CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
4. Cell Structure and Function
4.7. Exchange Through Membranes
If a cell is to stay alive, it must be able to exchange materials with its surroundings. Because all cells are surrounded by a plasma membrane, the nature of the membrane influences what materials can pass through it. There are six ways in which materials enter and leave cells: diffusion, osmosis, facilitated diffusion, active transport, endocytosis, and exocytosis. The same mechanisms are involved in the movement of materials across the membranes of the various cellular organelles such as golgi, mitochondria, and chloroplasts.
Diffusion
A basic principle of physics states that all molecules are in a constant state of motion. Although in solids molecules tend to vibrate in place, in liquids and gases they are able to move past one another. Because the motion of molecules is random, there is a natural tendency in gases and liquids for molecules of different types to mix completely with each other.
Consider a bottle of perfume or cologne. When you open the bottle, the perfume molecules and air molecules begin to mix and you smell the perfume. Perfume molecules leave the bottle and enter the bottle. Molecules from the air enter and leave the bottle. However, more perfume molecules leave the bottle than enter it. This overall movement is termed net movement, the movement in one direction minus the movement in the opposite direction. The direction in which the greatest number of molecules of a particular kind moves (net movement) is determined by the difference in concentration of the molecules in different places. Diffusion is the net movement of a kind of molecule from a place where that molecule is in higher concentration to a place where that molecule is less concentrated. The difference in concentration of the molecules over a distance is known as a concentration gradient or diffusion gradient (figure 4.21). When no concentration gradient exists, the movement of molecules is equal in all directions, and the system has reached a state of dynamic equilibrium. There is an equilibrium because there is no longer a net movement (diffusion), because the movement in one direction equals the movement in the other. It is dynamic, however, because the system still has energy, and the molecules are still moving.
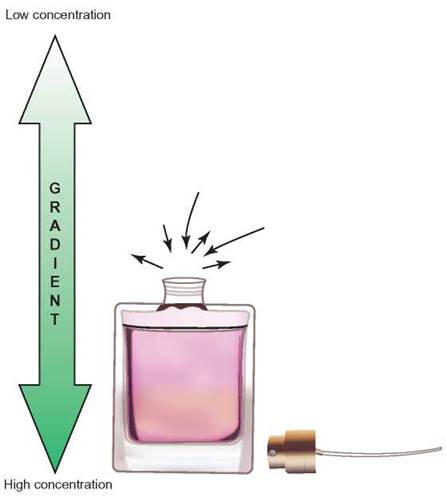
FIGURE 4.21. Concentration Gradient
The difference in concentrations of molecules over a distance is called a concentration gradient. When the top of this perfume bottle is removed, a concentration gradient is formed. The highest concentration is inside, decreasing as you measure farther away from the bottle.
The rate at which diffusion takes place is determined by several factors. Diffusion occurs faster if the molecules are small, if they are moving rapidly, and if there is a large concentration gradient.
Diffusion in Cells
Diffusion is an important means by which materials are exchanged between a cell and its environment. For example, cells constantly use oxygen in various chemical reactions. Consequently, the oxygen concentration in cells always remains low. The cells, then, contain a lower concentration of oxygen than does the environment outside the cells. This creates a concentration gradient, and the oxygen molecules always diffuse from the outside of the cell to the inside.
Diffusion can take place as long as there are no barriers to the free movement of molecules. In the case of a cell, the plasma membrane surrounds the cell and serves as a partial barrier to the movement of molecules through it. Because the plasma membrane allows only certain molecules to pass through it, it is selectively permeable. A molecule’s ability to pass through the membrane depends on its size, electrical charge, and solubility in the phospholipid membrane. In general, the membrane allows small molecules, such as oxygen or water, to pass through but prevents the passage of larger molecules. The membrane also regulates the passage of ions. If a particular portion of the membrane has a large number of positive ions on its surface, positively charged ions in the environment will be repelled and prevented from crossing. Molecules that are able to dissolve in phospholipids, such as vitamins A and D, can pass through the membrane rather easily; however, many molecules cannot pass through at all.
