5 Steps to a 5: AP Biology - Mark Anestis 2021
STEP 4 Review the Knowledge You Need to Score High
CHAPTER 5 Chemistry of Life
Exam Weight: 8—11%
IN THIS CHAPTER
Summary: This chapter introduces the chemical principles that are related to understanding the AP Biology topics covered throughout the course.

Key Ideas
![]() Organic compounds contain essential elements, including carbon, oxygen, hydrogen, nitrogen, and phosphorus.
Organic compounds contain essential elements, including carbon, oxygen, hydrogen, nitrogen, and phosphorus.
![]() The structure and function of macromolecules (proteins, carbohydrates, lipids, and nucleic acids) are key to the organization of living systems.
The structure and function of macromolecules (proteins, carbohydrates, lipids, and nucleic acids) are key to the organization of living systems.
![]() Water and its properties are instrumental in the organization and survival of all living organisms and systems.
Water and its properties are instrumental in the organization and survival of all living organisms and systems.
![]() Hydrolysis and dehydration reactions are vital in the formation and cleavage of bonds between monomer units.
Hydrolysis and dehydration reactions are vital in the formation and cleavage of bonds between monomer units.
Introduction
What is the name of the test you are studying for? The AP Biology exam. Then why in tarnation are we starting your review with a chapter titled Chemistry of Life?!?!? Because it is important that you have an understanding of the key chemical principles before we dive into the deeper biological material. We will keep it short, don’t worry. ![]()
Elements, Compounds, Atoms, and Ions
By definition, matter is anything that has mass and takes up space; an element is defined as matter in its simplest form; an atom is the smallest form of an element that still displays its particular properties. (Terms boldfaced in text are listed in the Glossary at the end of the book.) For example, sodium (Na) is an element mentioned often in this book. The element sodium can exist as an atom of sodium, in which it is a neutral particle containing an equal number of protons and electrons. It can also exist as an ion, which is an atom that has a positive or negative charge. Ions such as sodium that take on a positive charge are called cations, and are composed of more protons than electrons. Ions with a negative charge are called anions, and are composed of more electrons than protons.
Elements can be combined to form molecules, for example, an oxygen molecule (O2) or a hydrogen molecule (H2). Molecules that are composed of more than one type of element are called compounds, for example H2O. The two major types of compounds you need to be familiar with are organic and inorganic compounds. Organic compounds contain carbon and usually hydrogen; inorganic compounds do not. Some of you are probably skeptical, at this point, as to whether any of what we have said thus far matters for this exam. Bear with me because it does. You will deal with many important organic compounds later on in this book, including carbohydrates, proteins, lipids, and nucleic acids.
Before moving onto the next section, where we discuss these particular organic compounds in more detail, we would like to cover a topic that many find confusing and therefore ignore in preparing for this exam. This is the subject of functional groups. These poorly understood groups are responsible for the chemical properties of organic compounds. They should not intimidate you, nor should you spend a million hours trying to memorize them in full detail.
The following is a list of the functional groups you should study for this exam:
John (11th grade): “My teacher wanted me to know these structures . . . she was right!”
SYI-1
Living systems are organized in a hierarchy of structural levels that interact.
1. Amino group. An amino group has the following formula:
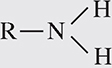
The symbol R stands for “rest of the compound” to which this NH2 group is attached. One example of a compound containing an amino group is an amino acid. Compounds containing amino groups are generally referred to as amines. Amino groups act as bases and can pick up protons from acids.
2. Carbonyl group. This group contains two structures:
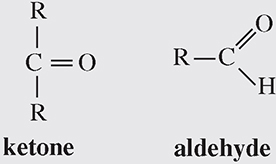
If the C=O is at the end of a chain, it is an aldehyde. Otherwise, it is a ketone. (Note: in aldehydes, there is an H at the end; there is no H in the word ketone.) A carbonyl group makes a compound hydrophilic and polar. Hydrophilic means water-loving, reacting well with water. A polar molecule is one that has an un-equal distribution of charge, which creates a positive side and a negative side to the molecule.
3. Carboxyl group. This group has the following formula:
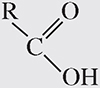
A carboxyl group is a carbonyl group that has a hydroxide in one of the R spots and a carbon chain in the other. This functional group shows up along with amino groups in amino acids. Carboxyl groups act as acids because they are able to donate protons to basic compounds. Compounds containing carboxyl groups are known as carboxylic acids.
4. Hydroxyl group. This group has the simplest formula of the bunch:
R — OH
A hydroxyl group is present in compounds known as alcohols. Like carbonyl groups, hydroxyl groups are polar and hydrophilic.
