Medical Microbiology
Section 1 The adversaries – microbes
8 The host–parasite relationship
Introduction
The preceding chapters have focused primarily on organisms that are quite clearly disease agents. Small numbers may be found in healthy individuals, but their presence in large numbers is usually associated with pathologic changes. The organisms covered in the first section of this chapter may cause disease under certain circumstances (e.g. in the newborn or in stressed, traumatized or immunocompromised individuals), but usually coexist quite peacefully with their host. Many of these form what is termed the ‘indigenous’ or ‘normal’ flora of the body – a collection of species routinely found in the normal healthy individual and which, in some cases, are necessary for normal functioning of the human body. Their relationship with the host makes an interesting comparison with that of species that are considered as true parasites or pathogens and is discussed later in this chapter in the broader context of symbiotic relationships and the evolution of host–parasite relationships.
The normal flora
Why is it called the normal flora?
The term flora is used for the collective bacteria and other microorganisms in an ecosystem such as the human host. It has been estimated that humans have approximately 1013 cells in the body and something like 1014 bacteria associated with them, the majority in the large bowel. Members of groups such as viruses, fungi and protozoa are also regularly found in healthy individuals, but form only a minor component of the total population of resident organisms.
The organisms occur in those parts of the body that are exposed to, or communicate with, the external environment, namely the skin, nose and mouth and intestinal and urinogenital tracts. The main organisms found in these sites are shown in Figure 8.1. Internal organs and tissues are normally sterile.
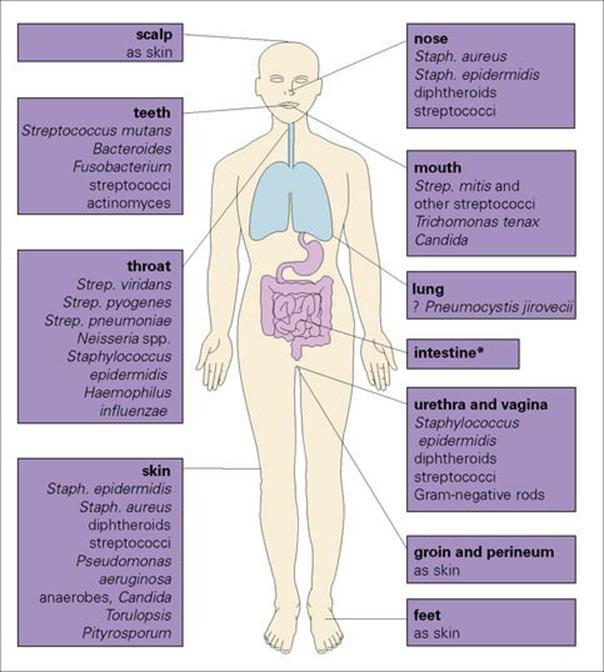
Figure 8.1 Examples of organisms that occur as members of the normal flora and their location on the body.
(*Those found in the intestine are detailed in Fig. 8.2.)
The normal flora is acquired rapidly during and shortly after birth and changes continuously throughout life
The organisms present at any given time reflect the age, nutrition and environment of the individual. It is therefore difficult to define the normal flora very precisely because it is to a large extent environmentally determined. This is well illustrated by data from NASA astronauts who were rendered relatively bacteriologically sterile by antibiotic treatment before their space flights. It took only 6 weeks after the flight for their flora to re-populate, and the re-populating species were precisely those of their immediate neighbours. The bowel flora of children in developing countries is quite different from that of children in developed countries. In addition, breast-fed infants have lactic acid streptococci and lactobacilli in their gastrointestinal tract, whereas bottle-fed children show a much greater variety of organisms.
Different regions of the skin support different flora
Exposed dry areas are not a good environment for bacteria and consequently have relatively few resident organisms on the surface, whereas moister areas (axillae, perineum, between the toes, scalp) support much larger populations. Staphylococcus epidermidis is one of the commonest species, making up some 90% of the aerobes and occurring in densities of 103–104/cm2; Staphylococcus aureus may be present in the moister regions.
Anaerobic diphtheroids occur below the skin surface in hair follicles, sweat and sebaceous glands, Propionibacterium acnes being a familiar example. Changes in the skin occurring during puberty often lead to increased numbers of this species, which can be associated with acne.
A number of fungi, including Candida, occur on the scalp and around the nails. They are infrequent on dry skin, but can cause infection in moist skinfolds (intertrigo).
Both the nose and mouth can be heavily colonized by bacteria
The majority of bacteria here are anaerobes. Common species colonizing these areas include streptococci, staphylococci, diphtheroids and Gram-negative cocci. Some of the aerobic bacteria found in healthy individuals are potentially pathogenic (e.g. Staph. aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Neisseria meningitidis); Candida is also a potential pathogen.
The mucous membranes of the mouth can have the same microbial density as the large intestine, numbers approaching 1011/g wet weight of tissue.
