Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT VI: Storage and Expression of Genetic Information
Chapter 32. Regulation of Gene Expression
I. OVERVIEW
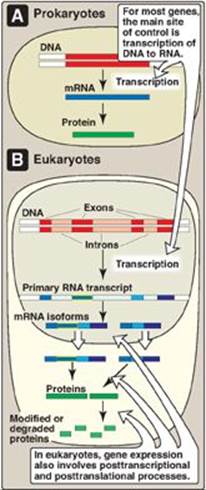
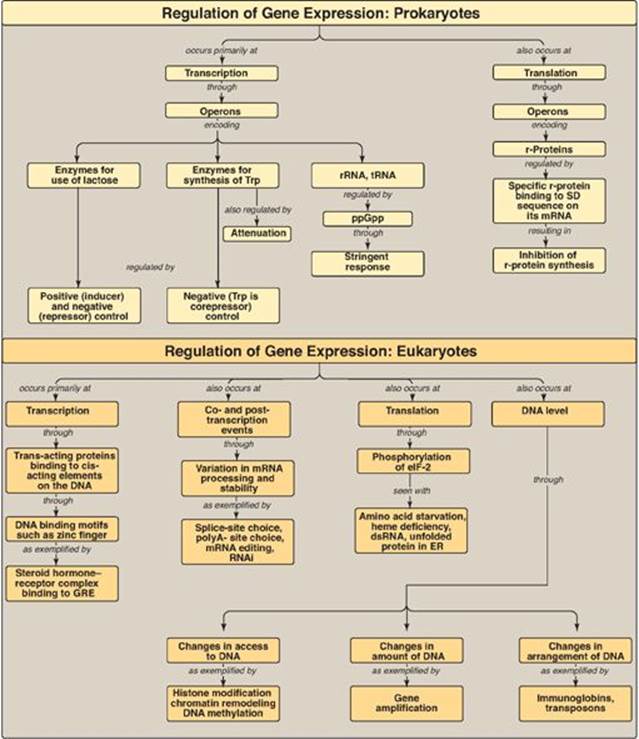
Gene expression refers to the multistep process that ultimately results in the production of a functional gene product, either ribonucleic acid (RNA) or protein. The first step in gene expression, the use of deoxyribonucleic acid (DNA) for the synthesis of RNA (transcription), is the primary site of regulation in both prokaryotes and eukaryotes. In eukaryotes, however, gene expression also involves extensive posttranscriptional and posttranslational processes as well as actions that influence access to particular regions of the DNA. Each of these steps can be regulated to provide additional control over the kinds and amounts of functional products that are produced.
Not all genes are regulated. For example, genes described as constitutive encode products required for basic cellular functions and so are continually expressed. They are also known as “housekeeping” genes. Regulated genes, however, are expressed only under certain conditions. They may be expressed in all cells or in only a subset of cells, for example, hepatocytes. The ability to regulate gene expression (that is, to determine if, how much, and when particular gene products will be made) gives the cell control over structure and function. It is the basis for cellular differentiation, morphogenesis, and adaptability of any organism. Control of gene expression is best understood in prokaryotes, but many themes are repeated in eukaryotes. Figure 32.1 lists some common strategies employed in gene regulation.
Figure 32.1 Control of gene expression. mRNA = messenger RNA.
II. REGULATORY SEQUENCES AND MOLECULES
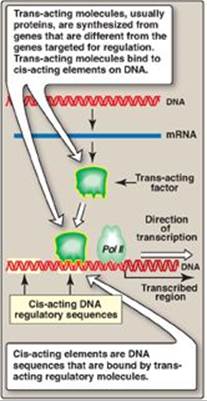
Regulation of transcription, the initial step in all gene expression, is controlled by regulatory sequences of DNA, usually embedded in the noncoding regions of the genome. The interaction between these DNA segments and regulatory molecules, such as transcription factors, can engage or repress the transcriptional machinery, influencing the kinds and amounts of products that are produced. These DNA sequences flanking a gene are called cis-acting because they influence expression of genes only on the same chromosome (see p. 423). A trans-acting factor is the regulatory molecule itself, which can transit (diffuse) through the cell from its site of synthesis to its DNA-binding site (Figure 32.2). For example, a protein transcription factor (a trans-acting molecule) that regulates a gene on chromosome 6 might itself have been produced from a gene on chromosome 11. The binding of proteins to DNA is through structural motifs such as the zinc finger (Figure 32.3), leucine zipper, or helix-turn-helix in the protein. [Note: Some trans-acting factors can negatively affect gene expression.]
