplest epoxide shown in Figure 5-15 is ethylene oxide). Epoxides are found in epoxy resins and in epoxy glues, and are common as intermediates in multistep syntheses.
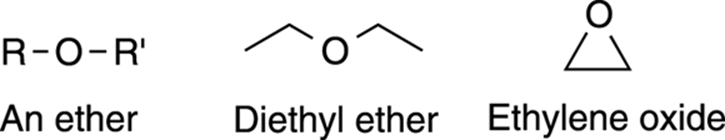
FIGURE 5-15: Ethers.
Carbonyl Compounds
The chemistry of living things is largely the chemistry of carbonyl compounds. A carbonyl group is a C=O group — in other words, a carbon atom double-bonded to oxygen. A carbonyl group is not considered a functional group in itself; instead, it’s considered a component in some of the most important functional groups, including the aldehydes, ketones, esters, amides, and carboxylic acids. If you’re considering taking a course in biochemistry, you should have a good understanding of the reactions and properties of carbonyl compounds because most of the reactions in the body are carbonyl reactions (including the reactions in the Krebs Cycle, in glycolysis, and in the synthesis of fatty acids and polyketides).
Living on the edge: Aldehydes
Aldehydes are the simplest of the carbonyl compounds. In an aldehyde (see Figure 5-16), the carbonyl group is flanked by one hydrogen and one R group. It may be helpful to think of an aldehyde as a carbonyl group at the end of an organic molecule.
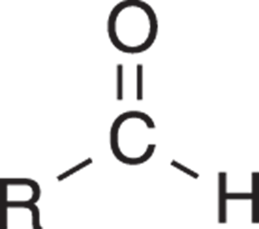
FIGURE 5-16: An aldehyde.
The simplest aldehyde, formaldehyde — in which the R group is hydrogen — has many uses, including as a preservative. Often the smells that waft from a biology laboratory come from the formaldehyde used to preserve specimens (not that chemists have much right to complain, though, considering some of the smells emitting from their laboratories). Retinal is a large aldehyde; it’s one of the pigments that traps light in the eyes of humans, making vision possible. Benzaldehyde is a wonderfully sweet-smelling compound that gives almonds its odor. Both retinal and benzaldehyde are shown in Figure 5-17.
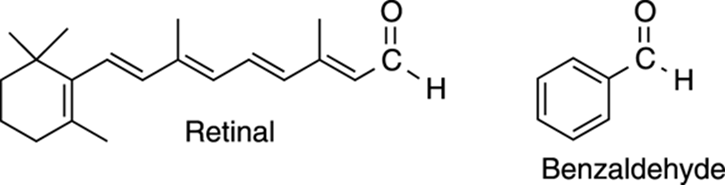
FIGURE 5-17: Two important aldehydes.
 You’ll often see aldehydes represented as R-CHO. Don’t confuse this condensed form with an alcohol, which is represented as R-OH.
You’ll often see aldehydes represented as R-CHO. Don’t confuse this condensed form with an alcohol, which is represented as R-OH.
Stuck in the middle: Ketones
Compounds that contain a carbonyl group sandwiched between two carbons are called ketones. If an aldehyde can be thought of as a carbonyl at the end of a molecule, then a ketone is a carbonyl somewhere in the middle of a molecule. The simplest ketone, in which both R groups are CH3 units, is called acetone, a common laboratory solvent (see Figure 5-18). Acetone is the smelly stuff found in nail polish remover, and is the reason why polished nails don’t stay polished for very long in organic laboratories. The names of ketones generally end with the suffix –one.
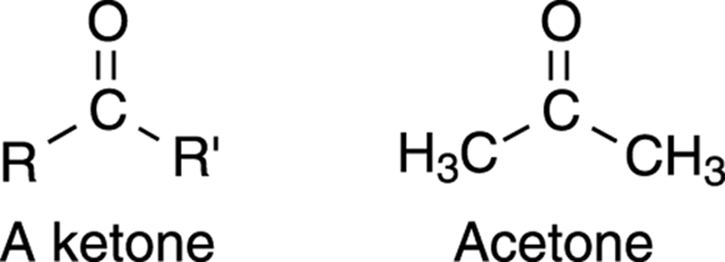
FIGURE 5-18: The general structure of a ketone and the specific structure of acetone.
Carboxylic acids
The carboxylic acid functional group is made up of a carbonyl group attached to an OH group. The general form of carboxylic acids is shown in Figure 5-19.