Dental caries is one of the most common infectious diseases in developed countries
The surfaces of the teeth and the gingival crevices carry large numbers of anaerobic bacteria. Plaque is a film of bacterial cells anchored in a polysaccharide matrix, which the organisms secrete. When teeth are not cleaned regularly, plaque can accumulate rapidly and the activities of certain bacteria, notably Streptococcus mutans, can lead to dental decay (caries), as acid fermented from carbohydrates can attack dental enamel. The prevalence of dental decay is linked to diet.
The pharynx and trachea carry their own normal flora
The flora of the pharynx and trachea may include both α- and β-haemolytic streptococci as well as a number of anaerobes, staphylococci (including Staph. aureus), Neisseria and diphtheroids. The respiratory tract is normally quite sterile, despite the regular intake of organisms by breathing. However, substantial numbers of clinically normal people may carry the fungus Pneumocystis jirovecii (previously known as P. carinii) in their lungs.
In the gut the density of microorganisms increases from the stomach to the large intestine
The stomach normally harbours only transient organisms, its acidic pH providing an effective barrier. However, the gastric mucosa may be colonized by acid-tolerant lactobacilli and streptococci. Helicobacter pylori, which can cause gastric ulcers (see Ch. 22), is carried without symptoms by large numbers of people, the bacterium being in mucus and neutralizing the local acidic environment. The upper intestine is only lightly colonized (104 organisms/g), but populations increase markedly in the ileum, where streptococci, lactobacilli, enterobacteriaceae and Bacteroides may all be present. Bacterial numbers are very high (estimated at 1011/g) in the large bowel, and many species can be found (Fig. 8.2). The vast majority (95–99%) are anaerobes, Bacteroides being especially common and a major component of faecal material; E. coli is also carried by most individuals. Bacteroides and E. coli are among the species capable of causing severe disease when transferred into other sites in the body. Harmless protozoans can also occur in the intestine (e.g. Entamoeba coli) and these can be considered as part of the normal flora, despite being animals.
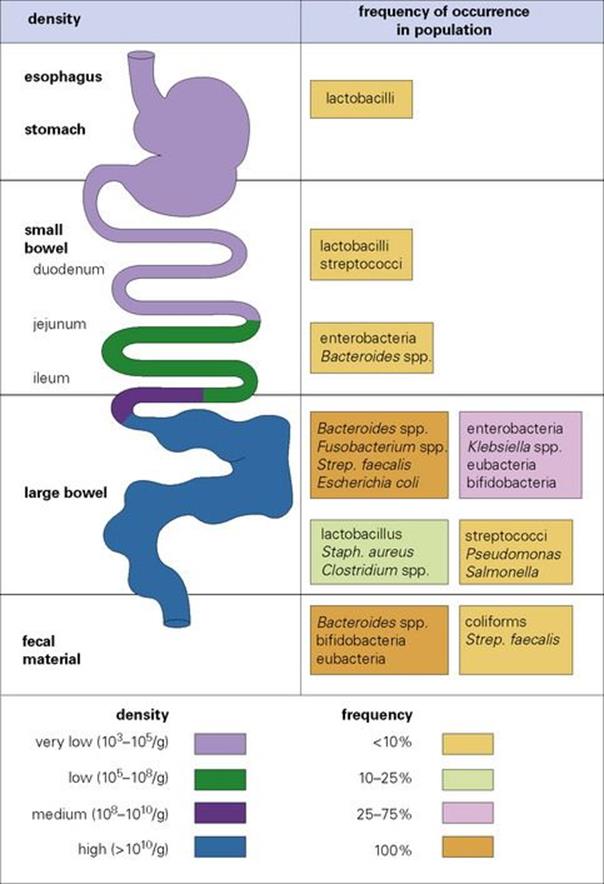
Figure 8.2 The longitudinal distribution, frequency of occurrence and densities of the bacteria making up the normal flora of the human gastrointestinal tract.
The urethra is lightly colonized in both sexes, but the vagina supports an extensive flora of bacteria and fungi
The urethra in both sexes is relatively lightly colonized, although Staph. epidermidis, Strep. faecalis and diphtheroids may be present. In the vagina, the composition of the bacterial and fungal flora undergoes age-related changes:
• Before puberty, the predominant organisms are staphylococci, streptococci, diphtheroids and E. coli.
• Subsequently, Lactobacillus aerophilus predominates, its fermentation of glycogen being responsible for the maintenance of an acid pH, which prevents overgrowth by other vaginal organisms.
A number of fungi occur, including Candida, which can overgrow to cause the pathogenic condition ‘thrush’ if the vaginal pH rises and competing bacteria diminish. The protozoan Trichomonas vaginalis may also be present in healthy individuals.
Advantages and disadvantages of the normal flora
Some of the species of the normal flora are positively beneficial to the host
The importance of these species for health is sometimes revealed quite dramatically under stringent antibiotic therapy. This can drastically reduce their numbers to a minimum, and the host may then be over-run by introduced pathogens or by overgrowth of organisms normally present in small numbers. After treatment with clindamycin, overgrowth by Clostridium difficile, which survives treatment, can give rise to antibiotic-associated diarrhea or, more seriously, pseudomembranous colitis.
Ways in which the normal flora prevents colonization by potential pathogens include the following:
• Skin bacteria produce fatty acids, which discourage other species from invading.