The cell has no control over the rate or direction of diffusion. The direction of diffusion is determined by the relative concentration of specific molecules on the two sides of the membrane. Diffusion is a passive process that does not require any energy expenditure on the part of the cell. The energy that causes diffusion to occur is supplied by the kinetic energy of the molecules themselves.
Diffusion in Large Organisms
In large animals, many cells are buried deep within the body. If it were not for the animals’ circulatory systems, cells would have little opportunity to exchange gases or other molecules directly with their surroundings. Oxygen can diffuse into blood through the membranes of the lungs, gills, or other moist surfaces of an animal’s body. The circulatory system then transports the oxygen-rich blood throughout the body, and the oxygen automatically diffuses into cells. This occurs because the concentration of oxygen inside cells is lower than that of the blood. The opposite is true of carbon dioxide. Animal cells constantly produce carbon dioxide as a waste product, so there is always a high concentration of it within the cells. These molecules diffuse from the cells into the blood, where the concentration of carbon dioxide is kept constantly low, because the blood is pumped to the moist surfaces (e.g., gills, lungs) and the carbon dioxide again diffuses into the surrounding environment. In a similar manner, many other types of molecules constantly enter and leave cells.
The health of persons who have difficulty getting enough oxygen to their cells can be improved by increasing the concentration gradient. Oxygen makes up about 20 percent of the air. If this concentration is artificially raised by supplying a special source of oxygen, diffusion from the lungs to the blood will take place more rapidly. This will help assure that oxygen reaches the body cells that need it, and some of the person’s symptoms can be controlled (figure 4.22).
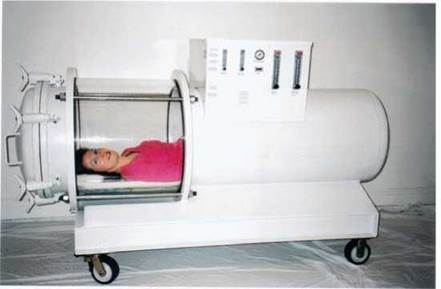
FIGURE 4.22. Diffusion
As a result of molecular motion, molecules move from areas where they are concentrated to areas where they are less concentrated. The machine in the photo is called a hyperbaric (hyper = above; baric = pressure) chamber. It is used to treat people who have certain kinds of infections (e.g., gangrene) or other conditions in which high concentrations of oxygen are beneficial. The concentration of the oxygen in the chamber is higher than in the atmospheric pressure, encouraging diffusion, and the gas pressure in the chamber is higher than atmospheric pressure. Both contribute to getting oxygen into the gangrenous tissue.
Osmosis
Water molecules easily diffuse through cell membranes. Osmosis is the net movement (diffusion) of water molecules through a selectively permeable membrane. Although osmosis is important in living things, it will take place in any situation in which there is a selectively permeable membrane and a difference in water concentration in the solutions on opposite sides of the membrane. For example, consider a solution of 90% water and 10% sugar separated by a selectively permeable membrane from a sugar solution of 60% water and 40% sugar (figure 4.23). The membrane allows water molecules to pass freely but prevents the larger sugar molecules from crossing. There is a higher concentration of water molecules in one solution, compared with the concentration of water molecules in the other. Therefore, more of the water molecules move from the solution with 90% water to the other solution, with 60% water. Be sure that you recognize (1) that osmosis is really diffusion in which the diffusing substance is water and (2) that the regions of different concentrations are separated by a membrane that is more permeable to water than the substance dissolved in the water.