5. Phosphate group. This group has the following formula:
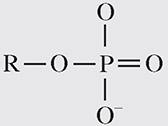
Phosphate groups are vital components of compounds that serve as cellular energy sources: ATP, ADP, and GTP. Like carboxyl groups, phosphate groups are acidic molecules.
6. Sulfhydryl group. This group also has a simple formula:
R — SH
This functional group does not show up much on the exam, but you should recognize it when it does. This group is present in the amino acids methionine and cysteine and assists in structure stabilization in many proteins.
Water
Water is an inorganic compound consisting of one oxygen molecule covalently bonded to two hydrogen bonds. The electrons shared between the hydrogen and oxygen molecules are closer to the oxygen molecule due to its electronegativity, resulting in the oxygen molecule being negatively charged and the hydrogen molecule being positively charged. Water molecules are polar because they have a positive and a negative side. Nonpolar molecules have a neutral charge due to equal sharing of electrons.
Hydrogen bonding is the attraction between a positively charged hydrogen atom and any other electronegative atom, such as oxygen. These bonds may form between atoms within the same molecule or between two separate molecules. Water is a molecule that contains slightly positive charged hydrogens and slightly negative oxygen molecules. This allows water molecules to form up to two hydrogen bonds with other water molecules, leading to a variety of properties unique to water (Figure 5.1.
Properties of Water
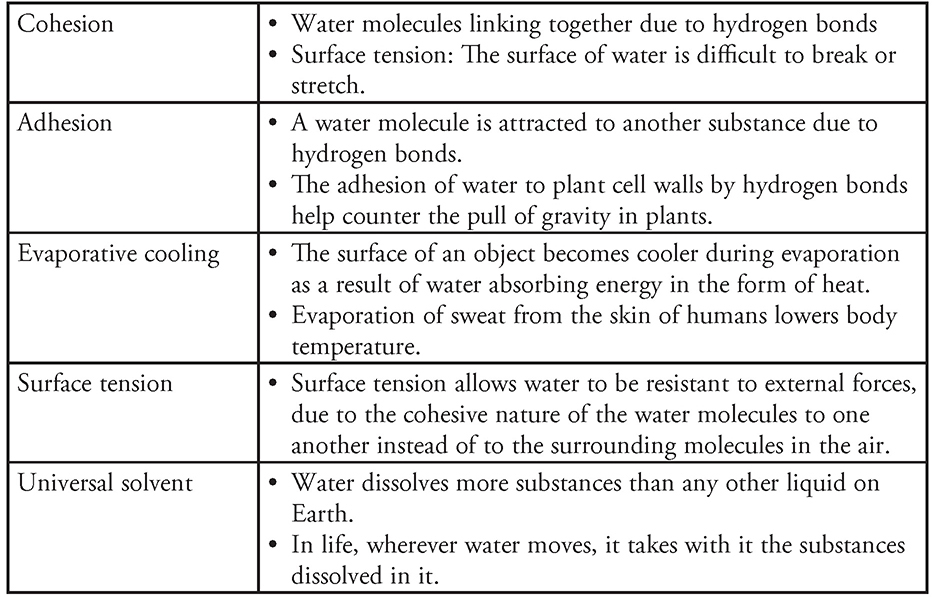
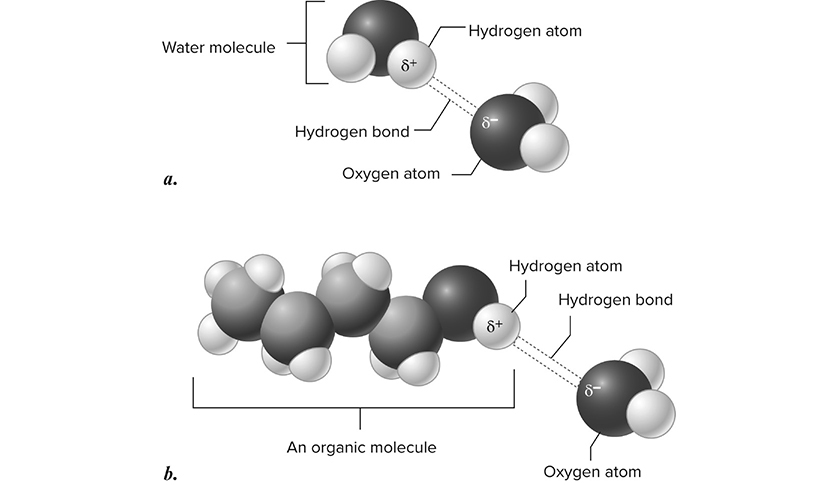
Figure 5.1 Structure of a hydrogen bond. a. Hydrogen bond between two water molecules. b. Hydrogen bond between an organic molecule (n-butanol) and water. H in n-butanol forms a hydrogen bond with oxygen in water. This kind of hydrogen bond is possible any time H is bound to a more electronegative atom. (Reproduced with permission from Raven P, Johnson G, Mason K, Losos J, Duncan T; Biology, 12th ed. New York: McGraw Hill; 2020)

Macromolecules
Monomers and Polymers
Macromolecules are made up of single units called monomers that are joined together via covalent bonds to form large polymers, such as carbohydrates, nucleic acids, and proteins. Due to their large size, lipids are also classified as macromolecules even though they lack the repeating monomer subunits seen in the other molecules.