• Gut bacteria release a number of factors with antibacterial activity (bacteriocins, colicins) as well as metabolic waste products that help prevent the establishment of other species.
• Vaginal lactobacilli maintain an acid environment, which suppresses growth of other organisms.
• The sheer number of bacteria present in the normal flora of the intestine means that almost all of the available ecologic niches become occupied; these species therefore out-compete others for living space.
Gut bacteria also release organic acids, which may have some metabolic value to the host; they also produce B vitamins and vitamin K in amounts that are large enough to be valuable if the diet is deficient. The antigenic stimulation provided by the intestinal flora helps to ensure the normal development of the immune system.
What happens when the normal flora is absent?
Germ-free animals tend to live longer, presumably because of the complete absence of pathogens, and develop no caries (see Ch. 18). However, their immune system is less well developed and they are vulnerable to introduced microbial pathogens. At the time of birth, humans are germ free, but acquire the normal flora during and immediately after birth, with the accompaniment of intense immunologic activity.
The disadvantages of the normal flora lie in the potential for spread into previously sterile parts of the body
This may happen:
• when the intestine is perforated or the skin is broken
• during extraction of teeth (when Streptococcus viridans may enter the bloodstream)
• when organisms from the perianal skin ascend the urethra and cause urinary tract infection.
Members of the normal flora are important causes of hospital-acquired infection when patients are exposed to invasive treatments. Patients suffering burns are also at risk.
Overgrowth by potentially pathogenic members of the normal flora can occur when the composition of the flora changes (e.g. after antibiotics) or when:
• the local environment changes (e.g. increases in stomach or vaginal pH)
• the immune system becomes ineffective (e.g. AIDS, clinical immunosuppression).
Under these conditions, the potential pathogens take advantage of the opportunity to increase their population size or invade tissues, so becoming harmful to the host. An account of diseases associated with such opportunistic infections is given in Chapter 30.
Symbiotic associations
All living animals are used as habitats by other organisms; none is exempt from such invasion – bacteria are invaded by viruses (bacteriophages) and protozoans have their own flora and fauna – for example, amoeba are natural hosts for Legionella pneumophila infection. As evolution has produced larger, more complex and better regulated bodies, it has increased the number and variety of habitats for other organisms to colonize. The most complex bodies, those of birds and mammals (including humans), provide the most diverse environments, and are the most heavily colonized. Relationships between two species – interspecies associations or symbiosis – are therefore a constant feature of all life.
As the normal flora demonstrates, disease is not the inevitable consequence of interspecies associations between humans and microbes. Many factors influence the outcome of a particular association, and organisms may be pathogenic in one situation but harmless in another. To understand the microbiologic basis of infectious disease, host–microbe associations that can be pathogenic need to be placed firmly in the context of other symbiotic relationships, such as commensalism or mutualism, where the outcome for the host does not normally involve any damage or disadvantage.
Commensalism, mutualism and parasitism are categories of symbiotic association
All associations in which one species lives in or on the body of another can be grouped under the general term ‘symbiosis’ (literally ‘living together’). Symbiosis has no overtones of benefit or harm and includes a wide diversity of relationships. Attempts have been made to categorize types of association very specifically, but these have failed because all associations form part of a continuum (Fig. 8.3). Three broad categories of symbiosis – commensalism, mutualism and parasitism – can be identified on the basis of the relative benefit obtained by each partner. None of these categories of association is restricted to any particular taxonomic group. Indeed, some organisms can be commensal, mutualist or parasitic depending upon the circumstances in which they live (Fig. 8.4).
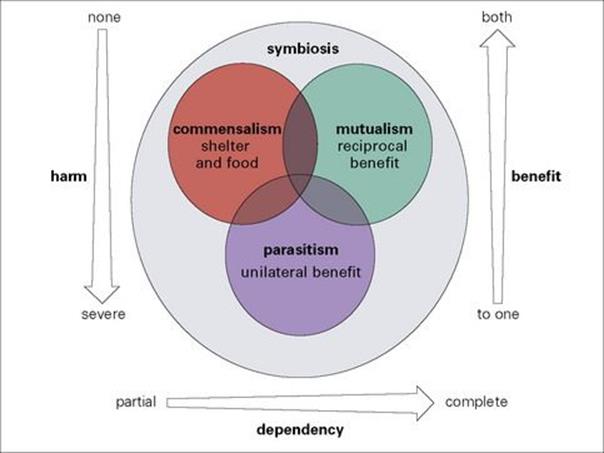
Figure 8.3 The relationships between symbiotic associations. Most species are independent of other species or rely on them only temporarily for food (e.g. predators and their prey). Some species form closer associations termed ‘symbioses’ and there are three major categories – commensalism, mutualism and parasitism – though each merges with the other and no definition separates one absolutely from the others.
