Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 8
Big Idea #6: Equilibrium, Acids and Bases, Titrations, and Solubility
LE CHÂTELIER’S PRINCIPLE
At equilibrium, the rates of the forward and reverse reactions are equal. A “shift” in a certain direction means the rate of the forward or reverse reaction increases. Le Châtelier’s Principle states that whenever a stress is placed on a system at equilibrium, the system will shift in response to that stress. If the forward rate increases, we say the reaction has shifted right, which will create more products. If the reverse rate increases, we say the reaction has shifted left, which will create more reactants.
Let’s use the Haber process, which is used in the industrial preparation of ammonia, as an example.
N2(g)+3H2(g) 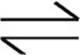 2NH3(g) ∆H°=−92.6 kJ/molrxn
2NH3(g) ∆H°=−92.6 kJ/molrxn
Concentration
When the concentration of a reactant or product is increased, the reaction will shift in the direction that allows it to use up the added substance. If N2 or H2 is added, the reaction shifts right. If NH3 is added, the reaction shifts left.
When the concentration of a species is decreased, the reaction will shift in the direction that allows it to create the substance that has been removed. If N2 or H2 is removed, the reaction shifts left. If NH3 is removed, the reaction shifts right.
Pressure
When the external pressure is increased, the reaction will shift to the side with fewer gas molecules. When the external pressure is decreased, the reaction will shift to the side with more gas molecules. In this case, an increase in pressure would cause a shift to the right, and a decrease in pressure would cause a shift to the left.
A common way to cause a pressure shift on a reaction is to change the volume of the container that the reaction is occurring in. A decrease in volume will lead to an increase in pressure (provided temperature remains constant), and thus cause a shift to the side with fewer gas molecules. If there is no gas involved in the reaction, or if both sides of the equilibrium have the same number of moles of gas, then changing the pressure and/or volume has no effect on the reaction.
Temperature
There’s a trick to figure out what happens when the temperature changes. First, rewrite the equation to include the heat energy on the side that it would be present on. The Haber process is exothermic, so heat is generated:
N2(g)+3H2(g)↔2NH3(g)+energy
Then, if the temperature goes up, the reaction will proceed in the backward direction (shifting away from the added energy). If the temperature goes down, the reaction will proceed in the forward direction (creating more energy). The reverse would be true in an endothermic reaction, as the energy would be part of the reactants.