Biology For Dummies
Part IV Systems Galore! Animal Structure and Function
Chapter 17
Fighting Back: Human Defenses
In This Chapter
Distinguishing the good microbes from the bad
Checking out the components of your innate and adaptive immune systems
Helping your immune system out when it needs it
Seeing how age affects your internal defense system
You encounter bacteria and viruses all the time, some of which have the potential to make you very sick. (On the other hand, some bacteria are actually quite beneficial to human existence.) Whether these potential pathogens cause you harm depends on a complicated give and take between their invasion tools and your defenses. You emerge the winner from the vast majority of your microbial encounters due to the combination of your innate immunity (a built-in immune system that all humans have) and your adaptive immunity (the part of your immune system that develops as you encounter microbes).
In this chapter, we present the structures and cells that keep you safe from microbes and review the options available to you when your body’s defenses need a helping hand. We also explain what happens to your immune system as you age.
Microbial Encounters of the Best and Worst Kinds
Microbes (bacteria and viruses) exist on every surface. They’re in the air, the water, the soil — even your body. So how can you tell a good microbe from a bad one? The next sections solve the puzzle.
Good bacteria: Health helpers
The bacteria that normally live in and on your body are your normal microbiota, and they play an important role in your general health, in large part because they protect you from disease-causing microbes called pathogens.
 Your normal microbiota are beneficial to you because they
Your normal microbiota are beneficial to you because they
Aid the digestive process and assist with blood clotting by releasing vitamin K. (You can’t get vitamin K from foods, only from the beneficial bacteria inside your digestive tract. So consider vitamin K the rent the bacteria pay for using your intestines as their home.)
Take up space and nutrients so pathogens can’t easily colonize you.
Make chemicals called bacteriocins that inhibit the growth of other bacteria.
Bad bacteria: Health harmers
Some bacteria really live up to the bad reputation they’ve developed over time. These bad bacteria are pathogens, the microbes that cause infectious disease.
To cause disease, bad bacteria must be able to do three things:
Enter and colonize the body: Bad bacteria can enter your body when you breathe or consume food and drink. They can also enter through a wound or be passed along through sexual contact. One of the more infamous pathogens in history is Yersinia pestis, the bacterium that caused the Black Death, a wave of bubonic plague that killed about two-thirds of the people living in Europe during the 13th century. This bacterium normally lives and reproduces inside rodents, but if infected rodents and humans are living near each other, the plague bacterium can be transferred to humans by fleas that bite the rodents and then bite the humans.
Overcome your defenses: Your body’s defenses are pretty darn good, but some bacteria have tricks to get past them. Other bacteria take advantage of you when you’re down. For example, the Streptococcus pneumoniae bacteria exist in the throats of normal, healthy people all the time. Most of the time, the bacteria are well-behaved, just hanging about in warm, dark crevices of the throat. But if the host is weakened by a cold or flu, things get ugly. The bacteria get a little power hungry and begin reproducing rapidly, which can lead to a sinus or ear infection, or even pneumonia.
Damage the body: Pathogens produce toxins and enzymes that damage your tissues. If, for example, food is improperly processed, bacterial toxins may become the secret ingredient in your meals. One good example of this is botulism. This illness is usually caused by improper canning, which allows the bacteria Clostridium botulinum to grow in the food and release their toxins. The toxins, not the bacteria, are what make you sick.
Viruses: All bad, all the time
Viruses are the pirate raiders of the microbial world. They’re tiny particles containing genetic information that hijack your cells, forcing them to make more viruses. Although, viruses are very different in structure than bacteria, they can also make you sick.
 Here’s how viruses attack cells (see Figure 17-1 for the visual):
Here’s how viruses attack cells (see Figure 17-1 for the visual):
1. The virus attaches its proteins to a receptor on a cell.
You can think of this like inserting your key into the door when you get home. If your key doesn’t fit, you can’t get in. In #2 in Figure 17-1, you can see the HIV virus attaching to a protein called CD4 that’s found on the surface of certain human white blood cells.