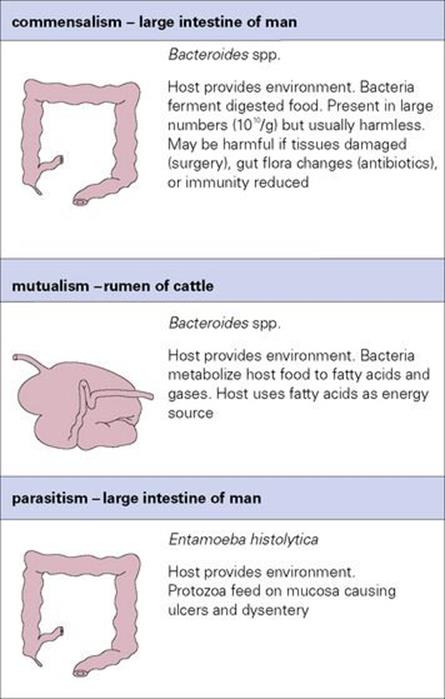
Figure 8.4 Examples of commensalism, mutualism and parasitism. These examples show how difficult it is to categorize any organism as entirely harmless, entirely beneficial or entirely harmful.
Commensalism
In commensalism, one species of organism lives harmlessly in or on the body of a larger species
At its simplest, a commensal association is one in which one species of organism uses the body of a larger species as its physical environment and may make use of that environment to acquire nutrients.
Like all animals, humans support an extensive commensal microbial flora on the skin, in the mouth and in the alimentary tract. The majority of these microbes are bacteria, and their relationship with the host may be highly specialized, with specific attachment mechanisms and precise environmental requirements. Normally, such microbes are harmless, but they can become harmful if their environmental conditions change in some way (e.g. Bacteroides, E. coli, Staphylococcus aureus). Conversely, commensal microbes can benefit the host by preventing colonization by more pathogenic species (e.g. the intestinal flora), an interaction which could also be considered mutualistic. Thus, the normal definition of commensalism is not very exact, as the association can merge into mutualism or parasitism.
Mutualism
Mutualistic relationships provide reciprocal benefits for the two organisms involved
Frequently, the relationship is obligatory for at least one member, and may be for both. Good examples are the bacteria and protozoa living in the stomachs of domestic ruminants, which play an essential role in the digestion and utilization of cellulose, receiving in return both the environment and the nutrition essential for their survival. The dividing line between commensalism and mutualism can be hard to draw. In humans, good health and resistance to colonization by pathogens can depend upon the integrity of the normal commensal enteric bacteria, many of which are highly specialized for life in the human intestine, but there is certainly no strict mutual dependence in this relationship.
Parasitism
In parasitism, the symbiotic relationship benefits only the parasite
The terms ‘parasites’ and ‘parasitism’ are sometimes thought to apply only to protozoans and worms, but all pathogens are parasites. Parasitism is a one-sided relationship in which the benefits go only to the parasite, the host providing parasites with their physicochemical environment, their food, respiratory and other metabolic needs, and even the signals that regulate their development. Although parasites are thought of as necessarily harmful, this is a view coloured by human and veterinary clinical medicine, and by the results of laboratory experimentation. In fact, many ‘parasites’ establish quite innocuous associations with their natural hosts but may become pathogenic if there are changes in the host’s health or they infect an unnatural host; the rabies virus, for example, coexists harmlessly with many wild mammals but can cause fatal disease in humans. This state of ‘balanced pathogenicity’ is sometimes explained as the outcome of selective pressures acting upon a relationship over a long period of evolutionary time. It may reflect selection of an increased level of genetically determined resistance in the host population and decreased pathogenicity in the parasite (as has happened with myxomatosis in rabbits). Alternatively, it may be the evolutionary norm, and ‘unbalanced pathogenicity’ may simply be the consequence of organisms becoming established in ‘unnatural’ (i.e. new) hosts. Thus, like the other categories of symbiosis, parasitism is impossible to define exclusively except in the context of clear-cut and highly pathogenic organisms. The belief that the ability to cause harm is a necessary characteristic of a parasite is difficult to sustain in any broader view (though it is a convenient assumption for those working with infectious diseases) and the reasons for this are discussed in more detail below.
The characteristics of parasitism
Many different groups of organisms are parasitic and all animals are parasitized
Parasitism as a way of life has been adopted by many different groups of organisms. Some groups, such as viruses, are exclusively parasitic (see below), but the majority include both parasitic and free-living representatives. Parasites occur in all animals, from the simplest to the most complex, and are an almost inevitable accompaniment of organized animal existence. We can see, then, that parasitism has been an evolutionary success; as a way of life, it must confer very considerable advantages.
Parasitism has metabolic, nutritional and reproductive advantages
The most obvious advantage of parasitism is metabolic. The parasite is provided with a variety of metabolic requirements by the host, often at no energy cost to itself, so it can devote a large proportion of its own resources to replication or reproduction. This one-sided metabolic relationship shows a broad spectrum of dependence, both within and between the various groups of parasites. Some parasites are totally dependent upon the host, while others are only partly dependent.