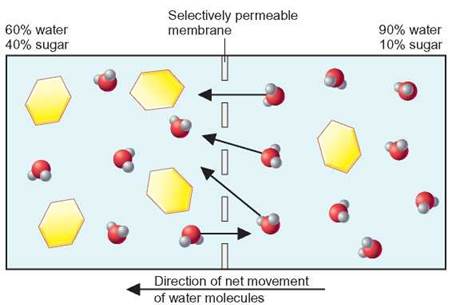
FIGURE 4.23. Osmosis
When two solutions with different percentages of water are separated by a selectively permeable membrane, there will be a net movement of water from the solution with the highest percentage of water to the one with the lowest percentage of water.
It is important to understand that, when one adds something to a water solution, the percentage of the water in the solution declines. For example, pure water is 100% water. If you add salt to the water, the solution contains both water and salt and the percentage of water is less than 100%. Thus, the more material you add to the solution, the lower the percentage of water.
Osmosis in Cells
A proper amount of water is required if a cell is to function efficiently. Too much water in a cell may dilute the cell contents and interfere with the chemical reactions necessary to keep the cell alive. Too little water in the cell may result in a buildup of poisonous waste products. As with the diffusion of other molecules, osmosis is a passive process, because the cell has no control over the diffusion of water molecules. This means that the cell can remain in balance with an environment only if that environment does not cause the cell to lose or gain too much water.
If cells contain a concentration of water and dissolved materials equal to that of their surroundings, the cells are said to be isotonic to their surroundings. For example, the ocean contains many kinds of dissolved salts. Organisms such as sponges, jellyfishes, and protozoa are isotonic to the ocean, because the amount of material dissolved in their cellular water is equal to the amount of salt dissolved in the ocean’s water.
If an organism is to survive in an environment that has a different concentration of water than does its cells, it must expend energy to maintain this difference. Organisms that live in freshwater have a lower concentration of water (a higher concentration of dissolved materials) than their surrounding and tend to gain water by osmosis very rapidly. They are said to be hypertonic to their surroundings, and the surroundings are hypotonic, compared with the cells. These two terms are always used to compare two different solutions. The hypertonic solution is the one with more dissolved material and less water; the hypotonic solution has less dissolved material and more water.
The concept of osmosis is important in medical situations. Often, people are given materials by intravenous injections. However, the solutions added must have the right balance between water and dissolved substances, or red blood cells may be injured (figure 4.24). Similarly, during surgery organs are bathed in a solution that is isotonic to the cells of the body.
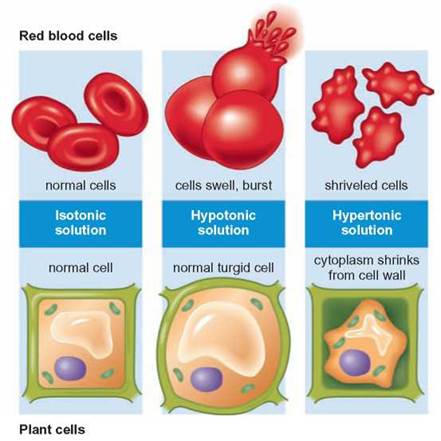
FIGURE 4.24. Osmotic Influences on Cells
Cells are affected by the amount of dissolved materials in the water that surrounds them. When in an isotonic situation the cells neither gain nor lose water. In a hypotonic solution water diffuses from the surroundings into the cell. Animal cells will swell and burst but plant cells have a tough cell wall surrounding the cell contents and the pressure generated on the inside of the cell causes it to become rigid. Both plant and animal cells shrink when in a hypertonic solution because water moves from the cells which have the higher water concentration to the surroundings.
Regulating Water Balance
If an organism is to survive in an environment that has a different concentration of water than does its cells, it must expend energy to maintain this difference.
Organisms whose cells gain water by osmosis must expend energy to eliminate any excess if they are to keep from swelling and bursting. Many kinds of freshwater protozoa have special organelles called contractile vacuoles that fill with water and periodically empty, forcing the water from the cell. The kidneys of freshwater fish are designed to get rid of the water they constantly receive as a result of osmosis from their surroundings. Similarly, organisms that are hypotonic to their surroundings (have a higher concentration of water than their surroundings) must drink water or their cells will shrink. Most ocean fish are in this situation. They lose water by osmosis to their salty surroundings and must drink seawater to keep their cells from shrinking. Because they are taking in additional salt with the seawater they drink, they must expend energy to excrete this excess salt.