2. The virus shoots its nucleic acid into the cell, taking over the cell.
The viral nucleic acid reprograms the cell, turning it into a viral production factory. Instead of doing its job for the body, the cell starts making viral nucleic acid and proteins. The cell even uses its own molecules and energy reserves (ATP) to produce the viral parts. In #3 in Figure 17-1, you can see the viral genetic material of HIV entering the human cell, and in #6, you can see more viral genetic material being made by the cell.
3. The viral components pull themselves together to form mature viruses.
Eventually, this viral replication creates too much of a crowd for the cell to handle and the cell explodes, releasing viral particles to go wreak havoc in other cells in the host’s body. The number of viruses that go on attack at this point can range from ten to tens of thousands, depending on the type of virus. In #8 in Figure 17-1, you can see completed viral particles exiting the human cell.
Figure 17-1:How viruses attack cells.
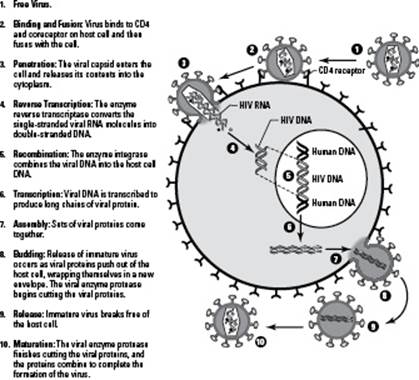
 Because viruses need a host in order to replicate, they’re called obligate intracellular parasites (obligate means they need it, intracellular means they go inside cells, and parasite means they use the host’s resources for their own benefit and destroy the host in the process).
Because viruses need a host in order to replicate, they’re called obligate intracellular parasites (obligate means they need it, intracellular means they go inside cells, and parasite means they use the host’s resources for their own benefit and destroy the host in the process).
Built to Protect You: Innate Human Defenses
Usually you’re not aware of all the microbes roaming the world because a) you can’t see them and b) your innate immunity keeps most of them from bothering you. Innate immunity is the built-in immunity that you have due to the way the human body is constructed. Like the walls of a fortress, your innate defenses can repel all attackers (meaning they’re not specifically targeted for one particular pathogen).
You only notice microbes if they manage to get past your innate defenses. When that happens, you need your adaptive immunity to come to your rescue. (See the later section “Learning a Lesson: Adaptive Human Defenses” for more on this part of your immune system.)
 Your innate defenses have several ways of fending off the potential pathogens you encounter:
Your innate defenses have several ways of fending off the potential pathogens you encounter:
Physical barriers: Your skin and mucous membranes are the barriers that physically block access to your tissues and organs. You can think of these barriers as the walls surrounding your castle.
Chemical barriers: The pH of your stomach acid is a chemical barrier that prevents microbial growth. In other words, your stomach acid is kind of like the boiling oil that castle defenders dump over the walls onto invaders.
Dendritic cells: These cells patrol your body in search of microbes and alert your immune system of impending invasions. Like sentinels patrolling a castle’s grounds, they run back to the interior of the castle and alert the commander-in-chief of the army (in this case, the helper T cell) to the presence of invaders.
Phagocytes: These are certain white blood cells that seek and destroy microbes that have successfully entered your body. They actually wrap around invading microbes and eat them alive. Think of phagocytes as your body’s hand-to-hand combat specialists.
Inflammation: This is the alarm call that gathers white blood cells to the site of an invasion and helps contain and destroy the microbes, similar to how a warning cry rallies a castle’s defenders to fight an invasion.
Filters: The mucus in your nose and throat and the hairs in your nose act as filters that trap microbes and prevent them from getting deeper into your body. But that’s not the only filtration system at work in your body. Your lymphatic system screens your body fluids for the presence of microbes and destroys any it finds. All of these filters act a little like a moat around a castle, slowing the enemy down.
We cover each of these innate defense mechanisms in greater detail in the sections that follow.
Your body’s best blockers: Skin and mucous membranes
Your skin and mucous membranes have an important job: serving as your body’s first line of defense against bad bacteria and viruses. Both the skin and mucous membranes are epithelia, tissues composed of multiple cell layers that are packed tightly together in order to prevent microbes from sneaking in.
 Your skin is the largest organ in your body, but its size and tightly packed layers aren’t the only characteristics that make it a particularly good microbe repellent. Your skin is good at keeping microbes out for these reasons as well:
Your skin is the largest organ in your body, but its size and tightly packed layers aren’t the only characteristics that make it a particularly good microbe repellent. Your skin is good at keeping microbes out for these reasons as well:
It’s dry. Microbes need water in order to grow, so many things simply can’t grow on your dry skin.