Viruses are completely dependent upon the host for all their metabolic needs
Viruses are at one extreme of the ‘parasite dependency’ spectrum. They are obligate parasites, possessing the genetic information required for production of new viruses, but none of the cellular machinery necessary to transcribe or translate this information, to assemble new virus particles or to produce the energy for these processes. The host provides not only the basic building blocks for the production of new viruses, but also the synthetic machinery and the energy required (Fig. 8.5). Retroviruses go one stage further in dependence, inserting their own genetic information into the host’s DNA in order to parasitize the transcription process. Viruses therefore represent the ultimate parasitic condition and are qualitatively different from all other parasites in the nature of their relationship with the host.
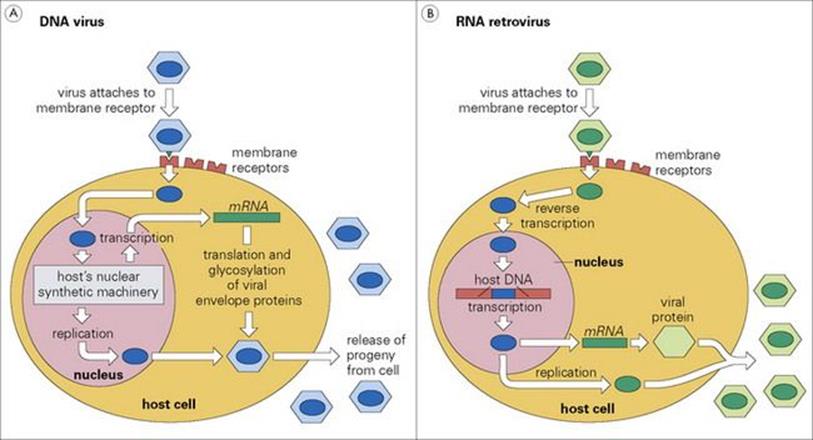
Figure 8.5 How DNA and RNA viruses invade and infect cells. (A) DNA viruses such as the herpes viruses have their own DNA, and use only the host’s cellular machinery to make more DNA and more virus protein and glycoprotein. These are then reassembled into new virus particles before they are released from the cell. (B) RNA retroviruses (e.g. HIV) first make viral DNA, using their reverse transcriptase, insert this DNA into the host’s genetic material so that viral RNA can be transcribed, and then translate some of the RNA into virus protein. The viral protein and RNA are then reassembled into new particles and released.
The basis for the fundamental difference between viruses and other parasites is the difference between virus organization and the cellular organization of prokaryotic and eukaryotic parasites. Non-viral parasites have their own genetic and cellular machinery, and multi-enzyme systems for independent metabolic activity and macromolecular synthesis. The degree of reliance on the host for nutritional requirements varies considerably and follows no consistent pattern between the various groups, nor does it follow that smaller parasites tend to be more dependent; e.g. some of the largest parasites, the tapeworms, are wholly reliant upon the host’s digestive machinery to provide their nutritional needs. All, of course, receive nutrition from the host but, whereas some use macromolecular material (proteins, polysaccharides) of host origin and digest it using their own enzyme systems, others rely on the host for the process of digestion as well, being able to take up only low molecular weight materials (amino acids, monosaccharides). Nutritional dependence may also include host provision of growth factors that the parasite is unable to synthesize itself. All internal parasites rely upon the host’s respiratory and transport systems to provide oxygen, although some respire anaerobically in either a facultative or obligate manner.
Parasite development can be controlled by the host
The advantage that parasitism confers in reproductive terms makes it vital to coordinate parasite development with the availability of suitable hosts. Indeed, one of the characteristic features of parasites is that their development may be controlled partly or completely by the host, the parasite having lost the ability to initiate or to regulate its own development. At its simplest, host control is limited to providing the cell surface molecules necessary for parasite attachment and internalization. Many parasites, from viruses to protozoa, rely on the recognition of such molecular signals for their entry into host cells, and this process provides the trigger for their replicative or reproductive cycles.
Other parasites, primarily the eukaryotes, require more comprehensive and sophisticated signals, often a complex of signals, to initiate and regulate their entire developmental cycle. The complexity of the signal required for development is one of the factors determining the specificity of the host–parasite relationship. Where the availability of one of the signals entails that parasite development can occur in only one species, host specificity is high. Where many host species are capable of providing the necessary signals for a parasite, specificity is low.
Disadvantages of parasitism
The most obvious disadvantage of parasitism arises from the fact that the host controls the development of the parasite. No development is possible without a suitable host, and many parasites will die if no host becomes available. For this reason, several adaptations have evolved to promote prolonged survival in the outside world and so maximize the chances of successful host contact (e.g. virus particles, bacterial spores, protozoan cysts and worm eggs). The prolific replication of parasites is another device to achieve the same end. Nevertheless, where parasites fail to make contact with a host, their powers of survival are ultimately limited. Adaptation to host signals can therefore have a reproductive cost (i.e. the loss of many potential parasites).