Macromolecules are assembled via dehydration synthesis, a reaction that forms a covalent bond between two monomer units while releasing a water molecule in the process.
Hydrolysis is the process by which the covalent bonds between monomer units are broken by the addition of water (Figure 5.2.
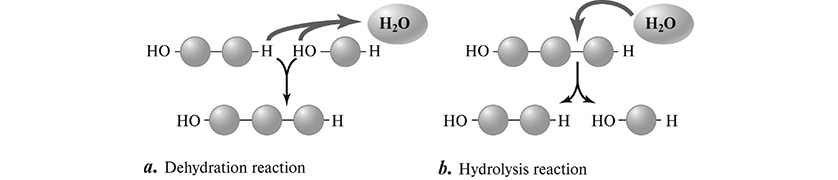
Figure 5.2 Making and breaking macromolecules. a. Biological macromolecules are polymers formed by linking monomers together through dehydration reactions. This process releases a water molecule for every bond formed. b. Breaking the bond between subunits involves hydrolysis, which reverses the loss of a water molecule by dehydration. (Reproduced with permission from Raven P, Johnson G, Mason K, Losos J, Duncan T; Biology, 12th ed. New York: McGraw Hill; 2020)
Lipids
Lipids are organic compounds used by cells as long-term energy stores or building blocks. Lipids are hydrophobic and insoluble in water because they contain a hydrocarbon tail of CH2S that is nonpolar and repellant to water. The most important lipids are fats, oils, steroids, and phospholipids.
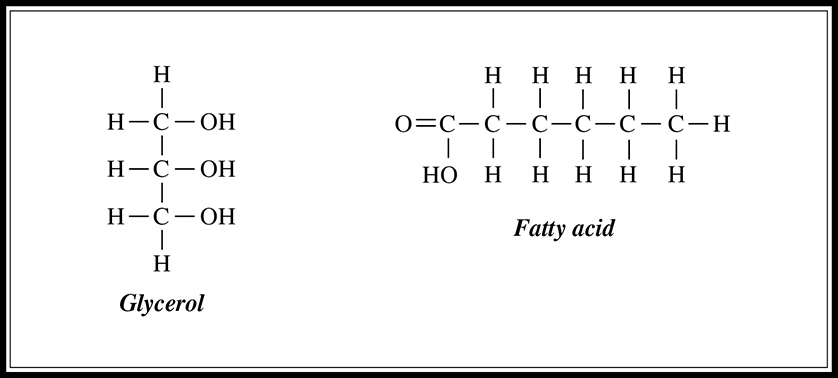
Figure 5.3 Structure of glycerol and fatty acids.
Fats, which are lipids made by combining glycerol and three fatty acids (Figure 5.3, are used as long-term energy stores in cells. They are not as easily metabolized as carbohydrates, yet they are a more effective means of storage; for instance, one gram of fat provides two times the energy of one gram of carbohydrate. Fats can be saturated or unsaturated. Saturated fat molecules contain no double bonds. Unsaturated fats contain one (mono-) or more (poly-) double bonds, which means that they contain fewer hydrogen molecules per carbon than do saturated fats. Saturated fats are the bad guys and are associated with heart disease and atherosclerosis. Most of the fat found in animals is saturated, whereas plants tend to contain unsaturated fats. Fat is formed when three fatty-acid molecules connect to the OH groups of the glycerol molecule. These connecting bonds are formed by dehydration synthesis reaction (Figure 5.4.
