Biology Premium, 2024: 5 Practice Tests + Comprehensive Review + Online Practice - Wuerth M. 2023
UNIT 2 Cell Structure and Function
6 Movement of Water in Cells
Learning Objectives
In this chapter, you will learn:
➜Water Potential
➜Osmolarity and Its Regulation
Overview
Maintaining water balance in cells is a critical component of homeostasis, and life depends upon it. This chapter provides an overview of water potential, discusses how to calculate water potential, and explains how differences in water potential affect the movement of water into and out of cells.
Water Potential
In an introductory biology course, you may have learned the terms hypotonic, hypertonic, and isotonic. These three terms focus on the relative concentrations of solute in a solution. A hypotonic solution has a lower concentration of solute. A hypertonic solution has a higher concentration of solute. An isotonic solution has the same concentration of solute as that of another solution.
Since hypotonic, hypertonic, and isotonic are relative terms, how a solution is characterized with these terms is dependent on what you are comparing the solution to. For example, a cup of coffee may be considered hypertonic if it is being compared to a cup of water. However, the same cup of coffee would be considered hypotonic if compared to a cup of coffee to which two sugar cubes have been added.
TIP
Learning prefixes can help you remember terms and decode the definitions of unfamiliar terms. The prefix hyper- means “more,” the prefix hypo- means “less,” and the prefix iso- means “same.”
An advantage of using water potential to describe solutions is that water potential is not a relative term; rather, water potential can be calculated using a few simple equations. The calculated water potential values of solutions make comparing these solutions more exact. This helps in predicting how water will move between solutions.
Water potential can be defined as the potential energy of water in a solution, or the ability of water to do work. The more water there is in a solution, the higher its water potential will be. The less water there is in a solution, the lower its water potential will be. Water flows from areas of higher water potential to areas of lower water potential. This is similar to how a ball at the top of a hill, which has higher potential energy, will roll down a hill to an area of lower potential energy. (See Figure 6.1.)
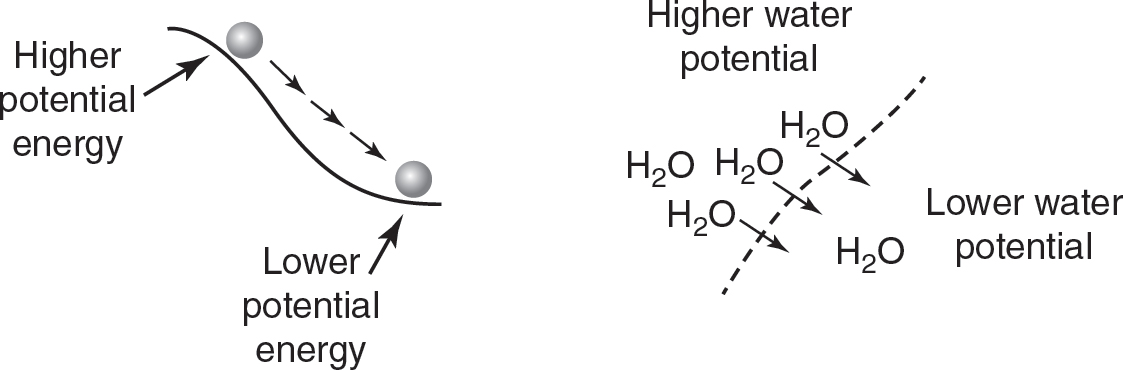
Figure 6.1 Water Potential
TIP
Remember, hypertonic solutions have more solute and less water, so their water potential is lower. Hypotonic solutions have less solute and more water, so their water potential is higher.
Water potential focuses on the concentration of water in a solution. If there is more solute in a solution, the water is less concentrated in that solution. A hypertonic solution that has more solute will have less concentrated water and a lower water potential. A hypotonic solution has less solute and therefore more concentrated water and a higher water potential. Since water flows from areas of higher water potential to areas of lower water potential, water will flow from hypotonic solutions (which have a higher water potential) to hypertonic solutions (which have a lower water potential).
