Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 5
Big Idea #3: Chemical Reactions, Energy Changes, and Redox Reactions
OXIDATION-REDUCTION REACTIONS
In an oxidation-reduction (or redox, for short) reaction, electrons are exchanged by the reactants, and the oxidation states of some of the reactants are changed over the course of the reaction. Look at the following reaction:
Fe + 2 HCl → FeCl2 + H2
The oxidation state of Fe changes from 0 to +2.
The oxidation state of H changes from +1 to 0.
· When an atom gains electrons, its oxidation number decreases, and it is said to have been reduced.
In the reaction above, H was reduced.
· When an atom loses electrons, its oxidation number increases, and it is said to have been oxidized.
Here’s a mnemonic device that might be useful.
LEO the lion says GER
LEO: you Lose Electrons in Oxidation
GER: you Gain Electrons in Reduction
In the reaction above, Fe was oxidized.
Oxidation and reduction go hand in hand. If one atom is losing electrons, another atom must be gaining them.
An oxidation-reduction reaction can be written as two half-reactions: one for the reduction and one for the oxidation. For example, the reaction
Fe + 2HCl → FeCl2 + H2
can be written as
|
Fe → Fe2+ + 2e− |
Oxidation |
|
2H+ + 2e− → H2 |
Reduction |
Another helpful mnemonic
for oxidation-reduction
reactions is OIL RIG.
Oxidation Is Loss
Reduction Is Gain
Reduction Potentials
Every half-reaction has an electric potential, or voltage, associated with it. You will be given the necessary values for the standard reduction potential of half-reactions for any question in which they are required. Potentials are always given as reduction half-reactions, but you can read them in reverse and flip the sign on the voltage to get oxidation potentials.
Look at the reduction potential for Zn2+.
|
Zn2+ + 2e− → Zn(s) |
E° = −0.76 V |
Read the reduction half-reaction in reverse and change the sign on the voltage to get the oxidation potential for Zn.
|
Zn → Zn2+ + 2e− |
E° = +0.76 V |
The larger the potential for a half-reaction, the more likely it is to occur.
|
F2(g) + 2e− → 2 F− |
E° = +2.87 V |
F2( g) has a very large reduction potential, so it is likely to gain electrons and be reduced.
|
Li(s) → Li+ + e− |
E° = +3.05 V |
Li(s) has a very large oxidation potential, making it very likely to lose electrons and be oxidized.
You can calculate the potential of a redox reaction if you know the potentials for the two half-reactions that constitute it. There are two important things to remember when calculating the potential of a redox reaction.
· Add the potential for the oxidation half-reaction to the potential for the reduction half-reaction.
· Never multiply the potential for a half-reaction by a coefficient.
Let’s look at the following reaction:
Zn + 2Ag+ → Zn2+ + 2Ag(s)
The two half-reactions are
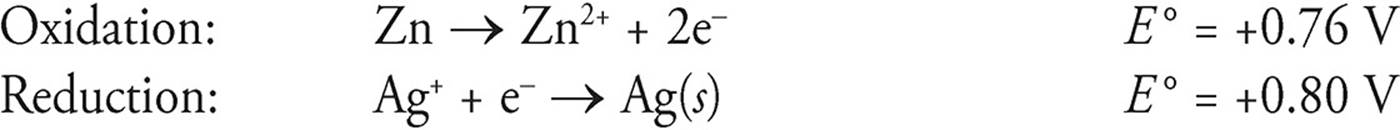
E = Eoxidation + Ereduction
E = 0.76 V + 0.80 V = 1.56 V
Notice that we ignored that silver has a coefficient of 2 in the balanced equation.
The relative reduction strengths of two different metals can also be determined qualitatively. In the above reaction, if Zn(s) were placed in a solution containing Ag+ ions, the silver ions have a high enough reduction potential that they would take electrons from the zinc and start forming solid silver, which would precipitate out on the surface of the zinc.
However, if Ag(s) were placed in a solution containing Zn2+ ions, zinc does not have a high enough reduction potential to take electrons from silver and so no reaction would occur. So, when a solid metal is placed into a metallic solution and a new solid starts to form, the reduction potential of the metal in solution is greater than that of the solid. If no solid forms, the reduction potential of the solid metal is higher.