ENE-1
The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules
SYI-1
Living systems are organized in a hierarchy of structural levels that interact
IST-1
Heritable information provides for the continuity of life
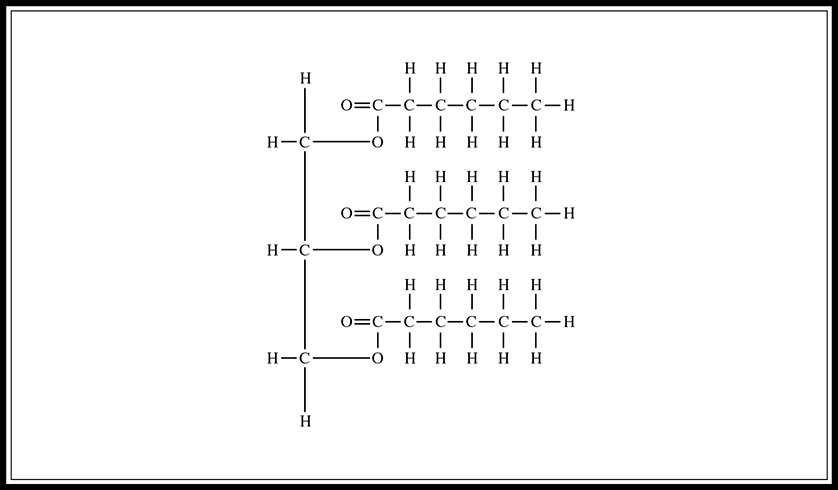
Figure 5.4 Fat structure (glycerol plus three fatty acids).
Steroids are lipids composed of four carbon rings that look like chicken-wire fencing in pictorial representations. One example of a steroid is cholesterol, an important structural component of cell membranes that serves as a precursor molecule for another important class of steroids: the sex hormones (testosterone, progesterone, and estrogen). You should be able to recognize the structures shown in Figure 5.5 for the AP exam.
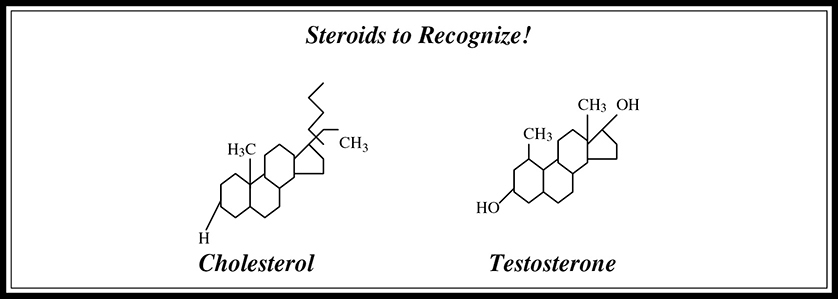
Figure 5.5 Steroid structures.
A phospholipid is a lipid formed by combining a glycerol molecule with two fatty acids and a phosphate group (Figure 5.6. Phospholipids are amphipathic structures; they have both a hydrophobic tail (a hydrocarbon chain) and a hydrophilic head (the phosphate group) (Figure 5.7. They are the major component of cell membranes; the hydrophilic phosphate group forms the outside portion, and the hydrophobic tail forms the interior of the wall.
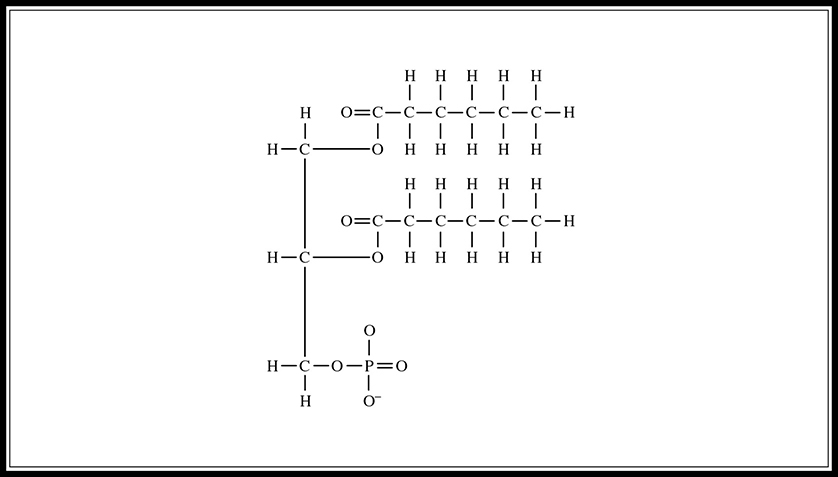
Figure 5.6 Structure of phospholipid.
Carbohydrates
Carbohydrates can be simple sugars or complex molecules containing multiple sugars. Carbohydrates are used by the cells of the body in energy-producing reactions and as structural materials. Carbohydrates have the elements C, H, and O. Hydrogen and oxygen are present in a 2:1 ratio. The three main types of carbohydrates you need to know are monosaccharides, disaccharides, and polysaccharides.