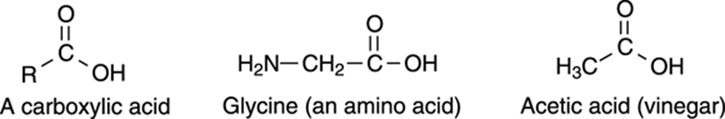
FIGURE 5-19: Common carboxylic acids.
 Don’t confuse a carboxylic acid with a ketone or an alcohol. Carboxylic acids have entirely different properties and reactivities than either ketones or alcohols. In particular, the proton (H+) on the oxygen in a carboxylic acid is unusually acidic (hence the name!), for reasons I talk about in Chapter 4.
Don’t confuse a carboxylic acid with a ketone or an alcohol. Carboxylic acids have entirely different properties and reactivities than either ketones or alcohols. In particular, the proton (H+) on the oxygen in a carboxylic acid is unusually acidic (hence the name!), for reasons I talk about in Chapter 4.
SNEAKY ORCHIDS, KETONES, AND CHEMICAL SIREN SONGS
In 2003, German and Australian scientists first reported their discovery of the diketone (a molecule with two ketone functional groups) chiloglottone (see the figure), produced by an Australian orchid flower. This orchid, they discovered, uses this diketone to reproduce, to get its pollen to another orchid for pollination. But how, you ask, can a chemical be responsible for transporting the orchid’s pollen to another orchid, sometimes over several miles? By trickery, deception, and bamboozling, of course. This deception forms yet another interesting episode of the soap opera between plants and insects, a soap opera that mostly goes undetected by humans.
Chiloglottone, it turns out, not only is produced by the Australian orchid, but also is the sex pheromone of a species of wasp native to Australia. When a female wasp emits this pheromone, she signals to all eligible male wasps in the area that she’s ready to mate. When a male wasp detects the pheromone in the air, he does the equivalent of a wasp “hubba hubba hubba,” and wastes no time in tracing the chemical trail back to its source for a little wasp hanky-panky. The orchid, however, has cracked the chemical code of communication between the wasps, and takes advantage of their instant-messaging system for its own devious purposes. The orchid makes its mischief by producing and transmitting the same sex pheromone that the wasps produce, and uses the chemical as a lure.
When they detect the pheromone produced by the orchid, the wasps — in typical male fashion — are so swooned by the possibility of wasp love that they become irrational, and in the height of their passion, they neglect to realize that the orchid is not a female wasp. Swooping down, the male wasp tries his best to mate with the orchid’s flower head, and when he’s finished his business, he flies away from the orchid with a thin dusting of orchid pollen on his legs. Coated with pollen, the wasp flies off until he’s attracted by another orchid emitting the sex pheromone, and, feeling amorous yet again, he “mates” with the second orchid. When the pollen from the first orchid rubs off the wasp’s legs, the second orchid becomes pollinated.
In this way, the orchid tricks the male wasp into becoming a postman for its pollen, free of charge, no stamps required. This kind of bamboozling is actually quite common in nature, and is called sexual deception. Though insects get revenge in some ways on plants — munching on them, for instance! — this round belongs to the plants.
Carboxylic acids are abundant in nature. In fact, each of the amino acids that our body uses as building blocks to make proteins contains a carboxylic acid (such as the amino acid glycine in Figure 5-19), as do all the fatty acids. The names of carboxylic acids generally end with –oic acid,like ethanoic acid (more commonly called acetic acid), which is the carboxylic acid responsible for the flavor and bitterness of vinegar.
Sweet-smelling compounds: Esters
Esters are very similar in structure to carboxylic acids. An ester is basically a carboxylic acid with the hydrogen snipped off, and an R group glued in its place. (In fact, esters are made from carboxylic acids.) Esters are generally sweet-smelling compounds, and many of the lovely smells from fruits are esters. (See Figure 5-20 for the structures of some esters.) Because of this, they’re generally found in deodorants and used as artificial flavorings in food. The names of esters generally end with the suffix –oate.
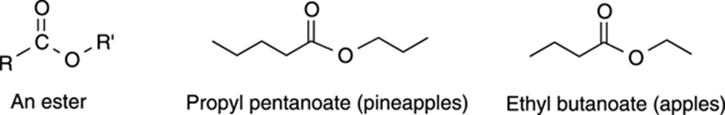
FIGURE 5-20: Common esters.
Nitrogen-containing functional groups
Just as the carbonyl compounds play an important role in nature, so do nitrogen-containing compounds. Illicit drugs and painkillers, which are often alkaloids (nitrogen-containing rings), often contain nitrogen as well.