Calculating Water Potential
The water potential of a solution has two components: a component that depends on the amount of solute and a component that depends on pressure, as shown in the following equation:
![]()
ψs is the water potential due to the solute concentration (solute potential). ψp is the water potential due to the pressure on the system (pressure potential).
TIP
You do not need to memorize the formula for water potential; it will be on the AP Biology Equations and Formulas sheet.
When you drink a liquid through a straw, you are using negative pressure potential. As you suck air from the straw, you create a negative pressure in the straw. The liquid in your drink then moves from an area of higher pressure potential (in the cup) into an area of lower pressure potential (in the straw). An example of positive pressure potential would be Super Soaker water toys. In those toys, you create positive pressure inside the water toy by pumping air into the toy. When you pull the trigger on the water toy, water flows out of the higher pressure potential environment in the toy and into the lower pressure potential environment outside of the toy.
Most biological systems are open to the atmosphere and are in pressure equilibrium with their environment. So in most cases, the ψp is 0 and the water potential will depend solely on the solute potential. In this case, the water potential becomes simply:
![]()
The solute potential depends on the concentration of the solute, how many particles the solute forms when in solution, and the temperature of the solution. The solute potential is calculated with this formula:
![]()
The ionization constant is i, which is a function of how many particles or ions a solute will form in solution. For covalent compounds, which do not separate into ions in solution (for example, glucose and sucrose), the ionization constant is 1 since one molecule of glucose will form one particle in solution. For ionic compounds, which do separate into ions in solution (for example, sodium chloride NaCl or calcium chloride CaCl2), the ionization constant depends upon how many ions the compound will form in solution. NaCl forms two ions when in solution, Na+ and Cl—, so the ionization constant for NaCl is 2. CaCl2 forms three ions when in solution, Ca2+ and two Cl— ions, so the ionization constant for CaCl2 is 3.
C refers to the concentration of solute in the solution. Since there is a negative sign in the solute potential formula, as the concentration of solute increases, the solute potential decreases. Solutions with more solute (higher solute concentrations) will have lower water potentials if all other conditions are equal.
R is the pressure constant, ![]() . You do not need to memorize this constant; it will be on the AP Biology Equations and Formulas sheet that you will have access to during the exam.
. You do not need to memorize this constant; it will be on the AP Biology Equations and Formulas sheet that you will have access to during the exam.
T is the temperature of the solution. The temperature is in Kelvin, NOT degrees Celsius. There are two reasons why temperature must be stated in Kelvin:
§ The pressure constant, R, uses temperature units of Kelvin, so consistent units for temperature are needed throughout the formula.
§ The Kelvin temperature scale is an absolute scale, meaning there are no negative numbers in the Kelvin scale. Using negative temperatures in this formula would result in incorrect water potential calculations.
Converting temperatures from Celsius to Kelvin is simple: Kelvin = °C + 273.
The reference solution for solute potential is distilled water. Because distilled water has no solutes, C = 0, and the solute potential for distilled water is 0 bars at any temperature. As soon as any amount of solute is added to distilled water, the solution’s solute potential will become negative. The more solute that is added, the more negative the solute potential becomes.
Try the following sample calculation for water potential.
A solution of 0.50 molar glucose is at a temperature of 21°C and open to the atmosphere. What is its water potential?
Because the solution is open to the atmosphere, its pressure potential is zero. So its total water potential is its solute potential.
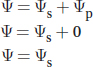
Glucose is a covalent compound, so i = 1. Convert 21°C to Kelvin:
![]()
Using the formula for solute potential:
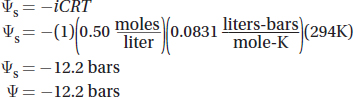
Here is an example in which the pressure potential is not 0 bars:
A plant cell has a pressure potential of 1.0 bars and a solute potential of —6.50 bars. What is its water potential?
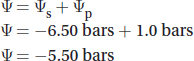
Osmolarity and Its Regulation
Osmolarity is the total concentration of solutes in a solution. Living organisms need to closely regulate their internal solute concentration and their water potential. If an organism became too dehydrated, it might die. If too much water moved into a cell, the pressure potential from the water moving into the cell could cause the cell to burst.