Since terrestrial animals like us are not bathed in a watery solution, we do not gain and lose water through our surfaces by osmosis. However, we do lose water due to evaporation. Thus, we must drink water to replace that lost. Our desire to drink is directly related to the osmotic condition of the cells in our body. If we are dehydrated, we develop a thirst and drink some water. This is controlled by cells in the brain. Under normal conditions, when we drink small amounts of water, the cells of the brain swell a little, and signals are sent to the kidneys to rid the body of excess water. By contrast, persons who are dehydrated, such as marathon runners, may drink large quantities of water in a very short time following a race. This rapid addition of water to the body may cause abnormal swelling of brain cells, because the excess water cannot be gotten rid of rapidly enough. If this happens, the person may lose consciousness or even die because the brain cells have swollen too much.
Water Balance in Plant Cells
Plant cells also experience osmosis. If the water concentration outside the plant cell is higher than the water concentration inside, more water molecules enter the cell than leave. This creates internal pressure within the cell. But plant cells do not burst, because they are surrounded by a strong cell wall. Lettuce cells that are crisp are ones that have gained water so that there is high internal pressure. Wilted lettuce has lost some of its water to its surroundings, so that it has only slight internal pressure. Osmosis occurs when you put salad dressing on a salad. Because the dressing has a very low water concentration, water from the lettuce diffuses from the cells into the surroundings. Salad that has been “dressed” too long becomes limp and unappetizing (table 4.1).
TABLE 4.1. Effects of Osmosis on Various Cell Types
Cell Type |
What Happens When Cell Is Placed in Hypotonic Solution |
What Happens When Cell Is Placed in Hypertonic Solution |
With cell wall (e.g., bacteria, fungi, plants) |
Water enters the cell, causing it to swell and generate pressure. However, the cell does not burst because the presence of an inelastic cell wall on the outside of the plasma (cell) membrane prevents the membrane from stretching and rupturing. |
Water leaves the cell and the cell shrinks. The plasma membrane pulls away from inside the cell wall; the cell contents form a small mass. |
Without cell wall (e.g., human red blood cells) |
Water enters the cell and it swells, causing the plasma membrane to stretch and rupture. |
Water leaves the cell and it shrinks into a compact mass. |
Controlled Methods of Transporting Molecules
So far, we have considered only situations in which cells have no control over the movement of molecules. Cells cannot rely solely on diffusion and osmosis, however, because many of the molecules they require either cannot pass through the plasma membrane or occur in relatively low concentrations in the cell’s surroundings.
Facilitated Diffusion
Some molecules move across the membrane by interacting with specific membrane proteins. When the rate of diffusion of a substance is increased in the presence of such a protein, it is called facilitated diffusion. Because this movement is still diffusion, the net direction of movement is from high to low concentration. The action of the carrier does not require an input of energy other than the molecules’ kinetic energy. Therefore, this is considered a passive transport method, although it can occur only in living organisms with the necessary proteins. There are two groups of membrane proteins involved in facilitated diffusion: (1) carrier proteins and (2) ion channels. When a carrier protein attaches to the molecule to be moved across the membrane, the combination molecule changes shape. This shape change enables the molecule to be shifted from one side of the membrane to the other. The carrier then releases the molecule and returns to its original shape (figure 4.25a). Ion channels do not really attach to the molecule being transported through the membrane, but operate like gates. The opening and closing of a channel is controlled by changes in electrical charge at the pore, or “gate-keeping” signal molecules (figure 4.25b).
