Cracking the AP Biology Exam
2
The Chemistry of Life
SUBATOMIC PARTICLES
If you break down an element into smaller pieces, you’ll eventually come to the atom—the smallest unit of an element that retains its characteristic properties. Atoms are the building blocks of the physical world.
Within atoms, there are even smaller subatomic particles called protons, neutrons, and electrons. Let’s take a look at a typical atom:
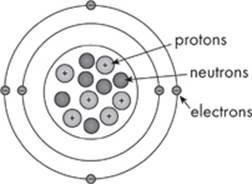
Protons and neutrons are particles that are packed together in the core of an atom called the nucleus. You’ll notice that protons are positively charged (+) particles, whereas neutrons are uncharged particles.
Electrons, on the other hand, are negatively charged (–) particles that spin around the nucleus. Electrons are pretty small compared to protons and neutrons. In fact, for our purposes, electrons are considered massless. Most atoms have the same number of protons and electrons, making them electrically neutral. Some atoms have the same number of protons but differ in the number of neutrons in the nucleus. These atoms are called isotopes.