Figure 32.2 Cis-acting elements and trans-acting molecules. mRNA = messenger RNA; Pol II = RNA polymerase II.
III. REGULATION OF PROKARYOTIC GENE EXPRESSION
In prokaryotes such as Escherichia coli (E. coli), regulation of gene expression occurs primarily at the level of transcription and, in general, is mediated by the binding of trans-acting proteins to cis-acting regulatory elements on their single DNA molecule (chromosome). [Note: Regulating the first step in the expression of a gene is an efficient approach, insofar as energy is not wasted making unneeded gene products.] Transcriptional control in prokaryotes can involve the initiation or premature termination of transcription.
A. Transcription of messenger RNA from bacterial operons
In bacteria, the structural genes that code for proteins involved in a particular metabolic pathway are often found sequentially grouped on the chromosome along with the cis-acting regulatory elements that determine the transcription of these genes. The transcription product is a single polycistronic messenger RNA (mRNA) (see p. 418). The genes are, thus, coordinately controlled (that is, turned on or off as a unit). This entire package is referred to as an operon.
B. Role of operators in prokaryotic transcription
Prokaryotic operons contain an operator, a segment of DNA that regulates the activity of the structural genes of the operon. If the operator is not bound by a repressor molecule, RNA polymerase passes over the operator and reaches the protein-coding genes which it transcribes to mRNA. If a repressor molecule is bound to the operator, the polymerase is blocked and does not produce mRNA. As long as the repressor is bound to the operator, no proteins are made. However, when an inducer molecule is present, it binds to the repressor, causing the repressor to change shape so that it no longer binds the operator. When this happens, the RNA polymerase can proceed with transcription. One of the best-understood examples is the inducible lactose operon of E. coli that illustrates both positive and negative regulation (Figure 32.4).
C. The lactose operon
The lactose (lac) operon contains the genes that code for three proteins involved in the catabolism of the disaccharide lactose: The lacZ gene codes for β-galactosidase, which hydrolyzes lactose to galactose and glucose; the lacYgene codes for a permease, which facilitates the movement of lactose into the cell; and the lacA gene codes for thiogalactoside transacetylase, which acetylates lactose. [Note: The physiologic function of this acetylation is unknown.] All of these proteins are maximally produced only when lactose is available to the cell and glucose is not. [Note: Bacteria use glucose, if available, as a fuel in preference to any other sugar.] The regulatory portion of the operon is upstream of the three structural genes and consists of the promoter region where RNA polymerase binds and two additional sites, the operator (O) site and the CAP site, where regulatory proteins bind. The lacZ, lacY, and lacA genes are expressed only when the O site is empty, and the CAP site is bound by a complex of cyclic adenosine monophosphate ([cAMP] see p. 94) and the catabolite activator protein (CAP), sometimes called the cAMP regulatory protein (CRP). A regulatory gene, the lacI gene, codes for the repressor protein (a trans-acting factor) that binds to the O site with high affinity. [Note: The lacI gene has its own promoter.]
Figure 32.3 Zinc (Zn) finger is a common motif in proteins that bind DNA. Cys = cysteine; His = histidine.
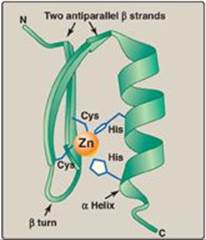
Figure 32.4 The lactose operon of E. coli. *[Note: Even when the operon has been turned off by catabolite repression, the repressor transiently dissociates from the operator at a slow rate, allowing a very low level of expression. The synthesis of a few molecules of permease (and β-galactosidase) allows the organism to respond rapidly should glucose become unavailable.] CAP = catabolite activator protein; cAMP = cyclic adenosine monophosphate; mRNA = messenger RNA.