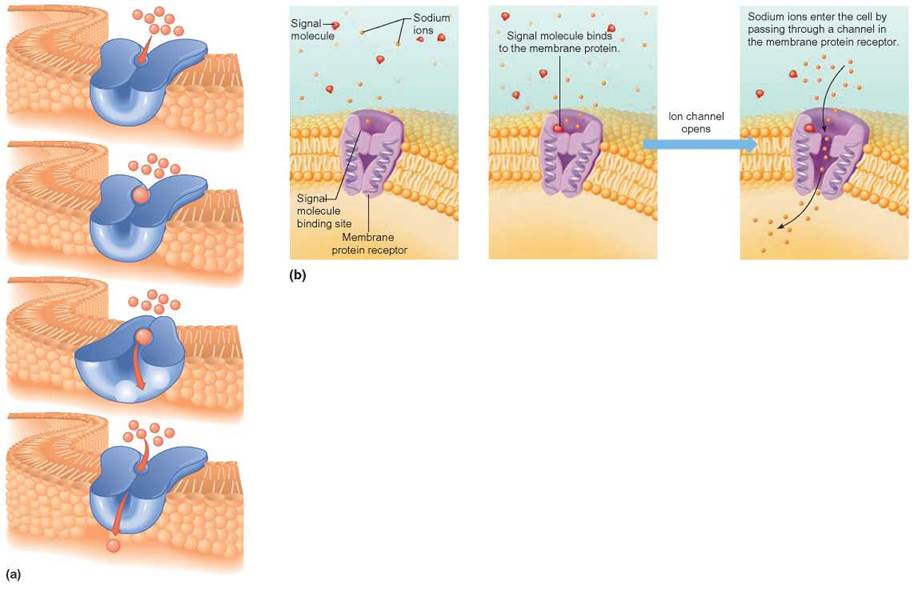
FIGURE 4.25. Mechanisms for Facilitated Diffusion
(a) The molecules being moved through the membrane attach to a specific transport carrier protein in the membrane. This causes a change in the shape of the protein, which propels the molecule or ion from inside to outside or from outside to inside. (b) Ion channels can be opened or closed to allow these sodium ions to be transported to the other side of the membrane. When the signal molecule binds to the ion channel protein, the gate is opened.
Active Transport
When molecules are moved across the membrane from an area of low concentration to an area of high concentration, the cell must expend energy. This is the opposite direction molecules move in osmosis and diffusion. The process of using a carrier protein to move molecules up a concentration gradient is called active transport (figure 4.26). Active transport is very specific: Only certain molecules or ions can be moved in this way, and they must be carried by specific proteins in the membrane. The action of the carrier requires an input of energy other than the molecules’ kinetic energy; therefore, this process is termed active transport. For example, some ions, such as sodium and potassium, are actively pumped across plasma membranes. Sodium ions are pumped out of cells up a concentration gradient. Potassium ions are pumped into cells up a concentration gradient.
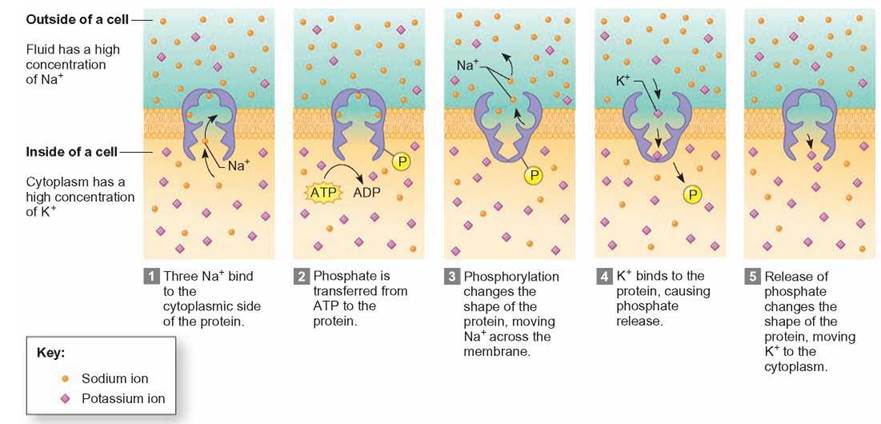
FIGURE 4.26. Active Transport
The action of the carrier protein requires an input of energy (the compound ATP) other than the kinetic energy of the molecules; therefore, this process is termed active transport. Active transport mechanisms can transport molecules or ions up a concentration gradient from a low concentration to a higher concentration.