A paramecium living in freshwater has a higher internal solute concentration and a lower water potential than its environment. Because water flows from areas of higher water potential to areas of lower water potential, water will be constantly flowing from the higher water potential in the freshwater environment into the paramecium. If this were unregulated, excess water would build up inside the paramecium and eventually the cell would burst. To counter this, paramecia have a specialized organelle, a contractile vacuole, in which this excess water entering the cell is stored and then pumped out of the cell. This allows the paramecium to maintain its internal solute concentration.
The cells in a saltwater fish have a lower solute concentration and a higher water potential than their marine environment. In this scenario, water would be constantly flowing out of the fish cells (which have a higher water potential) into the saltwater surrounding the fish (which has a lower water potential). To counteract this, the saltwater fish is constantly drinking large quantities of saltwater. The fish retains the water, and specialized salt-secreting organs in the fish excrete the excess solute. This allows the saltwater fish to regulate its internal solute concentration and water potential.
Practice Questions
Multiple-Choice
1.The solute potential of distilled water is
(A)negative.
(B)zero.
(C)positive.
(D)dependent on the temperature.
2.A solution has a solute concentration of 0.25 moles per liter and is at a temperature of 37°C. The ionization constant of the solute is 1. What is the solute potential of this solution?
(A)—0.64 bars
(B)—0.77 bars
(C)—6.44 bars
(D)—7.70 bars
3.A cell has a solute potential of —5.42 bars and a pressure potential of 0.48 bars. What is its total water potential?
(A)—5.42 bars
(B)—4.94 bars
(C)0.48 bars
(D)4.94 bars
4.A blood cell with a water potential of —7.7 bars is placed in distilled water. Which of the following correctly describes what will occur?
(A)Water will flow out of the blood cell because the blood cell has a higher water potential than distilled water.
(B)Water will flow into the blood cell because the blood cell has a higher water potential than distilled water.
(C)Water will flow out of the blood cell because the blood cell has a lower water potential than distilled water.
(D)Water will flow into the blood cell because the blood cell has a lower water potential than distilled water.
5.A plant cell with a solute potential of —4.0 bars and a pressure potential of 0.5 bars is placed into a solution with a water potential of —5.0 bars. What will happen to the plant cell in this solution?
(A)Water will flow into the plant cell because the plant cell has a total water potential that is lower than that of the surrounding solution.
(B)Water will flow into the plant cell because the plant cell has a total water potential that is higher than that of the surrounding solution.
(C)Water will flow out of the plant cell because the plant cell has a total water potential that is lower than that of the surrounding solution.
(D)Water will flow out of the plant cell because the plant cell has a total water potential that is higher than that of the surrounding solution.
6.Four solutions of covalent compounds are in beakers that are open to the atmosphere. Which of the following solutions has the highest water potential?
(A)0.5 molar glucose at a temperature of 21°C
(B)0.75 molar fructose at a temperature of 21°C
(C)1.0 molar sucrose at a temperature of 21°C
(D)1.25 molar lactose at a temperature of 21°C
7.A freshwater fish is placed in a saltwater aquarium. Predict the most likely effect this will have on the fish, and justify your prediction.
(A)The fish will gain water because it likely has a lower water potential than its new surroundings.
(B)The fish will lose water because it likely has a lower water potential than its new surroundings.
(C)The fish will gain water because it likely has a higher water potential than its new surroundings.
(D)The fish will lose water because it likely has a higher water potential than its new surroundings.
8.Potato slices that are immersed in distilled water for 24 hours become stiff and hard. Potato slices that are immersed in 0.5 molar sucrose solution for 24 hours become limp and soft. Which of the following is the most logical conclusion based on this information?
(A)The potato slices are hypotonic to both distilled water and the 0.5 molar sucrose solution.
(B)The potato slices are hypertonic to both distilled water and the 0.5 molar sucrose solution.
(C)The potato slices are hypotonic to distilled water and hypertonic to the 0.5 molar sucrose solution.
(D)The potato slices are hypertonic to distilled water and hypotonic to the 0.5 molar sucrose solution.
9.Human cells have an approximate NaCl concentration of 0.15 moles per liter. Seawater has an approximate NaCl concentration of 0.45 moles per liter. Which of the following is the most likely effect if a person drank seawater?