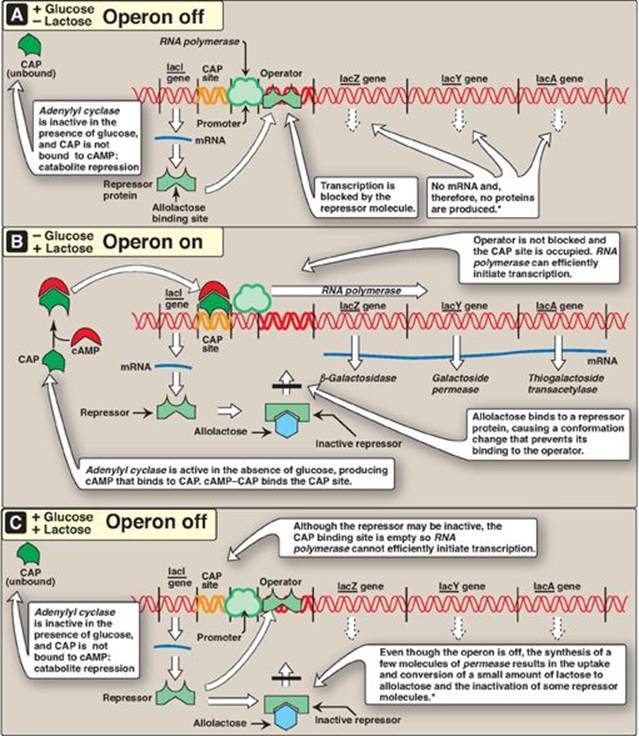
1. When only glucose is available: In this case, the lac operon is repressed (turned off). Repression is mediated by the repressor protein binding via a helix-turn-helix motif (Figure 32.5) to the operator site, which is downstream of the promoter region (see Figure 32.4A). Binding of the repressor interferes with the progress of RNA polymerase and blocks transcription of the structural genes. This is an example of negative regulation.
2. When only lactose is available: In this case, the lac operon is induced (maximally expressed, or turned on). A small amount of lactose is converted to an isomer, allolactose. This compound is an inducer that binds to the repressor protein, changing its conformation so that it can no longer bind to the operator. In the absence of glucose, adenylyl cyclase is active, and sufficient quantities of cAMP are made and bind to the CAP protein. The cAMP–CAP trans-acting complex binds to the CAP site, causing RNA polymerase to more efficiently initiate transcription at the promoter site (see Figure 32.4B). This is an example of positive regulation. The transcript is a single polycistronic mRNA molecule that contains three sets of start and stop codons. Translation of the mRNA produces the three proteins that allow lactose to be used for energy production by the cell. [Note: In contrast to the inducible lacZ, lacY, and lacA genes, whose expression is regulated, the lacI gene is constitutive. Its gene product, the repressor protein, is always made and is active unless the inducer is present.]
3. When both glucose and lactose are available: In this case, transcription of the lac operon is negligible, even if lactose is present at a high concentration. Adenylyl cyclase is inhibited in the presence of glucose (a process known as catabolite repression) so no cAMP–CAP complex forms, and the CAP site remains empty. RNA polymerase is, therefore, unable to effectively initiate transcription, even though the repressor may not be bound to the operator region. Consequently, the three structural genes of the operon are not expressed (see Figure 32.4C).
Figure 32.5 Helix-turn-helix motif of the lac repressor protein.
D. Tryptophan operon
The tryptophan (trp) operon contains five structural genes that code for enzymes required for the synthesis of the amino acid, tryptophan. As with the lac operon, the trp operon is subject to negative control. However, for the repressible trp operon, negative control includes Trp itself binding to a repressor protein and facilitating the binding of the repressor to the operator: Trp is a corepressor. Because repression by Trp is not always complete, unlike the lac operon, the trp operon is also regulated by a process known as attenuation. With attenuation, transcription is initiated but is terminated well before completion (Figure 32.6). If Trp is plentiful, transcription initiation that escaped repression by Trp is attenuated (stopped) by the formation at the 5ʹ-end of the mRNA of a hairpin (stem–loop) structure like that seen in rho-independent termination (see p. 421). [Note: Transcription and translation are temporally linked in prokaryotes (see p. 438), and, therefore, attenuation also results in the formation of a truncated, nonfunctional peptide product that is rapidly degraded.] If Trp becomes scarce, the operon is expressed. The 5ʹ-end of the mRNA contains two adjacent codons for Trp. The lack of Trp causes ribosomes to stall at these codons, covering regions of the mRNA required for formation of the attenuation hairpin. This prevents attenuation and allows transcription to continue.