It’s flaky. Skin cells regularly fall right off of you, taking microbes with them.
It’s tough. Skin cells are reinforced with the protein keratin — the same strong protein that forms your hair and fingernails.
It’s acidic. The fatty acids from your sebum (the oily stuff secreted by the glands in your skin) give your skin a slightly acidic pH, which prevents many microbes from growing.
Just like your skin guards the exterior of your body, your mucous membranes protect your wet interior surfaces. Mucous membranes line your respiratory, gastrointestinal, urinary, and reproductive systems. Although your mucous membranes aren’t quite as tough as your skin, they have some unique defenses of their own:
They’re sticky. Mucus traps invading microbes as they land on the membranes.
They move stuff out. Mechanical washing removes microbes from the surfaces of several mucous membranes. For example, tears wash the eyes, urine flushes out the urethra, and peristalsis moves material through the intestines.
 In the respiratory tract, a blanket of cilia called the ciliary escalator moves mucus upward in the throat just like an escalator moves people upward at the mall. The mucus moves to a location where you can cough it out, protecting your lower respiratory tract from infection (see Chapter 4 for more on cilia).
In the respiratory tract, a blanket of cilia called the ciliary escalator moves mucus upward in the throat just like an escalator moves people upward at the mall. The mucus moves to a location where you can cough it out, protecting your lower respiratory tract from infection (see Chapter 4 for more on cilia).
Tiny but mighty: Molecular defenders
The fluids in your body contain a variety of defensive proteins that help prevent infection. These tiny, invisible defenders bind to microbes, breaking them down and just generally making it difficult for them to get a foothold in your body.
These invisible chemical defenders are found in your tears, saliva, mucus, blood, and tissue fluids. Here are the names of a few of ’em:
Lysozyme is a protein that breaks down one of the chemicals found in bacterial cell walls (lyse- is to break, and zyme means “enzyme,” so a lysozyme is an enzyme that breaks down bacteria). It’s one of the most common molecular defenders in your body; basically, when bacteria land on you or in you, they encounter lysozyme.
Transferrin in your blood binds iron so microbes don’t have enough iron for their growth.
Complement proteins in your blood and tissue fluids bind to microbes and target them for destruction.
Interferons are proteins that are released by cells infected with viruses. They travel to cells all around the infected cell and warn them about the virus. Cells that receive a warning from interferon produce proteins to help protect themselves against viral attack.
Microbe seeker-outers: Dendritic cells
All around your body, white blood cells called dendritic cells look for potentially dangerous microbes with their special cell receptors designed for detecting molecules typical of microbial cells. Because of these special receptors, called toll-like receptors, dendritic cells are very good at recognizing foreign microbes. Molecules from bacteria and viruses bind to the toll-like receptors and activate the dendritic cells.
 Dendritic cells do two things that are key to your ability to fight infections:
Dendritic cells do two things that are key to your ability to fight infections:
They release communicating molecules called cytokines. Cytokines spread through your body and bind to other cells in your immune system (cyto- means “cell,” and kinesis means “movement,” so cytokines are literally molecules that travel between cells). The cytokines tell your immune system that microbes have been detected and help activate the cells you need to fight back.
They break down microbes into little bits called antigens and then show the antigens to helper T cells. Helper T cells are very important to your pathogen-fighting ability because they recognize foreign antigens and send signals directing other cells of your immune system to fight.
Invader eaters, big and small: Phagocytes
Phagocytes are white blood cells that patrol your body looking for microbes. When they find them, they grab them and eat them alive (phago- means “eat,” and -cyte means “cell,” so phagocytes are “eater-cells”). Like dendritic cells (covered in the preceding section), phagocytes activate helper T cells by showing them antigens from the destroyed microbes.
The two types of phagocytes are
Neutrophils: These phagocytes multiply early during an infection and are the first ones to arrive on the scene during inflammation.
Macrophages: These phagocytes live in particular tissues. (Macro- means “big,” and phage means “eat,” so macrophages are literally “big eaters.”)