(A)The lower water potential in the human cells would cause water to flow out of the human cells into the seawater that was consumed.
(B)The lower water potential in the human cells would cause NaCl to flow out of the human cells into the seawater that was consumed.
(C)The higher water potential in the human cells would cause water to flow out of the human cells into the seawater that was consumed.
(D)The higher water potential in the human cells would cause NaCl to flow out of the human cells into the seawater that was consumed.
10.A dandelion plant is growing in an environment with a temperature of 21°C. The water potential in the root cells of a dandelion plant is —1.2 bars. If a 0.1 molar sodium chloride solution at 21°C was poured on the dandelion roots, what would be the most likely result?
(A)Sodium chloride would move into the dandelion cells, raising the water potential of the dandelion cells.
(B)Water would flow out of the dandelion cells because the water potential in the dandelion cells would be higher than the water potential of the 0.1 molar sodium chloride solution.
(C)There would be no effect on the dandelion cells because they would be open to the atmosphere and have a pressure potential of zero.
(D)Water would flow into the dandelion cells because the water potential in the dandelion cells would be lower than the water potential of the 0.1 molar sodium chloride solution.
Short Free-Response
11.The paramecium Paramecium aurelia has a contractile vacuole, which it uses to pump excess water out of its cell. P. aurelia was placed in four different salt solutions with concentrations of 0.02 molar, 0.04 molar, 0.08 molar, and 0.10 molar salt. The number of contractions of the contractile vacuole per minute was measured over a 10-minute period.
(a)Describe how the water potential of the surrounding solutions would affect the rate of contraction of the contractile vacuole.
(b)Identify the independent variable and dependent variable in this experiment.
(c)As a follow-up experiment, P. aurelia is placed in a beaker that contains distilled water. Predict what effect, if any, this would have on the rate of contraction of the contractile vacuole.
(d)Justify your prediction from part (c) using your knowledge of water potential.
12.If a person is in the hospital with severe dehydration, often an intravenous infusion of physiological saline (0.9% saline) will be administered.
(a)Describe how the water potential of a person’s cells would be affected by severe dehydration.
(b)Explain why 0.9% saline is used to rehydrate a person with severe dehydration and why distilled water is not used.
(c)During strenuous exercise under extreme heat, some athletes are advised to consume salt tablets. Predict whether consuming a salt tablet would lead to water loss or water conservation in the athlete’s body cells.
(d)Justify your prediction from part (c) using your knowledge of water potential.
Long Free-Response
13.A student conducts an experiment with four different root vegetables (carrots, beets, parsnips, and potatoes). Five cubes of equal sizes and surface areas are cut from each of the vegetables, and their masses are recorded. Each cube is placed in a beaker that contains a 0.35 molar sucrose solution for 24 hours. After 24 hours, the cubes were removed from the solutions and weighed, and the percent change in mass for each cube was calculated. The mean percent change in mass for each vegetable and the standard errors of the mean are shown in the table.
|
Vegetable |
Mean Percent Change in Mass |
Standard Error of the Mean |
Carrots |
+7.5% |
1.0% |
Beets |
+21.5% |
2.5% |
Parsnips |
—15.5% |
1.5% |
Potatoes |
—3.5% |
0.5% |
(a)Explain why some of the vegetables would have a positive percent change in mass while others would have a negative percent change in mass during the course of the experiment.
(b)Construct an appropriately labeled graph that shows the mean percent change in mass for each vegetable. Include 95% confidence intervals.
(c)Analyze the data to determine which vegetable has a water potential closest to the water potential of the 0.35 molar sucrose solution used in the experiment. Justify your answer with evidence from the experiment.
(d)It is determined that the sugar content in each vegetable is the major determinant of the vegetable’s water potential. Turnips have a higher sugar content than carrots but a lower sugar content than beets. If the experiment was repeated and included turnip cubes, predict the percent change in mass for the turnip cubes. Justify your prediction with evidence from the experiment.