Figure 32.6 Attenuation of transcription of the trp operon when tryptophan is plentiful. mRNA = messenger RNA.
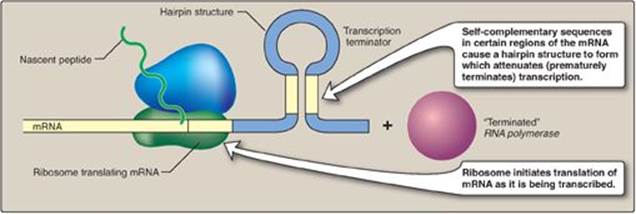
Transcriptional attenuation can occur in prokaryotes because translation of an mRNA begins before its synthesis is complete. This does not occur in eukaryotes because the presence of a membrane-bound nucleus spatially and temporally separates transcription and translation.
E. Coordination of transcription and translation in prokaryotes
Whereas transcriptional regulation of mRNA production is primary in bacteria, regulation at the level of ribosomal RNA (rRNA) and protein synthesis also occurs and plays important roles in the microbe’s ability to adapt to environmental stress.
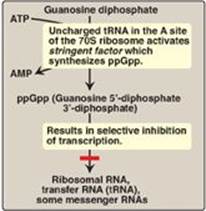
1. Stringent response: E. coli has seven operons that synthesize the rRNA needed for ribosome assembly, and each is regulated in response to changes in environmental conditions. Regulation in response to amino acid starvation is known as the stringent response. The binding of an uncharged transfer RNA (tRNA) to the A site of a ribosome (see p. 436) triggers a series of events that leads to the production of a polyphosphorylated guanosine, ppGpp. The synthesis of this unusual derivative of guanosine diphosphate (GDP) is catalyzed by stringent factor (RelA), an enzyme physically associated with ribosomes. Elevated levels of ppGpp result in inhibition of rRNA synthesis (Figure 32.7). [Note: In addition to rRNA, tRNA synthesis and some mRNA synthesis (for example, for ribosomal proteins) are also inhibited. However, synthesis of mRNAs for enzymes required for amino acid biosynthesis is not inhibited. ppGpp appears to alter promoter selection through use of different sigma-factors for RNA polymerase (see p.419.]
Figure 32.7 Regulation of transcription by the stringent response to amino acid starvation. S = Svedberg unit.
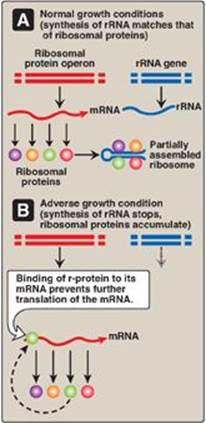
2. Regulatory ribosomal proteins: Operons for ribosomal proteins (r-proteins) can be inhibited by an excess of their own protein products. For each operon, one specific r-protein functions in the repression of translation of the polycistronic mRNA from that operon (Figure 32.8). The r-protein does so by binding to the Shine-Dalgarno (SD) sequence located on the mRNA just upstream of the first initiating AUG codon (see p. 439) and acting as a physical impediment to the binding of the small ribosomal subunit to the SD sequence. One r-protein thus inhibits synthesis of all the r-proteins of the operon. This same r-protein also binds to rRNA and with a higher affinity than for mRNA. If the concentration of rRNA falls, the r-protein then is available to bind its own mRNA and inhibit its translation. This coordinated regulation keeps the synthesis of r-proteins in balance with the transcription of rRNA, so that each is present in appropriate amounts for the formation of ribosomes.
Figure 32.8 Regulation of translation by an excess of ribosomal proteins. mRNA = messenger RNA; rRNA = ribosomal RNA; r-protein = ribosomal protein.
IV. REGULATION OF EUKARYOTIC GENE EXPRESSION
The higher degree of complexity of eukaryotic genomes, as well as the presence of a nuclear membrane, necessitates a wider range of regulatory processes. As with the prokaryotes, the primary site of regulation is at the level of transcription. Again, the theme of trans-acting molecules binding to cis-acting elements is seen. Operons, however, are generally not found in eukaryotes, which must use alternative strategies to solve the problem of how to coordinately regulate all the genes required for a specific response. In eukaryotes, gene expression is also regulated at multiple levels other than transcription. For example, the major modes of posttranscriptional regulation at the mRNA level are alternative mRNA splicing, control of mRNA stability, and control of translational efficiency. Additional regulation at the protein level occurs by mechanisms that modulate stability, processing, or targeting of the protein.