Damage control: Inflammation
When microbes do manage to invade, your body responds quickly to try and contain them. The microbes and your own damaged cells trigger a cascade of events that leads to inflammation, a local defensive response to cellular damage that’s characterized by redness, pain, heat, and swelling. Inflammation fights infection by destroying microbes, confining the infection to one location, and repairing damaged tissue.
Molecules such as histamine that are released during inflammation lead to vasodilation and increased blood vessel permeability.
Vasodilation causes blood vessels to widen, allowing more blood to flow to the affected area. The blood flowing to the infected area delivers clotting elements that trigger blood clots and help confine the infection to one location. It also brings more white blood cells, including phagocytes, to help fight the infection. As a result of the increased blood flow, the infected area becomes warm and red.
Increased blood vessel permeability means the walls of the blood vessels loosen up. This allows cells and materials to leave the blood and enter the tissues where the infection is happening. Phagocytes squeeze through the gaps in the blood vessel walls, crawl to the infection, and start eating the microbes. Fluid leaks from the blood vessels into the tissues and swelling occurs.
A fluid filterer: The lymphatic system
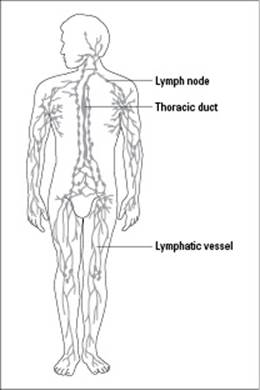
 Your blood constantly delivers materials such as food to your tissues. After fluid leaves the blood and enters the tissues, it needs to be cleaned before it’s returned to the blood. The lymphatic system (pictured in Figure 17-2) is responsible for checking these fluids for foreign materials, such as microbes, and cleaning them up.
Your blood constantly delivers materials such as food to your tissues. After fluid leaves the blood and enters the tissues, it needs to be cleaned before it’s returned to the blood. The lymphatic system (pictured in Figure 17-2) is responsible for checking these fluids for foreign materials, such as microbes, and cleaning them up.
Figure 17-2: The lymphatic system.
From LifeART®, Super Anatomy 1, © 2002, Lippincott Williams & Wilkins
Your lymphatic system has two main components:
Lymphatic vessels: These vessels carry fluid, called lymph, through a network of lymph nodes (which are lymphatic organs) and then back to the circulatory system. (Note: Lymphatic vessels form a circulatory system that’s similar to, but separate from, your blood vessels.) Fluid from your tissues drains into your lymphatic vessels and then flows in a one-way direction toward your heart. As lymph passes through the lymph nodes, defensive proteins and white blood cells clean it of any foreign material, including microbes. Fluid from the lymph then reenters the bloodstream near the heart.
Lymphatic organs: Lymph nodes, the spleen, the tonsils, and the thymus are all lymphatic organs. They’re full of white blood cells that help fight infection by destroying foreign material. Patches of lymphatic tissue are also scattered around in different organs of your body.
 The spleen is a little different from the other lymphatic organs because it’s connected to the circulatory system, so it filters and cleans blood rather than lymph. It’s considered a lymphatic organ, though, because it contains so many white blood cells and because its job is to clean up foreign material.
The spleen is a little different from the other lymphatic organs because it’s connected to the circulatory system, so it filters and cleans blood rather than lymph. It’s considered a lymphatic organ, though, because it contains so many white blood cells and because its job is to clean up foreign material.
Learning a Lesson: Adaptive Human Defenses
Your body’s innate defenses are incredible, and they prevent infection by most of the microbes that you encounter in your life. But every now and then, a microbe comes along that gets around your innate defenses and into your body. When your innate defenses are breached, it’s time for the troops of your adaptive immunity to rally and fight back.
Your adaptive immunity gets its name because it adapts and changes as you go through life and are exposed to specific microbes. If, for example, you’re infected with E. coli, only those white blood cells that recognize particular molecules on E. coli will be activated. If you face a different infection, say the bacteria Staphylococcus aureus, only the set of white blood cells that recognizes specific molecules on S. aureus will be activated. In other words, when your adaptive defenses come to your rescue, exactly the right team of white blood cells is activated to fight each pathogen. That means your adaptive defenses learn to recognize specific pathogens after you encounter them.