Answer Explanations
Multiple-Choice
1.(B)The solute potential of distilled water is defined as zero bars; it is the reference point to which other solutions are compared. Since the solute potential is zero, it is neither negative nor positive, so choices (A) and (C) are incorrect. The solute potential of distilled water is 0 bars, regardless of its temperature, so choice (D) is incorrect.
2.(C)ψs = —iCRT. In this example, i = 1, C = 0.25 moles per liter, ![]() , and
, and ![]() Choices (B) and (D) are incorrect because the Celsius temperature was used in the calculation instead of the Kelvin temperature. Avoid this common mistake by remembering to always convert the temperature to Kelvin in water potential calculations. Choice (D) also incorrectly used a value of 2.5 moles per liter instead of the correct value of 0.25 moles per liter in the calculation. Avoid this type of mistake by carefully inputting numbers into your calculator when working on calculations. In choice (A), an incorrect value of 0.025 moles per liter was used instead of 0.25 moles per liter. Again, be careful when inputting numbers into your calculator. If time allows, you may want to repeat calculations to confirm you inputted numbers correctly.
Choices (B) and (D) are incorrect because the Celsius temperature was used in the calculation instead of the Kelvin temperature. Avoid this common mistake by remembering to always convert the temperature to Kelvin in water potential calculations. Choice (D) also incorrectly used a value of 2.5 moles per liter instead of the correct value of 0.25 moles per liter in the calculation. Avoid this type of mistake by carefully inputting numbers into your calculator when working on calculations. In choice (A), an incorrect value of 0.025 moles per liter was used instead of 0.25 moles per liter. Again, be careful when inputting numbers into your calculator. If time allows, you may want to repeat calculations to confirm you inputted numbers correctly.
3.(B)Total water potential is ψ = ψs + ψp. Using the value —5.42 bars for ψs and 0.48 bars for ψp, then ψ = —5.42 bars + 0.48 bars = —4.94 bars. Choice (A) is incorrect because it is the value of ψs alone and doesn’t take into account the effect of ψp on the total water potential. Choice (C) is incorrect because it is the value of ψp alone and doesn’t take into account the effect of ψs on the total water potential. Choice (D) is incorrect because the total water potential is negative in this case, not positive.
4.(D)Water flows from areas of higher water potential to areas of lower water potential. Distilled water has a water potential of 0 bars. If the blood cell has a water potential of —7.7 bars, water will flow from the distilled water, which has a higher water potential, into the blood cell, which has a lower water potential. Choices (A) and (C) are incorrect because water will not flow out of the blood cell. Choice (B) is incorrect because even though that choice correctly states that water will flow into the blood cell, it incorrectly states that the blood cell has a higher water potential than distilled water.
5.(D)The total water potential of the plant cell is the sum of the solute potential and the pressure potential, in this case ψ = —4.0 bars + 0.5 bars = —3.5 bars, which is higher than the water potential of the surrounding solution (—5.0 bars). Water flows from higher water potential to lower water potential, so water will flow out of the plant cell into the surrounding solution. Choices (A) and (B) are incorrect because water will not flow into the plant cell. Choice (C) correctly states that water will flow out of the plant cell but incorrectly states that the plant cell has a lower water potential than that of the surrounding solution.
6.(A)The solutions are in beakers that are open to the atmosphere, so ψp is 0 and the total water potential will be equal to the solute potential. The formula for solute potential is
![]()
All four solutions are at the same temperature, and all four solutes are covalent compounds and have ionization constants that equal 1. R is constant for all four solutions. So the water potential will be inversely proportional to the concentration of the solute (if there is less concentrated solute, the water potential will be higher). The least concentrated solute in this question is 0.5 molar, so choice (A) would have the highest water potential. Note that if you read this question carefully and understand how each variable affects water potential, you don’t have to actually calculate the water potential for each solution.
7.(D)A fish that is adapted to a freshwater environment likely has a water potential that is more similar to freshwater than to saltwater. The freshwater fish would likely have a higher water potential than the saltwater. Water flows from areas of higher water potential to areas of lower water potential, so water would flow out of the fish into its new surroundings. Choices (A) and (C) are incorrect because the fish would likely lose water, not gain water. Choice (B) is incorrect because it is not likely that the freshwater fish would have a lower water potential than its new saltwater environment.