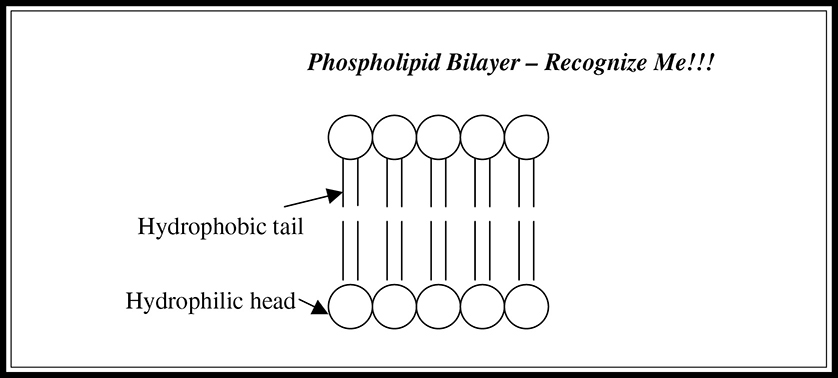
Figure 5.7 Bilayered structure of phospholipids.
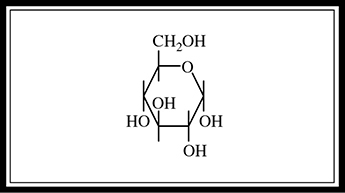
Figure 5.8 Glucose structure.
A monosaccharide, or simple sugar, is the simplest form of a carbohydrate. The most important monosaccharide is glucose (C6H12O6), which is used in cellular respiration to provide energy for cells. Monosaccharides with five carbons (C5H10O5) are used in compounds such as genetic molecules (RNA) and high-energy molecules (ATP). The structure of glucose is shown in Figure 5.8.
A disaccharide is a sugar consisting of two monosaccharides bound together. Com-mon disaccharides include sucrose, maltose, and lactose. Sucrose, a major energy carbohydrate in plants, is a combination of fructose and glucose; maltose, a carbohydrate used in the creation of beer, is a combination of two glucose molecules; and lactose, found in dairy products, is a combination of galactose and glucose.
A polysaccharide is a carbohydrate containing three or more monosaccharide molecules. Polysaccharides, usually composed of hundreds or thousands of monosaccharides, act as a storage form of energy and as structural material in and around cells. The most important carbohydrates for storing energy are starch and glycogen. Starch, made solely of glucose molecules linked together, is the storage form of choice for plants. Animals store much of their carbohydrate energy in the form of glycogen, which is most often found in liver and muscle cells. Glycogen is formed by linking many glucose molecules together.
Two important structural polysaccharides are cellulose and chitin. Cellulose, a compound composed of many glucose molecules, is used by plants in the formation of their cell walls. Chitin is an important part of the exoskeletons of arthropods such as insects, spiders, and shellfish.
Julie (11th grade): “Remembering these four came in handy on the test!”
Proteins
A protein is a compound composed of chains of amino acids. Proteins have many functions in the body—they serve as structural components, transport aids, enzymes, and cell signals, to name only a few. You should be able to identify a protein or an amino acid by sight if asked to do so on the test.
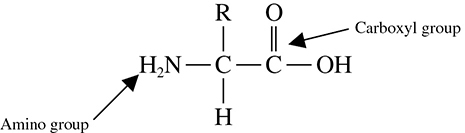
An amino acid consists of a carbon center surrounded by an amino group, a carboxyl group, a hydrogen, and an R group (see Figure 5.9). Remember that the R stands for “rest” of the compound, which provides an amino acid’s unique personal characteristics. For instance, acidic amino acids have acidic R groups, basic amino acids have basic R groups, and so forth.
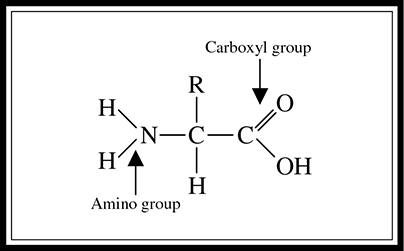
Figure 5.9 Structure of an amino acid.
Many students preparing for the AP exam wonder if they need to memorize the 20 amino acids and their structures and whether they are polar, nonpolar, or charged. This is a lot of effort for perhaps one multiple-choice question that you might encounter on the exam. We think that this time would be better spent studying other potential exam questions. If this is of any comfort to you, we have yet to see an AP Biology question that asks something to the effect of “Which of these 5 amino acids is nonpolar?” (Disclaimer: This does not mean that it will never happen ![]() .) It is more important for you to identify the general structure of an amino acid and know the process of protein synthesis.
.) It is more important for you to identify the general structure of an amino acid and know the process of protein synthesis.
A protein consists of amino acids linked together, as shown in Figure 5.10. They are most often much larger than that depicted here. Figure 5.10 is included to enable you to identify a peptide linkage on the exam. Most proteins have many more amino acids in the chain.