One of the awesome features of your adaptive immunity is that it can remember a pathogen it has encountered before. This immunologic memory allows your immune system to respond much more effectively when you reencounter a particular pathogen.
 Certain cells of your immune system, called memory cells, remain in a semiactivated state after your first encounter with a microbe. These memory cells and their descendants hang around for a long time after they’re activated in the first battle. When the same pathogen shows up again, these cells multiply quickly and efficiently destroy the pathogen before you even realize it came back. Memory cells are therefore the reason why you can get some illnesses just once and be protected from getting them again.
Certain cells of your immune system, called memory cells, remain in a semiactivated state after your first encounter with a microbe. These memory cells and their descendants hang around for a long time after they’re activated in the first battle. When the same pathogen shows up again, these cells multiply quickly and efficiently destroy the pathogen before you even realize it came back. Memory cells are therefore the reason why you can get some illnesses just once and be protected from getting them again.
We describe the other components of your adaptive immune system in the following sections.
Commanders-in-chief: Helper T cells
Helper T cells are white blood cells that coordinate your entire adaptive immune response. (They’re called T cells because they mature in the thymus, which is one of your lymphatic organs.) Helper T cells receive signals from the white blood cells of your innate defenses, such as dendritic cells and phagocytes, and relay those signals to the fighters of your adaptive defenses: the B cells and cytotoxic T cells (more on these in the next sections).
 Helper T cells are also called CD4 cells because they have a protein on their surface called CD4.
Helper T cells are also called CD4 cells because they have a protein on their surface called CD4.
Dendritic cells and phagocytes (which are both considered antigen-presenting cells) activate helper T cells by showing them the antigens from the microbes they’ve found. Here’s how the process works:
1. Antigen-presenting cells attach pieces of the foreign antigen to proteins that they display on their surface.
By putting antigens on their surfaces, the antigen-presenting cells can show the antigen to the helper T cells. They’re basically holding out the antigen to the helper T cells and saying, “Look what I’ve found!”
2. Antigen-presenting cells also release cytokines, signaling that they’ve detected a foreign antigen.
You can think of this as the antigen-presenting cells telling the helper T cells “This looks dangerous!”
3. Helper T cells bind to the displayed antigen using a receptor called a T cell receptor.
Only T cells whose T cell receptors are the right shape can bind a specific antigen. So, only the T cells that are the right T cells to fight this antigen will be activated.
Helper T cells also receive signals from antigen-presenting cells. Receptors on the helper T cells bind the signals, acting like ears so the T cells can “hear” the alarm call of the antigen-presenting cells.
After T cells bind to the antigens on antigen-presenting cells and receive the cells’ signals, they activate and begin releasing signals to the other cells of the adaptive immune system.
Soldiers on the march: B cells and antibodies
B cells are white blood cells that become activated when they detect foreign antigens with their B cell receptors or receive signals from helper T cells. They’re activated to form two types of cells: plasma cells and memory cells (for more on memory cells, see the earlier “Learning a Lesson: Adaptive Human Defenses” section).
 Plasma cells produce antibodies, defensive proteins that bind specifically to antigens. The antibodies produced by plasma cells are released into the blood, where they can circulate around the body. Anything in the body that’s tagged with antibodies — such as invading pathogens — is marked for destruction by the immune system.
Plasma cells produce antibodies, defensive proteins that bind specifically to antigens. The antibodies produced by plasma cells are released into the blood, where they can circulate around the body. Anything in the body that’s tagged with antibodies — such as invading pathogens — is marked for destruction by the immune system.
Following are the reasons why antibodies make it easier for the immune system to fight infection:
Phagocytes can bind to antibodies, so anything with antibodies bound to it is easier for the phagocytes to grab and eat.
Antibodies stick to pathogens and drag them into clumps. This makes phagocytes more efficient because it takes fewer “mouthfuls” to deal with the problem.
Antibodies stick to the surfaces of viruses, preventing them from binding to new host cells.
Each plasma cell makes antibodies that are specific to one antigen, so you can think of plasma cells like the archers of the immune system. Each archer is trained to hit just one type of target. When you have an infection, the helper T cells call out just the set of archers needed to fight that particular pathogen.