8.(D)Potato slices that are stiff and hard gained water, and potato slices that are limp and soft lost water. Since potato slices became stiff and hard in distilled water, distilled water has a higher water potential than the potato, and the potato is hypertonic to distilled water. Since the potato slices became limp and soft in 0.5 molar sucrose solution, the potatoes have a higher water potential than the 0.5 molar sucrose solution and are hypotonic to the sucrose solution. Choice (A) is incorrect because the potato slices are hypertonic to distilled water. The potato slices are hypotonic to the 0.5 molar sucrose solution, so choice (B) is incorrect. Choice (C) is incorrect because the potato slices are hypertonic to distilled water and hypotonic to the 0.5 molar sucrose solution.
9.(C)Human cells with an NaCl concentration of 0.15 moles per liter would have a higher water potential than seawater that has an NaCl concentration of 0.45 moles per liter. So water would flow out of the human cells into the seawater with the lower water potential. Choices (A) and (B) are incorrect because the human cells would have a higher water potential than the seawater. Choice (D) incorrectly states that NaCl would flow out of the human cells; it is in fact water that would flow out of the human cells.
10.(B)The water potential of a 0.1 molar NaCl solution at 21°C is —4.88 bars, which is calculated as follows:
![]()
Since the dandelion root cells have a water potential of —1.2 bars, water would flow from the higher water potential in the dandelion root cells into the lower water potential of the NaCl solution. Choice (A) is incorrect because if NaCl moved into the dandelion cells, it would lower, not raise, the water potential of those cells. The dandelion cells are open to the atmosphere and have a pressure potential of 0, but choice (C) fails to take into account the effect of the lower solute potential of the surrounding sodium chloride solution on the dandelion cells. Therefore, choice (C) is incorrect. Choice (D) incorrectly states that the water potential in the dandelion cells would be lower than that of the NaCl solution.
Short Free-Response
11.(a)If the water potential of the surrounding solution was higher than that of the paramecium, more water would enter the paramecium’s cell and the contractile vacuole would have to pump more often to remove the excess water from the cell.
(b)The independent variable is the concentration of the salt solution. The dependent variable is the number of contractions of the contractile vacuole per minute.
(c)The contractile vacuole would have to pump more times per minute to remove the excess water.
(d)Distilled water would have a higher water potential than P. aurelia. So more water would move into the paramecium and the contractile vacuole would have to work harder to remove the excess water.
12.(a)When people are severely dehydrated, their cells have less water and the water potential of their cells is lower.
(b)If distilled water were used to rehydrate a person, too much water would enter the person’s cells and there would be a risk of the blood cells bursting from the pressure caused by the excess water. A saline solution of 0.9%, which is the same concentration of saline that is normally seen in body cells, can rehydrate a severely dehydrated person without running the risk of bursting the blood cells.
(c)Consuming a salt tablet would lead to water conservation.
(d)Consuming salt lowers the water potential of the person’s body cells, so less water would leave the cells as sweat or urine.
Long Free-Response
13.(a)Vegetables with a lower water potential than the surrounding 0.35 molar sucrose solution would gain water and mass. Vegetables with a higher water potential than the surrounding 0.35 molar sucrose solution would lose water and mass.