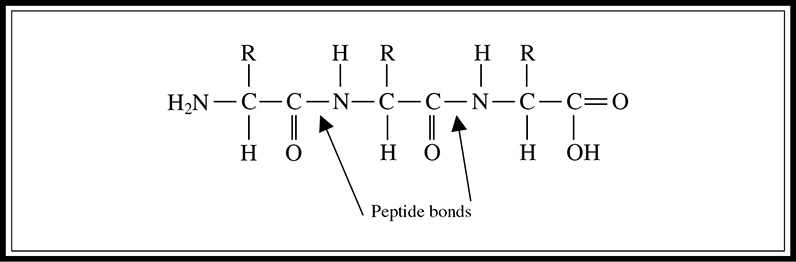
Figure 5.10 Amino acid structure exhibiting peptide linkage.
The AP exam may expect you to know about the structure of proteins:

Primary structure. The order of the amino acids that make up the protein.
Secondary structure. Three-dimensional arrangement of a protein caused by hydrogen bonding at regular intervals along the polypeptide backbone.
Tertiary structure. Three-dimensional arrangement of a protein caused by inter-action among the various R groups of the amino acids involved.
Quaternary structure. The arrangement of separate polypeptide “subunits” into a single protein. Not all proteins have quaternary structure; many consist of a single polypeptide chain.
Proteins with only primary and secondary structure are called fibrous proteins. Pro-teins with only primary, secondary, and tertiary structures are called globular proteins. Either fibrous or globular proteins may contain a quaternary structure if there is more than one polypeptide chain.
Nucleic Acids
DNA Structure and Function
Deoxyribonucleic acid, known to her peers as DNA, is composed of four nitrogenous bases: adenine, guanine, cytosine, and thymine. Adenine and guanine are a type of nitrogenous base called a purine, and contain a double-ring structure. Thymine and cytosine are a type of nitrogenous base called a pyrimidine, and contain a single-ring structure. Two scientists, James D. Watson and Francis H.C. Crick, spent a good amount of time devoted to determining the structure of DNA. Their efforts paid off, and they were the ones given credit for realizing that DNA was arranged in what they termed a double helix composed of two strands of nucleotides held together by hydrogen bonds. They noted that adenine always pairs with thymine (A=T) held together by two hydrogen bonds and that guanine always pairs with cytosine (C≡G) held together by three hydrogen bonds. Each strand of DNA consists of a sugar-phosphate backbone that keeps the nucleotides connected with the strand. The sugar is deoxyribose. (See Figure 5.11 for a rough sketch of what purine—pyrimidine bonds look like.)
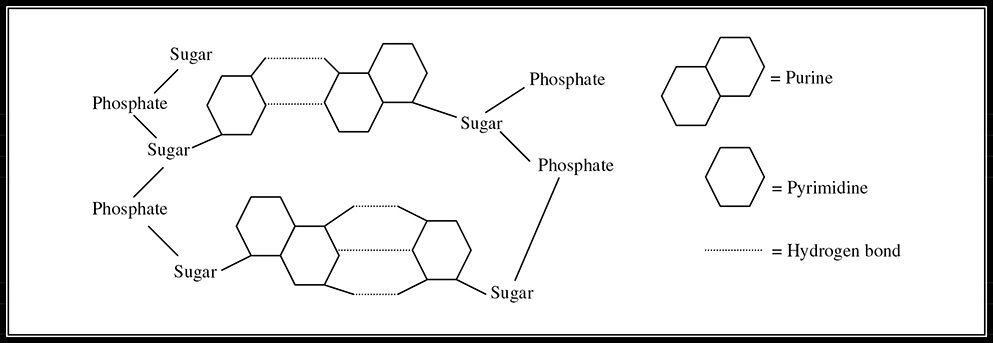
Figure 5.11 Purine—pyrimidine bonds.
One last structural note about DNA that can be confusing is that DNA has something called a 5′ end and a 3′ end (Figure 5.12. The two strands of a DNA molecule run antiparallel to each other; the 5′ end of one molecule is paired with the 3′ end of the other molecule, and vice versa.
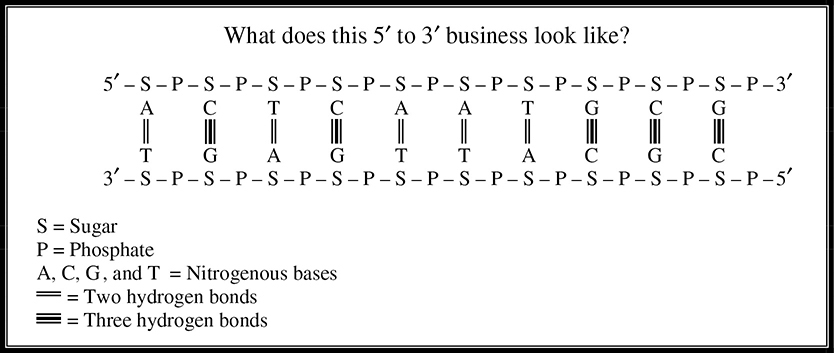
Figure 5.12 The 5′ and 3′ ends in DNA structure.