Even though the primary component in air is nitrogen gas (N2), most plants and animals cannot use this as a source of nitrogen because, in its gaseous state, nitrogen is so unreactive and stable. The N2 gas needs to be converted to ammonia (NH3) or nitrate (NO3–) for animals and plants to be able to use it as a building block, a process called nitrogen fixation. This job is left up to microorganisms, which have special enzymes that can tackle this reaction. (Lightning strikes also account for some nitrogen fixation, although only a small amount.)
I am what I amide
Amides are close relatives of esters, except that amides have a nitrogen (rather than an oxygen) next to the carbonyl group. Amides are quite often found in nature. Amide bonds hold all our proteins together (the amide bond in proteins is called a peptide bond). See Figure 5-21 for the structures of some common amides.
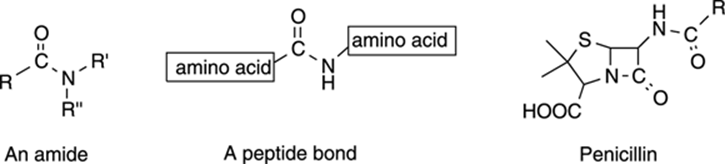
FIGURE 5-21: The general structure of an amide and a peptide, and the specific structure of penicillin.
 The famous antibiotic penicillin contains two amide groups. The amide in the four-membered ring (called a β-lactam ring) is responsible for penicillin’s bacteria-killing properties; this amide group reacts with enzymes responsible for building the cell walls of bacteria, which leads to bacterial death. (For more about penicillin, see Chapter 22.)
The famous antibiotic penicillin contains two amide groups. The amide in the four-membered ring (called a β-lactam ring) is responsible for penicillin’s bacteria-killing properties; this amide group reacts with enzymes responsible for building the cell walls of bacteria, which leads to bacterial death. (For more about penicillin, see Chapter 22.)
Be nice, don’t be amine person
Amines are nitrogen atoms that take the place of a carbon atom in an alkane (the three forms of an amine are R-NH2, R2NH, or R3N). Amines are not known for their pleasantness. For example, the smell of decaying animals comes from the diamine (a molecule with two amine functional groups) putrescine, for example. So, unless you’re a vulture, you probably don’t want to use these compounds as perfumes. Plants and animals and other organisms make many important amine compounds. Nicotine (see Figure 5-22), as well as many other familiar compounds like cocaine, mescaline, amphetamine, and morphine are all amine-containing compounds. Many drugs (both legal and illegal) contain amines that are essential to the drug’s activity.
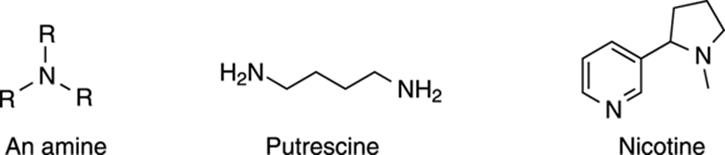
FIGURE 5-22: Common amines.
Nitriles
Nitriles (see Figure 5-23) are compounds that contain a carbon triply bonded to nitrogen. Nitriles are often useful in organic synthesis. Nitriles can be converted into carboxylic acids and amines by well-known procedures. Acetonitrile, in which the R group is a methyl group (CH3), is a common organic solvent. Nitriles generally end with the suffix –nitrile. As substituents on a molecule, they’re referred to as cyano groups.

FIGURE 5-23: Nitriles.
Test Your Knowledge
Figure 5-24 gives you a made-up molecule that contains some of the functional groups that I mention in this chapter. How many can you identify? (Hint: Draw out all the condensed portions — like COOH, CN, and CHO — to help you identify which functional group it is.)
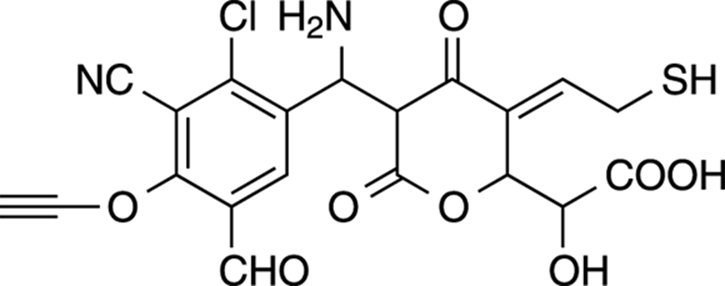
FIGURE 5-24: A hypothetical molecule with different functional groups.