Endocytosis and Exocytosis
Larger particles or collections of materials can be transported across the plasma membrane by being wrapped in membrane, rather than passing through the membrane molecule by molecule. When materials enter a cell in this manner, it is called endocytosis. When materials are transported out of cells in membrane-wrapped packages, it is known as exocytosis (figure 4.27). Endocytosis can be divided into three sorts of activities: phagocytosis, pinocytosis, and receptor mediated endocytosis.
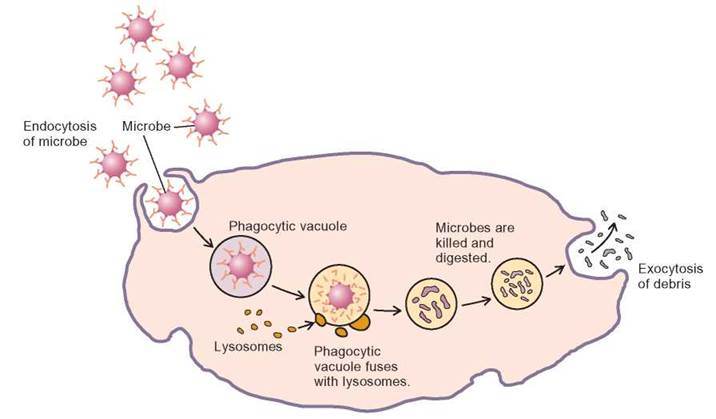
FIGURE 4.27. Endocytosis and Exocytosis
The sequence illustrates a white blood cell engulfing a microbe by endocytosis. The bacterium is surrounded by a portion of the plasma membrane. Once inside the cell, lysosomes add their digestive enzymes to the phagocytic vacuole, which speeds the breakdown of the contents of the vacuole. Finally, the vacuole containing the digested material moves to the inner surface of the plasma membrane, where the contents are discharged by exocytosis.
Phagocytosis is the process of engulfing large particles, such as cells. For example, protozoa engulf food and white blood cells engulf bacteria by wrapping them with membrane and taking them into the cell. Because of this, white blood cells often are called phagocytes. When phagocytosis occurs, the material to be engulfed touches the surface of the cell and causes a portion of the outer plasma membrane to be indented. The indented plasma membrane is pinched off inside the cell to form a sac containing the engulfed material. Recall that this sac, composed of a single membrane, is called a vacuole. Once inside the cell, the membrane of the vacuole fuses with the membrane of lysosomes, and the enzymes of the lysosomes break down the contents of the vacuole.
Pinocytosis is the process of engulfing liquids and the materials dissolved in the liquids. In this form of endocytosis, the sacs that are formed are very small, compared with those formed during phagocytosis. Because of their small size they are called vesicles. In fact, an electron microscope is needed to see vesicles.
Receptor mediated endocytosis is the process in which molecules from the cell’s surroundings bind to receptor molecules on the plasma membrane. The membrane then folds in and engulfs these molecules. Because receptor molecules are involved, the cell can gather specific necessary molecules from its surroundings and take the molecules into the cell.
Exocytosis occurs in the same manner as endocytosis. Membranous sacs containing materials from the cell migrate to the plasma membrane and fuse with it. This results in the sac contents’ being released from the cell. Many materials, such as mucus, digestive enzymes, and molecules produced by nerve cells, are released in this manner.
4.7. CONCEPT REVIEW
14. Describe what happens during the process of endocytosis.
15. How do diffusion, facilitated diffusion, osmosis, and active transport differ?
16. What will happen if an animal is placed in a hypertonic solution?