RNA Structure and Function
Ribonucleic acid is known to the world as RNA. There are some similarities between DNA and RNA. They both have a sugar-phosphate backbone. They both have four different nucleotides that make up the structure of the molecule. They both have three letters in their nickname—don’t worry if you don’t see that last similarity right away, . . . remember that we have been studying these things for years. These two molecules also have their share of differences. RNA’s nitrogenous bases are adenine, guanine, cytosine, and uracil. There is no thymine in RNA; uracil beat out thymine for the job (probably had a better interview during the hiring process). Another difference between DNA and RNA is that the sugar for RNA is ribose instead of deoxyribose. While DNA exists as a double strand, RNA has a bit more of an independent personality and tends to roam the cells as a single-stranded entity.
There are three main types of RNA that you should know about, all of which are formed from DNA templates in the nucleus of eukaryotic cells: (1) messenger RNA (mRNA), (2) transfer RNA (tRNA), and (3) ribosomal RNA (rRNA).
pH: Acids and Bases
The pH scale is used to indicate how acidic or basic a solution is. It ranges from 0 to 14; 7 is neutral. Anything less than 7 is acidic; anything greater than 7 is basic. The pH scale is a logarithmic scale and as a result, a pH of 5 is 10 times more acidic than a pH of 6. Following the same logic, a pH of 4 is 100 times more acidic than a pH of 6. Remember that as the pH of a solution decreases, the concentration of hydrogen ions in the solution increases, and vice versa. For the most part, chemical reactions in humans function at or near a neutral pH. The exceptions to this rule are the chemical reactions involving some of the enzymes of the digestive system.
![]() Review Questions
Review Questions
For questions 1—4, please use the following answer choices:
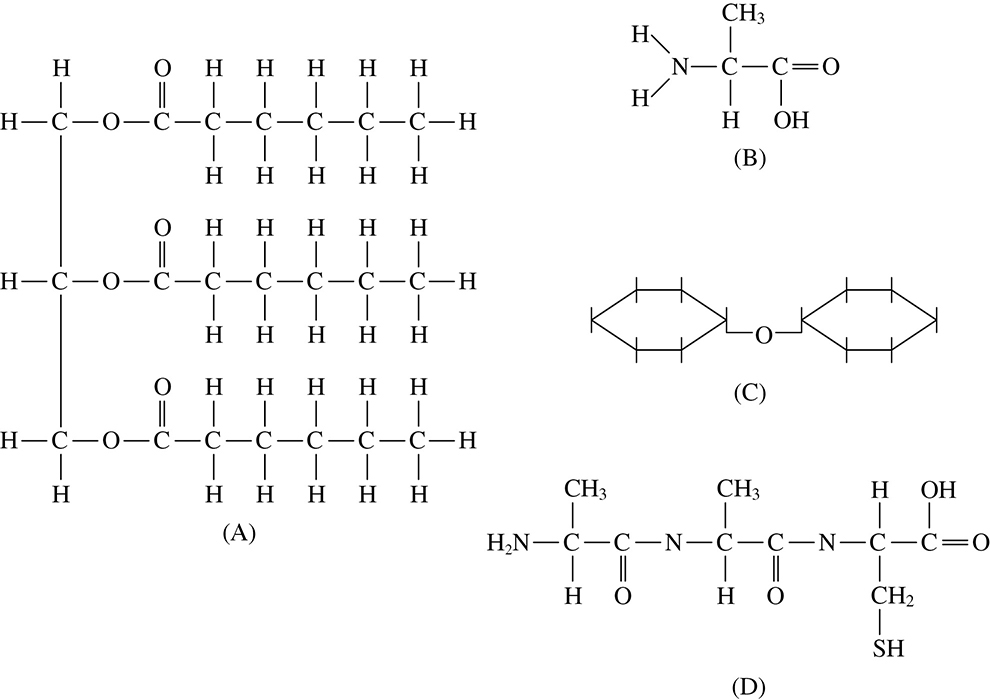
1. Which of the structures shown above is a polypeptide?
2. Which of these structures is a disaccharide?
3. Which of these structures is a fat?
4. Which of these structures is an amino acid?
5. Which of the following has both a hydrophobic portion and a hydrophilic portion?
A. Starch
B. Phospholipids
C. Proteins
D. Steroids
6. A solution that has a pH of 2 is how many times more acidic than one with a pH of 5?
A. 5
B. 10
C. 100
D. 1,000
7. The structure below contains which functional group?
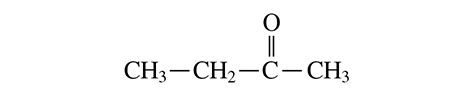
A. Aldehyde
B. Ketone
C. Amino
D. Hydroxyl
8. Which of the following will least affect the effectiveness of an enzyme?
A. Temperature
B. pH
C. Concentration of enzyme
D. Original activation energy of system
9. Which of the following is similar to the process of competitive inhibition?
A. When you arrive at work in the morning, you are unable to park your car in your (assigned) parking spot because the car of the person who parks next to you has taken up just enough space that you cannot fit your own car in.