The evolution of parasitism
As so many organisms are parasitic and every group of animals is subject to invasion by parasites, the development of parasitism as a way of life must have occurred at an early stage in evolution and at frequent intervals thereafter. How this occurred is not fully understood, and it may well have been different in different groups of organisms. In many, parasitism most probably arose as a consequence of accidental contacts between organism and host. Of many such contacts, some would have resulted in prolonged survival and, under favourable nutritional circumstances, prolonged survival would have been associated with enhanced replication, giving the organism a selective advantage within the environment. Many parasites of humans and other mammals may have originated via the route of accidental contact, but it is clear that others have become adapted to these hosts after initially becoming parasitic in other species. For example, parasites of blood-feeding arthropods have ready access to the tissues of the animals on which the arthropods feed. Where the parasite becomes specialized for the non-arthropod host it may lose the ability to be transmitted by blood feeding. Where the arthropod host is retained in the life cycle the parasite is faced by competing demands for survival in each host, which probably explains why, for example, arboviruses are restricted to only a few families of RNA viruses and a single DNA virus, African swine fever virus.
Bacterial parasites evolved through accidental contact
In the case of bacteria, it is easy to see how accidental contact in environments rich in free-living bacteria could lead to successful invasion of external openings such as the mouth and eventual colonization of the gastrointestinal tract. Initially, the organisms concerned would have had to be facultative parasites, capable of life both within or outside host organisms (many pathogenic bacteria still have this property, e.g. Legionella, Vibrio), but selective pressures would have forced others into obligatory parasitism. Such events are of course speculative, but are supported by the close relationship of enteric bacteria such as E. coli with free-living photosynthetic purple bacteria.
Many bacterial parasites have evolved to live inside host cells
Bacteria that became parasitic by accidental contact would have lived outside host cells at first and would not have had the advantages of being intracellular. The evolution of the intracellular habit required further modifications to allow survival within host cells, but could easily have been initiated by passive phagocytic uptake. Subsequent survival of the microbe would depend upon the possession of surface or metabolic properties that prevented digestion and destruction by the host cell. The success of intracellular life can be measured not only by the large number of bacteria that have adopted this habit, but also by the extent to which some organisms have integrated their biology with that of the host cell. The endpoint of such integration is perhaps to be seen in the evolution of the eukaryote mitochondrion, which may have evolved from symbiotically associated heterotrophic purple bacteria (Fig. 8.6).
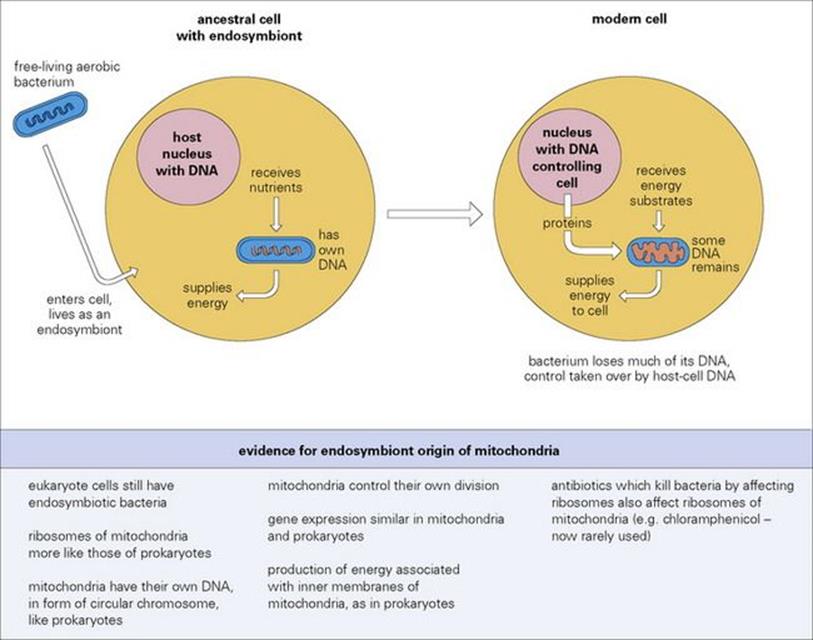
Figure 8.6 The evolution of mitochondria. Many lines of evidence suggest that mitochondria of modern eukaryote cells evolved from bacteria that established symbiotic (mutualistic) relationships with ancestral cells.
The pathway of virus evolution is uncertain
Clearly, parasitism by bacteria, which are undoubtedly ancient organisms (they can be traced back 3–5 billion years in the fossil record), depended upon the evolution of higher organisms to act as hosts. Whether the same is true of viruses is open to question, and depends upon whether viruses are considered primarily or secondarily simple. If viruses evolved from cellular ancestors by a process of secondary simplification, then parasitism must have evolved long after the evolution of prokaryotes and eukaryotes. If viruses are primitively non-cellular then it is possible that they became parasitic at a very early stage in the evolution of cellular life, at some point when, because of environmental change, independent existence became impossible. A third alternative is that viruses were never anything other than fragments of the nuclear material of other organisms and have in effect always been parasitic. Modern viruses may, in fact, have arisen by all three pathways.
Eukaryote parasites have evolved through accidental contact
The evolution of parasitism by eukaryotes is likely to have arisen much as it may have done in prokaryotes (i.e. through accidental contact and via blood-feeding arthropods). Examples can be found among protozoan and worm parasites to support this view:
• There are protozoa such as the free-living amoeba Naegleria which can opportunistically invade the human body and cause severe and sometimes fatal disease.