 Each adaptive immune response is tailored specifically to fight the invading pathogen. This specificity occurs because B cells (and T cells) activate only when their receptors recognize a specific foreign antigen. So, out of the thousands of different B (and T) cells your body can produce, only a small subset reacts to each pathogen.
Each adaptive immune response is tailored specifically to fight the invading pathogen. This specificity occurs because B cells (and T cells) activate only when their receptors recognize a specific foreign antigen. So, out of the thousands of different B (and T) cells your body can produce, only a small subset reacts to each pathogen.
Cellular assassins: Cytotoxic T cells
If the microbes try to hide inside your cells so the antibodies can’t find them, that’s when cytotoxic T cells come into play. Cytotoxic T cells are white blood cells that are experts at detecting infected host cells. When they detect foreign antigens on the surface of an infected host cell, they order the cell to commit suicide — a necessary sacrifice in order to destroy the hidden microbes.
 Cytotoxic T cells are also called CD8 cells because they have a protein on their surface called CD8.
Cytotoxic T cells are also called CD8 cells because they have a protein on their surface called CD8.
Giving Your Defenses a Helping Hand
As powerful as the human immune system is, microbes are amazingly clever and diverse, which means sometimes you may encounter a pathogen with the ability to get around all of your defenses. This is where science and medicine come in. Scientists study microbes and how they work, searching for ways to prevent pathogens from infecting and damaging the body. Doctors study how the body works and how to recognize the signs of different illnesses so they know which tools to use to help you fight off disease. Together, scientists and doctors have found ways to give the human immune system a helping hand when necessary. The next sections introduce you to these immune system helpers.
Killing bacteria with antibiotics
Antibiotics are molecules made by microbes that kill bacteria. The first and most famous antibiotic, penicillin, is produced by a mold that looks a lot like the green stuff you see on old bread in your kitchen. Many other antibiotics are produced by bacteria that live in the soil.
 The structures and enzymes that antibiotics target are unique to bacterial cells, so they have little effect on human cells (see Chapter 4 for more on the differences between bacterial and human cells).
The structures and enzymes that antibiotics target are unique to bacterial cells, so they have little effect on human cells (see Chapter 4 for more on the differences between bacterial and human cells).
Antibiotics worked so well after they were first discovered that people thought they’d won the war against bacteria. Funding for research on new antibiotics decreased because people thought they had enough weapons in their arsenal. But while people were celebrating their victory, the microbes continued to evolve. Today, humans are faced with a new microbial problem: antibiotic-resistant bacteria. These bacteria can’t be killed by some, or even all, of the existing antibiotics.
The conundrum modern doctors now face is that using antibiotics increases the chances that antibiotic-resistant strains of bacteria will develop. It’s purely natural selection (see Chapter 12 for more on this concept). In other words, when antibiotics are used, the most susceptible bacteria die first, leaving the more resistant bacteria to survive. The resistant bacteria multiply, creating new hordes that are more resistant to the antibiotic than the last horde. Repeat this cycle a few times, and the antibiotic no longer works at all.
Every year, nearly 100,000 Americans die from hospital-acquired infections (called nosocomial infections) related to antibiotic-resistant bacteria. And that’s just part of the problem. Infections that humans thought they had under control, including such nasty diseases as tuberculosis and bubonic plague, are rearing their ugly heads in developing countries around the world.
Scientists and doctors are teaming up again to fight the threat of antibiotic-resistant bacteria. Scientists are searching for new antibiotics and new bacteria-fighting strategies, while doctors are being careful about how they prescribe antibiotics. By saving antibiotics for when they’re really needed, doctors can slow down natural selection and help keep antibiotics working for as long as possible.
Using viruses to kill bad bacteria
It may seem strange to think of a helpful virus, but that’s exactly what a bacteriophage (or phage for short) is: a virus that attacks only bacterial cells.
Bacteria-blasting viruses were first discovered in the early years of the last century at the Pasteur Institute in Paris. Canadian microbiologist Félix d’Hérelle came across the little creatures while looking for a means of treating dysentery in Paris. He saw phages take on and completely destroy a whole colony of much larger bacteria. Logically enough, he hoped the microbes would help eliminate some of the world’s worst bacterial infections.