Figure 32.9 Combinatorial control of transcription.
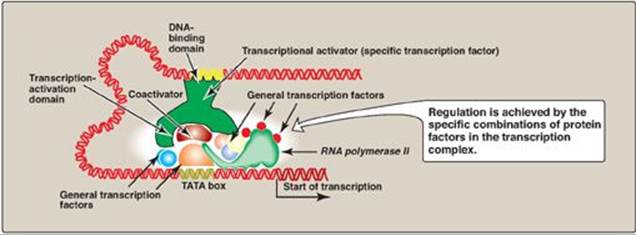
A. Trans-acting molecules
Specific transcription factors are trans-acting DNA-binding proteins that function as transcriptional activators. They have at least two binding domains: the DNA-binding domain, and the transcription-activation domain. The DNA-binding domain contains specific structural motifs, such as zinc fingers (see p. 450), that bind consensus sequences in DNA. The transcription-activation domain recruits other proteins, such as the general transcription factors ([GTFs] see p.423) and coactivators (for example, histone acetyltransferases [HATs]; see p. 422). These facilitate formation of the transcription initiation complex (RNA polymerase II plus the GTFs) at the promoter, and, thus, activate transcription (Figure 32.9). Regulation is achieved by the formation of a multiprotein complex bound to DNA, with protein–protein and protein–DNA interactions controlling assembly of the complex. Although activation domains recruit a variety of proteins, the specific effect of any one of them is dependent upon the protein composition of the complex. This is known as combinatorial control. [Note: DNA-binding proteins can also inhibit transcription.]
B. Cis-acting regulatory elements
The need to coordinately regulate a group of genes to cause a particular response is of key importance in multicellular organisms including humans. An underlying theme occurs repeatedly: A protein binds to a regulatory consensus element on each of the genes in the group and coordinately affects the expression of those genes, even if they are on different chromosomes. For example, hormone-response elements (HREs) are cis-acting DNA sequences that bind trans-acting protein factors and regulate gene expression in response to hormonal signals. In general, hormones bind either to intracellular receptors (steroid hormones are an example; see p. 240) or to cell-surface receptors (the peptide hormone glucagon is an example; see p. 314).
Figure 32.10 Transcriptional regulation by intracellular steroid hormone receptors. GRE = glucocorticoid-response element (an example of a hormone-response element); GR = glucocorticoid receptor.
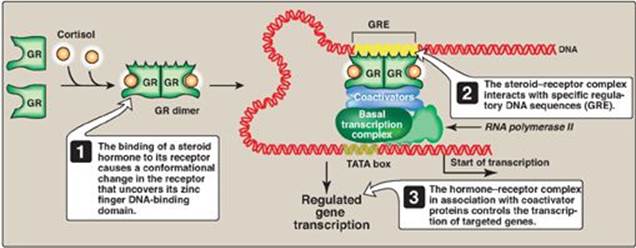
1. Regulatory signals mediated by intracellular receptors: Members of the nuclear receptor superfamily, which includes the steroid hormone (glucocorticoids, mineralocorticoids, androgens, and estrogens), vitamin D, retinoic acid, and thyroid hormone receptors, all directly influence gene expression by functioning as specific transcription factors. These receptors, therefore, contain a DNA-binding domain and an activation domain. They also contain a ligand-binding domain. For example, steroid hormones such as cortisol (a glucocorticoid) bind to soluble, intracellular receptors at the ligand-binding domain (Figure 32.10). Binding causes a conformational change in the receptor that activates it. The receptor–ligand complex enters the nucleus, dimerizes, and binds via a zinc finger motif to nuclear DNA at a cis-acting regulatory element, the glucocorticoid-response element (GRE), an example of an HRE. Binding allows recruitement of coactivators to the activation domain and results in increased expression of cortisol-responsive genes, each of which is under the control of its own GRE. Binding of the receptor–hormone complex to the GRE allows coordinate expression of a group of target genes, even when these genes are located on different chromosomes. The GRE can be located upstream or downstream of the genes it regulates and is able to function at great distances from those genes. The GRE, then, can function as a true enhancer (see p. 424). [Note: If associated with corepressors, hormone–receptor complexes inhibit transcription.]