• There are several species of nematode worms that can live either as parasites or as free-living organisms, Strongyloides stercoralis being the most important in humans.
• It is likely that trypanosomes (the protozoans responsible for sleeping sickness) were primarily adapted as parasites of blood-feeding flies and only secondarily became established as parasites of mammals, though most retain the arthropod in their life cycle.
Parasite adaptations to overcome host inflammatory and immune responses
We can view the evolution of parasitism and the adaptations necessary for life within another animal as being exactly analogous to the adaptations necessary for life within any other specialized habitat: the environment in which parasites live is merely one of the many to which organisms have become adapted in evolution (comparable with life in soil, freshwater, salt water, decaying material and so on). However, it is always necessary to remember that in one major respect parasitism is quite different from any other specialist mode of life. This difference is that the environment in which a parasite lives, the body of the host, is not passive; on the contrary, it is capable of an active response to the presence of the parasite.
The attractiveness of animal bodies as environments for parasites means that hosts are under continual pressures from infection, and these pressures are increased when hosts live:
• close together
• in insanitary conditions
• in climates that favour the survival of parasite stages in the external world.
Pressure of infection has been a major influence in host evolution
Pressure of infection has been a major selective influence in evolution, and there is little doubt that it has been largely responsible for the development of the sophisticated inflammatory and immune responses we see in humans and other mammals. In evolutionary terms, all infection has its costs to the host because it diverts valuable resources from the activities of survival and reproduction; there has therefore been pressure to develop means of overcoming infection whether or not it causes disease. Of course, this is not the focus of clinical microbiology, which legitimately places emphasis on the costs of infection in terms of frank disease, but it should be remembered because it explains more fully the nature of the continuing battle between host and parasite – the former attempting to contain or destroy, the latter attempting to evade or suppress – and why the emergence of new, and the return of old, infectious diseases are a constant threat.
Parasites are faced not only with the problems of surviving within the environment they experience initially, but also of surviving in that environment as it changes in ways that are likely to be harmful to them. The inflammatory and immune responses that follow the establishment of infection are the most important means by which the host can control infections by those organisms able to penetrate its natural barriers and survive within its body. These responses represent formidable obstacles to the continued survival of parasites, forcing them to evolve strategies to cope with harmful changes in their environment. The successful parasite is therefore one that can cope with, or evade, the host’s response in one of the ways shown in Table 8.1.
Table 8.1 Evasion strategies of parasites
|
Evasion strategies |
|
|
Strategy |
Example |
|
Elicit minimal response |
Herpes simplex virus – survives in host cells for long periods in a latent stage – no pathology |
|
Evade effects of response |
Mycobacteria – survive unharmed in granulomas designed to localize and destroy infection |
|
Depress host’s response |
HIV – destroys T cells; malaria – depresses immune responsiveness |
|
Antigenic change |
Viruses, spirochaetes, trypanosomes – all change target antigens so host response is ineffective |
|
Rapid replication |
Viruses, bacteria, protozoa – producing acute infections before recovery and immunity |
|
Survival in weakly responsive individuals |
Genetic heterogeneity in host population means some individuals respond weakly or not at all, allowing organism to reproduce freely; examples in all groups |
All of these adaptations are known to exist within different groups of parasites and they are well documented in the case of some of the major human pathogens. Indeed, they are often the very reason why such organisms are major pathogens. Nevertheless, transmission and survival of many parasites depends upon the existence of particularly susceptible host individuals (e.g. children) to provide a continuing reservoir of infective stages.
Changes in parasites create new problems for hosts
From what has been said above, it can be appreciated that there is no such thing as a static host–parasite relationship, and that concepts of unchanging ‘pathogenicity’ or ‘lack of pathogenicity’ cannot be justified. Each relationship is an ‘arms race’; changes in one member being countered by changes in the other. Quite subtle changes in either can completely change the balance of the relationship, towards greater or lesser pathogenicity, for example.
Perhaps the most important recent illustration of this situation is the dramatic and explosive appearance of HIV infections. This group of viruses was originally restricted to non-human primates, but changes in the virus have permitted extensive infections in humans. Similarly, changes in an avian influenza virus allowing human infection resulted in the major pandemic early in the twentieth century and the recent emergence of the new H1N1 flu in 2009 is another example; there is also current concern about the potential spread of avian virus such as H5N1. Of a different nature, but relevant to the general theme, is the acquisition of drug resistance in bacteria and protozoa (Fig. 8.7). Although the underlying genetic and metabolic changes do not by themselves influence pathogenicity, the expression of such changes in the face of intense and selective chemotherapy certainly does, so allowing overwhelming infection to occur. The problem of hospital-acquired MRSA infection is a perfect example.