Until about 1940, these tiny microbes (they’re only about a fortieth of the size of bacteria) were the miracle cure for many bacterial infections. Then antibiotics came on the scene, and the medical world turned its back on these little creatures. Because you have to find just the right bacteria to appeal to the palate of a specific phage or else the deal is off, antibiotics — which have a much broader spectrum — seemed like a better solution to the problem of infection.
Yet the rise of antibiotic-resistant bacteria (see the preceding section for more on this) has caused modern doctors and scientists to once again consider the beneficial features of phages. Here are the prominent ones:
Phages are among the most abundant creatures on Earth.
Phages survive and reproduce in the same neighborhoods that bacteria call home. They blissfully swim around in piles of sewage and hide in cozy little corners in your body.
Phages reproduce rapidly. After phages invade a bacterial cell, as many as 200 new phages are produced per hour. At that rate, it doesn’t take long before the rambunctious little phages burst right through the cell wall. Then it’s bye-bye bacterium. But that’s hardly the end of it. The infant phages move on to nearby bacteria, making short work of every cell in their path. Before long, a whole colony of bacteria is history.
Scientists are currently working to solve the problems that remain with phage therapy, which include the time it takes to identify which phages like to knock off which bacteria. Many scientists advise conservative use of phage therapy to help prevent the development of phage-resistant bacteria, but they also say that phage therapy could be a valuable treatment-of-last-resort, to be used when even the most powerful antibiotics fail.
Fighting viruses with antiviral drugs
Controlling viruses is even more difficult than controlling bacteria. Viruses aren’t cells, and they have very few molecules that can be targeted by drugs. They’re difficult to see and isolate, and they’re equally difficult to classify. And here’s another difficulty in the development of antiviral therapies: Because the host cell and viruses become so integrated, it’s sometimes hard to target the virus without destroying or upsetting the mechanics of the cell itself. So, in many cases, all doctors can do is treat the symptoms of viral illnesses rather than the illnesses themselves.
The medical world does have a couple options for fighting viruses, though:
Interferons and other cytokines can be used to stimulate the immune system’s response to viruses. These human proteins can now be made in a lab by scientists who genetically engineer bacteria (flip to Chapter 9 for more on genetic engineering).
Antiviral drugs that target specific viral proteins can be used to treat a number of viral diseases, including the flu, herpes, and HIV.
 The term antibiotic specifically applies to drugs that fight bacterial cells. When talking about drugs for viral infections, be sure to call them antiviral drugs and not antibiotics.
The term antibiotic specifically applies to drugs that fight bacterial cells. When talking about drugs for viral infections, be sure to call them antiviral drugs and not antibiotics.
Getting ahead of the game with vaccines
 Vaccines, solutions containing pieces of microbes that are introduced into the body, prevent diseases by generating immunologic memory. When you get a vaccine, the antigens from the pathogen enter your body and are processed by your immune system. The antigen-presenting cells pick up the pieces and show them to the helper T cells. The helper T cells then activate the B cells and cytotoxic T cells. In all the excitement of the adaptive immune response, the memory B and T cells are developed. They remember the antigens and are ready to defend you if the real pathogen ever shows up. If it does, your immune system kicks into gear so fast and hard that the pathogen is blown away before you even realize it was ever there.
Vaccines, solutions containing pieces of microbes that are introduced into the body, prevent diseases by generating immunologic memory. When you get a vaccine, the antigens from the pathogen enter your body and are processed by your immune system. The antigen-presenting cells pick up the pieces and show them to the helper T cells. The helper T cells then activate the B cells and cytotoxic T cells. In all the excitement of the adaptive immune response, the memory B and T cells are developed. They remember the antigens and are ready to defend you if the real pathogen ever shows up. If it does, your immune system kicks into gear so fast and hard that the pathogen is blown away before you even realize it was ever there.
Vaccine safety
One issue that challenges the success of vaccines today is people’s fears about vaccine safety. Because of these fears or mistrust of vaccines, some people are choosing not to vaccinate their children, a decision that ultimately puts the children at greater risk for infectious disease.
