CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
2. The Basics of Life
2.7. Water: The Essence of Life
Water seems to be a simple molecule, but it has several special properties that make it particularly important for living things. A water molecule is composed of two atoms of hydrogen and one atom of oxygen joined by covalent bonds. However, the electrons in these covalent bonds are not shared equally. Oxygen, with 8 protons, has a greater attraction for the shared electrons than does hydrogen, with its single proton. Therefore, the shared electrons spend more time around the oxygen part of the molecule than they do around the hydrogen. As a result, the oxygen end of the molecule is more negative than the hydrogen end.
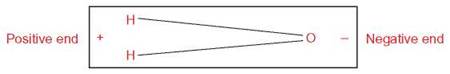
When the electrons in a covalent bond are not equally shared, the molecule is said to be polar and the covalent bonds are called polar covalent bonds.
FIGURE 2.11. Ethylene and the Ripening Process
The ancient Chinese knew from observation that fruit would ripen faster if placed in a container of burning incense, but they did not realize the incense released ethylene. We now know that ethylene stimulates the ripening process; it is used commercially to ripen fruits that are picked green.
When the negative end of a polar molecule is attracted to the positive end of another polar molecule, the hydrogen is located between the two molecules. Because in polar molecules the positive hydrogen end of one molecule is attracted to the negative end of another molecule, these attractive forces are often called hydrogen bonds. Hydrogen bonds can be intermolecular (between molecules) or intramolecular (within molecules) forces of attraction. They occur only between hydrogen and oxygen or hydrogen and nitrogen. As intramolecular forces, hydrogen bonds hold molecules together. Because they do not bond atoms together, they are not considered true chemical bonds. This attraction is usually represented as three dots between the attracted regions. This weak force of attraction is not responsible for forming molecules, but it is important in determining the threedimensional shape of a molecule. For example, when a very large molecule, such as a protein, has some regions that are slightly positive and others that are slightly negative, these areas attract each other and result in the coiling or folding of these threadlike molecules (figure 2.12). Because water is a polar covalent compound (it has slightly + and - ends), it has several significant physical and biological properties (Outlooks 2.1).
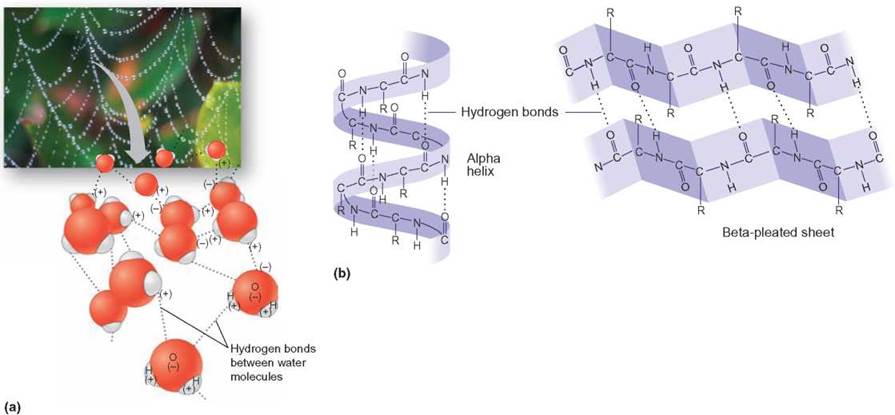
FIGURE 2.12. Hydrogen Bonds
(a) Water molecules arrange themselves so that their positive portions are near the negative portions of other water molecules. When enough gather together, water droplets form. It is this kind of intermolecular bonding that accounts for water’s unique chemical and physical properties. Without such bonds, life as we know it on Earth would be impossible. (b) The large protein molecules here also have polar areas. When the molecules are folded so that the partially positive areas are near the partially negative areas, a slight attraction forms and tends to keep them folded or twisted.
Mixtures and Solutions
A mixture is matter that contains two or more substances that are not in set proportions (figure 2.13). A solution is a liquid mixture of ions or molecules of two or more substances. For example, salt water can be composed of varying amounts of NaCl and H2O. If the components of the mixture are distributed equally throughout, the mixture is homogeneous. The process of making a solution is called dissolving. The amounts of the component parts of a solution are identified by the terms solvent and solute. The solvent is the component present in the larger amount. The solute is the component that dissolves in the solvent. Many combinations of solutes and solvents are possible. If one of the components of a solution is a liquid, it is usually identified as the solvent. An aqueous solution is a solution of a solid, liquid, or gas in water. When sugar dissolves in water, sugar molecules separate from one another. The molecules become uniformly dispersed throughout the molecules of water. In an aqueous salt solution, however, the salt dissociates into sodium and chlorine ions.
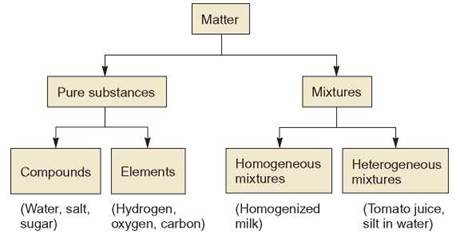
FIGURE 2.13. How Do Mixtures Compare?
Matter can be a pure substance or a mixture. The term homogeneous means “the same throughout" Homogenized milk has the same composition throughout the container. Before milk was homogenized (i.e., vigorously shaken to break fat into small globules), it was a heterogeneous mixture and it would “separate" The cream (which floats to the top) could be skimmed off the milk leaving skimmed milk. A heterogeneous mixture does not have the same composition throughout.
The relative amounts of solute and solvent are described by the concentration of a solution. In general, a solution with a large amount of solute is “concentrated,” and a solution with much less solute is “dilute,” although these are somewhat arbitrary terms.
2.7. CONCEPT REVIEW
17. What is the difference between a polar molecule and a nonpolar molecule?
18. What is different about a hydrogen bond in comparison with covalent and ionic bonds?
19. What is the difference between a solute and a solvent?
20. What relationship does kinetic energy have to homogeneous solutions?