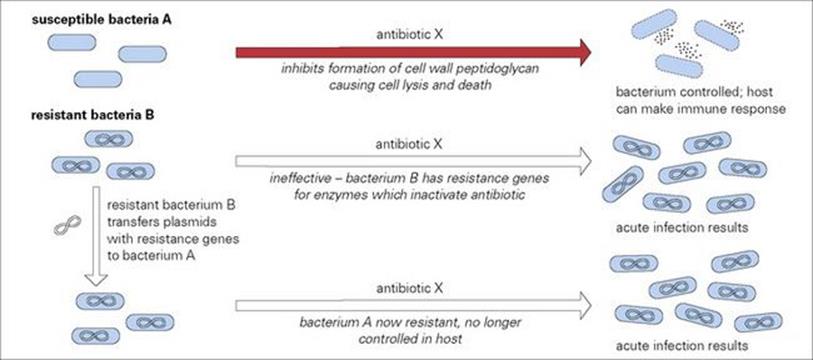
Figure 8.7 Antibiotic resistance in bacteria. The activity of many antibiotics can be blocked by bacterial enzymes coded for by genes located on cytoplasmic DNA in plasmids. The ability of bacteria to transfer plasmids between individual organisms means that strains or species previously susceptible to an antibiotic can acquire the ability to produce such enzymes and so gain antibiotic resistance directly from resistant organisms. These newly resistant forms are then differentially selected under antibiotic treatment, the susceptible individuals being deleted from the population. Primary antibiotic resistance also occurs through genetic mutations.
Host adaptations to overcome changes in parasites
Changes in the host can also alter the balance of a host–parasite relationship. A particularly dramatic example is the intense selection for resistant genotypes in rabbit populations exposed to the myxomatosis virus, which took place concurrently with selection for reduced pathogenicity in the virus itself (see Ch. 12). There are no exactly equivalent examples in humans, but in evolutionary time there have been major selective influences on populations prompting changes to permit survival in the face of life-threatening infections. A good example is the selective pressure exerted by Falciparum malaria, which has been responsible for the persistence in human populations of many alleles associated with haemoglobinopathies (e.g. sickle cell haemoglobin). Although these abnormalities are detrimental to varying degrees, they persist because they are (or were) associated with resistance to malarial infection. One study has suggested that malaria has also changed the frequency of certain HLA antigens in areas where infection was severe, although this has not been confirmed elsewhere.
Social and behavioural changes can be as important as genetic changes in altering host–parasite relations
Social and behavioural changes can alter host–parasite relations both positively and negatively (Table 8.2). Although many bacterial infections of the intestine have declined in importance with changes in human lifestyle, there are other contemporary microbiologic problems in the resource-rich world whose onset can be traced directly to sociologic, environmental and even medical change (Table 8.2). A particularly good example is disease arising from domestication of pets (e.g. toxoplasmosis) because it illustrates that human freedom from some infections arises primarily because of lack of contact with the organisms and not from any innate resistance to the establishment of the infection itself. Diseases arising from contact with infected animals or animal products (zoonotic infections) constitute a constant threat that can be realized by behavioural or environmental changes that alter established patterns of human–animal contact.
Table 8.2 Lifestyle changes and infectious diseases
|
Social and behavioral changes and infectious diseases |
|
|
The causes |
The results |
|
Altered environments (e.g. air conditioning) |
Water in cooling systems provides growth conditions for Legionella |
|
Changes in food production and food-handling practices |
Intensive husbandry under antibiotic protection leads to drug-resistant bacteria; deep-freeze, fast-food and inadequate cooking allow bacteria and toxins to enter body (e.g. Listeria, Salmonella) |
|
Routine use of antibiotics in medicine |
Emergence of antibiotic-resistant bacteria as hazards to hospitalized patients (e.g. MRSA – methicillin-resistant Staphylococcus aureus) |
|
Routine use of immunosuppressive therapy |
Development of opportunistic infections in patients with reduced resistance (e.g. Pseudomonas, Candida, Pneumocystis) |
|
Altered sexual habits |
Promiscuity increases sexually transmitted diseases (e.g. gonorrhoea, genital herpes, AIDS) |
|
Breakdown of filtration systems, overuse of limited water supplies |
Transmission of animal infections leading to diarrheal and other infections (e.g. cryptosporidiosis, giardiasis, leptospirosis) |
|
Increase in ownership of pets, particularly exotic species |
Transmission of animal infections (e.g. Chlamydia, Salmonella, Toxoplasma, Toxocara) |
|
Increased frequency of journeys to tropical and subtropical countries |
Exposure to exotic organisms and vectors (e.g. malaria, viral encephalitides) |
![]()
 Key Facts
Key Facts
• The body is colonized by many organisms (the normal flora) which can be positively beneficial. They live on or within the body without causing disease, and play an important role in protecting the host from pathogenic microbes.
• The normal flora is predominantly made up of bacteria, but includes fungi and protozoa.
• Members of the normal flora can be harmful if they enter previously sterile parts of the body. They can be important causes of hospital-acquired infections.
• The usual relationship between the normal flora and the body is an example of beneficial symbiosis; parasitism (in the broad sense, covering all pathogenic microbes) is a harmful symbiosis.
• The biological context of host–parasite relationships, and the dynamics of the conflict between two species in this relationship, provide a basis for understanding the causes and control of infectious diseases.
• Changes in medical practice, in human behaviour and, not least, in infectious organisms, are broadening the spectrum of organisms responsible for disease.
![]()