B. When you arrive at work in the morning, you are unable to park your car in your parking spot because someone with a car exactly like yours has already taken your spot, leaving you nowhere to park your car.
C. As you are about to park your car in your spot at work, a giant bulldozer comes along and smashes your car away from the spot, pre-venting you from parking your car in your spot.
D. When you arrive at work in the morning, you are unable to park your car in your park-ing spot because someone has placed a giant cement block in front of your spot.
10. All the following are carbohydrates except
A. starch.
B. glycogen.
C. chitin.
D. glycerol.
11. An amino acid contains which of the following functional groups?
A. Carboxyl group and amino group
B. Carbonyl group and amino group
C. Hydroxyl group and amino group
D. Carboxyl group and hydroxyl group
![]() Answers and Explanations
Answers and Explanations
1. D
2. C
3. A
4. B
5. B—A phospholipid has both a hydrophobic portion and a hydrophilic portion. The hydrocarbon portion, or tail, of the phospholipid dislikes water, and the phosphate portion, the head, is hydrophilic.
6. D—Because the pH scale is logarithmic, 2 is 1,000 times more acidic than 5.
7. B—This functional group is a carbonyl group. The two main types of carbonyl groups are ketones and aldehydes. In this case, it is a ketone because there are carbon chains on either side of the carbon double-bonded to the oxygen.
8. D—The four main factors that affect enzyme efficiency are pH, temperature, enzyme concentration, and substrate concentration.
9. B—Competitive inhibition is the inhibition of an enzyme—substrate reaction in which the inhibitor resembles the substrate and physically blocks the substrate from attaching to the active site. This parking spot represents the active site, your car is the substrate, and the other car already in the spot is the competitive inhibitor. Examples A and D more closely resemble noncompetitive inhibition.
10. D—Glycerol is not a carbohydrate. It is an alcohol. Starch is a carbohydrate stored in plant cells. Glycogen is a carbohydrate stored in animal cells. Chitin is a carbohydrate used by arthropods to construct their exoskeletons. Cellulose is a carbohydrate used by plants to construct their cell walls.
11. A
![]() Rapid Review
Rapid Review
Try to rapidly review the following material:
Organic compounds: contain carbon; examples include lipids, proteins, and carbs (carbohydrates).
Functional groups: amino (NH2), carbonyl (RCOR), carboxyl (COOH), hydroxyl (OH), phosphate (PO4), sulfhydryl (SH).
Fat: glycerol + 3 fatty acids.
Saturated fat: bad for you; animals and some plants have it; solidifies at room temperature.
Unsaturated fat: better for you, plants have it; liquifies at room temperature.
Steroids: lipids whose structures resemble chicken-wire fence; include cholesterol and sex hormones.
Phospholipids: glycerol + 2 fatty acids + 1 phosphate group; make up membrane bilayers of cells; have hydrophobic interiors and hydrophilic exteriors.
Carbohydrates: used by cells for energy and structure; monosaccharides (glucose), disaccharides (sucrose, maltose, lactose), storage polysaccharides (starch [plants], glycogen [animals]), structural polysaccharides (chitin [fungi], cellulose [arthropods]).
Proteins: made with the help of ribosomes out of amino acids; serve many functions (e.g., transport, enzymes, cell signals, receptor molecules, structural components, and channels).
Enzymes: catalytic proteins that react in an induced-fit fashion with substrates to speed up the rate of reactions by lowering the activation energy; effectiveness is affected by changes in pH, temperature, and substrate and enzyme concentrations.
Competitive inhibition: inhibitor resembles substrate and binds to active site.
Noncompetitive inhibition: inhibitor binds elsewhere on enzyme; alters active site so that substrate cannot bind.
pH: logarithmic scale <7 acidic, 7 neutral, >7 basic (alkaline); pH 4 is 10 times more acidic than pH 5.
Reaction types:
Hydrolysis reaction: breaks down compounds by adding water.
Dehydration reaction: two components brought together, producing H2O.
Endergonic reaction: reaction that requires input of energy.
Exergonic reaction: reaction that gives off energy.
Redox reaction: electron transfer reactions.