(b)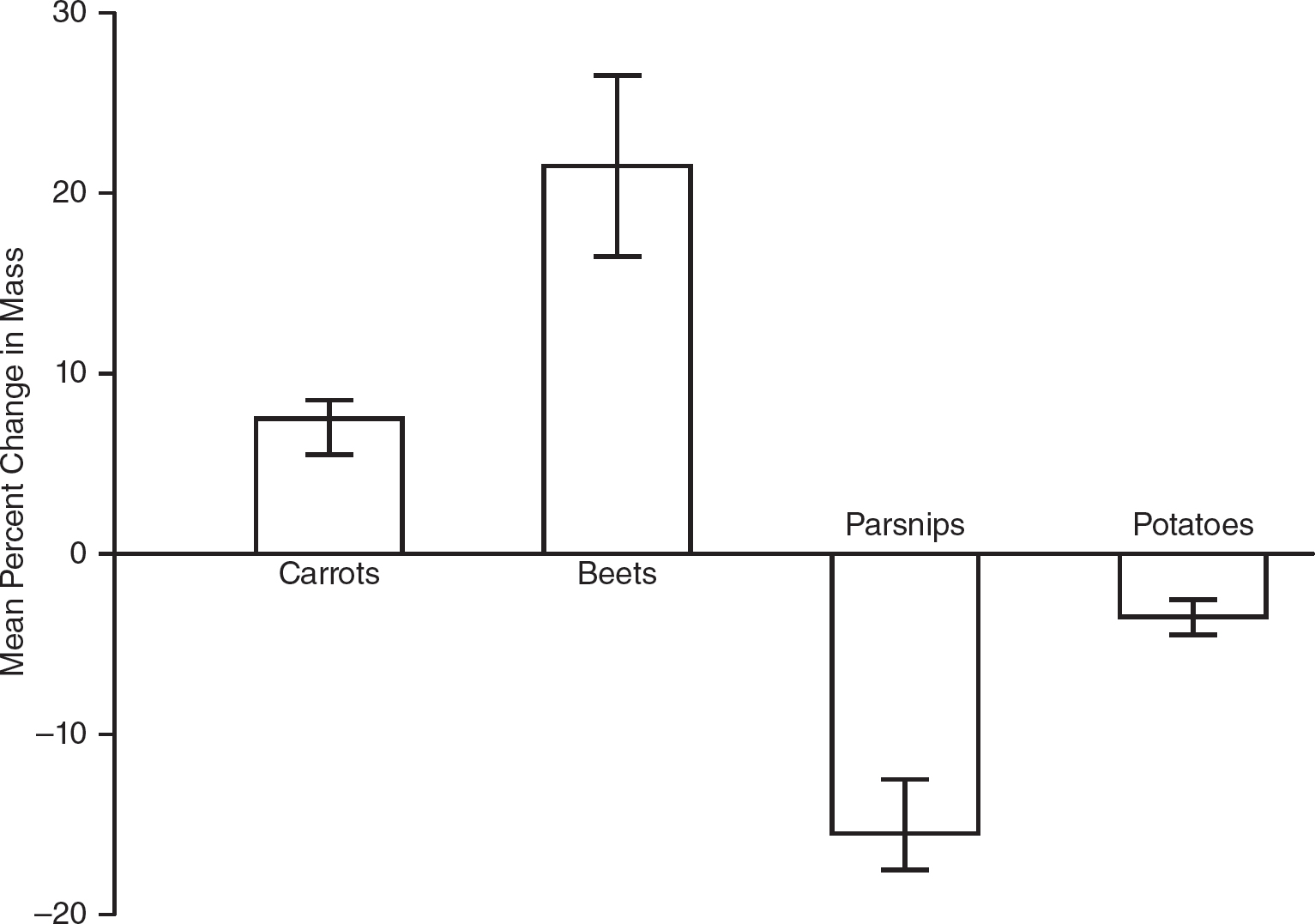
(c)The vegetable with a water potential that is closest to that of the 0.35 molar sucrose solution would have the smallest percent change in mass. If a vegetable had the same water potential as the 0.35 molar sucrose solution, its cells would be isotonic to the sucrose solution, and you would expect to find a 0% change in mass. The smaller the percent change in mass, the closer the vegetable’s water potential is to that of the 0.35 molar sucrose solution. The potato has a water potential that is closest to that of the 0.35 molar sucrose solution because its percent change in mass (—3.5%) is the smallest and closest to zero.
(d)Turnips would have a percent change in mass that is greater than +7.5% but less than +21.5%. Since turnips have a higher sugar content than carrots, the water potential of the turnip cells would be less than that of the carrot cells. So the turnips would gain more water from the surrounding 0.35 molar sucrose solution than the carrots did (more than 7.5%). Since the turnips have a lower sugar content than the beets, the water potential of the turnip cells would be higher than the water potential of the beet cells. So the turnips would gain less water from the surrounding 0.35 molar sucrose solution than the beets did (less than 21.5%).