2. Regulatory signals mediated by cell-surface receptors: Cell-surface receptors include those for insulin, epinephrine, and glucagon. Glucagon, for example, is a peptide hormone that binds its G protein–coupled plasma membrane receptor on glucagon-responsive cells. This extracellular signal is then transduced to intracellular cAMP (Figure 32.11; also see Figure 8.7 on p. 95), which can affect protein expression (and activity) through protein kinase A–mediated phosphorylation. In response to a rise in cAMP, a trans-acting factor (cAMP response element–binding [CREB] protein) is phosphorylated and activated. Active CREB protein binds via a leucine zipper motif to a cis-acting regulatory element, the cAMP response element (CRE), resulting in transcription of target genes with CREs in their promoters. [Note: The genes for phosphoenolpyruvate carboxykinase and glucose 6-phosphatase, key enzymes of gluconeogenesis (see p. 117), are examples of genes upregulated by the cAMP/CRE/CREB system.]
Figure 32.11 Transcriptional regulation by receptors located in the cell membrane. [Note: Cyclic AMP activates protein kinase A that phosphorylates cAMP response element-binding (CREB) protein.] CRE = cAMP response element.
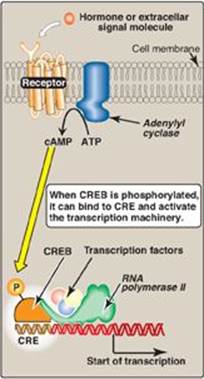
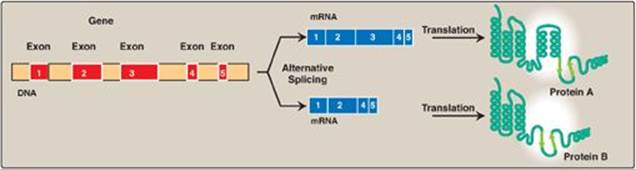
Figure 32.12 Tissue-specific alternative splicing produces multiple related proteins, or isoforms, from a single gene.
C. Regulation by processing of messenger RNA
Eukaryotic mRNA undergoes several modifications before it is exported from the nucleus to the cytoplasm for use in protein synthesis (see p. 418). Capping at the 5ʹ-end, polyadenylation at the 3ʹ-end, and splicing are essential processing events for the production of a functional eukaryotic messenger from most pre-mRNA (see p. 425), and variations in these events can affect gene expression. In addition, messenger stability also affects gene expression.
1. Splice-site choice: Tissue-specific protein isoforms can be made from the same pre-mRNA through differential cotranscriptional processing, particularly the use of alternative splice sites (Figure 32.12). For example, tropomyosin (TM) is an actin filament–binding protein that regulates the functions of actin in both muscle and nonmuscle cells. Its pre-mRNA undergoes tissue-specific differential splicing to yield a number of TM isoforms (see p. 427).
Over 60% percent of the approximately 25,000 genes in the human genome undergo differential splicing. The use of alternative polyadenylation and transcription start sites is also seen in many genes. This explains, at least in part, how 25,000 genes can give rise to hundreds of thousands of proteins.
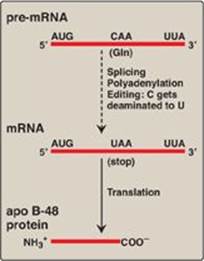
2. Messenger RNA editing: Even after mRNA has been fully processed, it may undergo additional posttranscriptional modification in which a base in the mRNA is altered. This is known as RNA editing. An important example in humans occurs with the transcript for apolipoprotein (apo) B, an essential component of chylomicrons (see p. 228) and very low density lipoproteins ([VLDL] see p. 231). Apo B mRNA is made in the liver and the small intestine. However, in the intestine only, the C residue in the CAA codon for glutamine is deaminated to U, changing the sense codon to a nonsense or stop codon (UAA), as shown in Figure 32.13. This results in a shorter protein (apo B-48, representing 48% of the message) being made in the intestine (and incorporated into chylomicrons) than is made in the liver (apo B-100, full-length, incorporated into VLDL).