Following are a few points to consider about the safety of vaccination:
The risks from a vaccine are less than the risks from the disease. All vaccines have risks and can cause side effects. However, in order for a vaccine to be licensed by the U.S. Food and Drug Administration, the side effects must be far less severe than the effects of the disease, and the risk of having side effects must be much lower than the risk of getting the disease.
Many diseases that are perceived as mere nuisances can actually have extremely serious complications. Measles, for example, which some people think of as a relatively harmless childhood disease, is the sixth most common killer of children worldwide. Measles infection can result in complications such as encephalitis (swelling of the brain) and pneumonia. According to the WHO, measles killed 777,000 children during 2000 alone. This statistic is especially tragic when you consider that measles is a vaccine-preventable disease.
Many people in rich nations have no firsthand knowledge of the full impacts of infectious disease. Most younger people who live in rich nations such as the United States or countries in Europe have grown up in a time when vaccinations were easily available. Few people who had polio are still alive in these countries, and hardly anyone remembers the days when people ended up in iron lungs because polio had paralyzed the muscles they needed to breathe. Because of a lack of knowledge, the fear of infectious diseases has declined in these countries, leading people to question the need for vaccinations.
The Internet spreads rumors like wildfire. The Internet brings a world of information right into your home. The problem with that information, however, is that it hasn’t all been checked for its accuracy. Books are checked by editors, and scientific and medical articles are carefully reviewed by groups of scientists and doctors before they’re published. All you need to put information on the Internet, however, is the cash for a domain name and host server. An official-looking Web site can fool people about the reliability of its information, so always check the source of your information.
Tip: Two organizations that have excellent information on vaccine safety are the Centers for Disease Control and Prevention (www.cdc.gov) and The Children’s Hospital of Philadelphia (www.chop.edu/service/vaccine-education-center).
Vaccines are the only tool people have to control the spread of some viral diseases. Following are the different types of vaccines:
Inactivated vaccines contain killed pathogens. The parts of the pathogen are included, but the pathogen can’t reproduce.
Attenuated (live) vaccines contain weakened pathogens. The pathogens have been altered in the lab so they have most of their original form, except for the parts that let them make you sick.
Subunit vaccines contain just bits of the pathogen, such as the antigens most recognized by the body.
Smallpox has been virtually wiped off the face of the Earth by vaccination. The World Health Organization (WHO), which works to control the spread of infectious disease around the world, is now working to eradicate polio through vaccination and also plans to target measles. Eradication of smallpox, polio, and measles is possible because humans are the only host species for these viruses, which means the viruses can’t multiply and mutate in animal hosts. So when you get vaccinated against these diseases, your immunologic memory takes over from there and you’re good to go.
Flu viruses are a bit different. They mutate so rapidly that new vaccines are required each year. When a virus mutates, it can continually adapt to new intracellular environments and escape from the host’s immune response. Mutations can change everything from the strength of a virus to its ability to latch on to new kinds of cell types or infect new animal hosts. Case in point: The swine flu pandemic of 2009 was caused by a strain of H1N1 influenza that had a unique mixture of genes from three different strains of flu, some of which hadn’t infected humans before. Because the H1N1 influenza had a unique combination of genes, it had a unique combination of antigens, and no humans had existing immunologic memory. This lack of immunity allowed the virus to spread far and wide, causing the pandemic.
Aging and Ailing: Changes in the Immune System
As your body grows, develops, and ages, your immune system changes. During the busy, trying time of puberty, the thymus starts to get smaller and smaller until it’s virtually nonexistent later in adulthood. Without the thymus, T cells don’t get differentiated as well or as often. Production of B cells starts to wane as well. As the cells in the bone marrow that are precursors to the T cells and B cells go through a slowdown in production, the bone marrow becomes less able to put cells through cell division.
 Weakening of the immune system may explain why people toward the high end of the average life span (78 years for people living in the United States) become more prone to infection.
Weakening of the immune system may explain why people toward the high end of the average life span (78 years for people living in the United States) become more prone to infection.
In a rather nasty paradox, older people also have an increased risk of autoimmune diseases, which are diseases in which the body’s healthy cells are attacked by the cells of its own immune system. One example of an autoimmune disease that’s much more common in older people than younger folk is arthritis. In people with arthritis, the cells of the immune system attack the cells lining the joint spaces, causing inflammation and deterioration (not to mention pain and swelling).