Figure 32.13 RNA editing of apolipoprotein (apo) B in the intestine and generation of the apo B-48 protein needed for chylomicron synthesis. Gln = glutamine; mRNA = messenger RNA.
3. Messenger RNA stability: How long an mRNA remains in the cytosol before it is degraded influences how much protein product can be produced from it. Regulation of iron metabolism and the gene-silencing process of RNA interference illustrate the importance of mRNA stability in the regulation of gene expression.
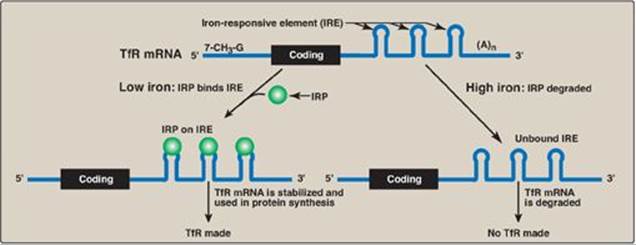
a. Iron metabolism: Transferrin is a plasma protein that transports iron. Transferrin binds to cell-surface receptors (transferrin receptors [TfRs]) that get internalized and provide cells, such as erythroblasts, with iron. The mRNA for the TfR has several cis-acting iron-responsive elements (IREs) at its 3-end. IREs have a short stem–loop structure that can be bound by trans-acting iron regulatory proteins ([IRPs] Figure 32.14). When the iron concentration in the cell is low, the IRPs bind to the 3-IREs and stabilize the mRNA for TfR, allowing TfR synthesis. When intracellular iron levels are high, the IRPs are degraded. The lack of IRPs bound to the mRNA hastens its destruction, resulting in decreased TfR synthesis. [Note: The mRNA for apoferritin, an intracellular protein of iron storage, has a single IRE at its 5ʹ-end. When iron levels in the cell are low, IRPs bind the 5ʹ-IRE and prevent the use of the mRNA, and less apoferritin is made. When iron accumulates in the cell, the IRP is degraded, allowing synthesis of apoferritin molecules to store the excess iron. ALAS2, the regulated enzyme of heme synthesis (see p. 279) in erythroblasts, also contains a 5-IRE.]
Figure 32.14 Regulation of transferrin receptor (TfR) synthesis. IRP = iron regulatory protein. [Note: The IREs are located in the 3ʹ UTR (untranslated region) of the TfR mRNA.]
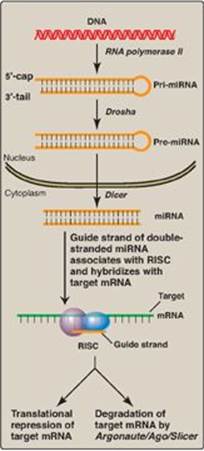
b. RNA interference: RNA interference (RNAi) is a mechanism of gene silencing through decreased expression of mRNA, either by repression of translation or by increased degradation. It is thought to play a key role in such fundamental processes as cell proliferation, differentiation, and apoptosis. RNAi is mediated by short (~22 bp), noncoding RNAs called microRNAs (miRNAs), which arise from far longer, genomically encoded nuclear transcripts, primary miRNA (pri-miRNA) that are partially processed in the nucleus to pre-miRNA by an endonuclease (Drosha) then transported to the cytoplasm. There, an endonuclease (Dicer) completes the processing and generates short, double-stranded miRNA. A single strand (the guide, or antisense strand) of the miRNA associates with a cytosolic protein complex known as the RNA-induced silencing complex (RISC). The guide strand hybridizes with a complementary sequence on a full length target mRNA, bringing RISC to the mRNA. This can result in repression of translation of the mRNA or its degradation by an endonuclease (Argonaute/Ago/Slicer) of the RISC. The extent of complementarity appears to be the determining factor (Figure 32.15). RNAi can also be triggered by double-stranded short interfering RNAs (siRNAs) introduced into a cell from exogenous sources. [Note: In vertebrates, the function of siRNAs that may arise from endogenous sources is unclear.]
Figure 32.15 Biogenesis and actions of miRNA. [Note: The extent of complementarity between the target messenger RNA (mRNA) and the microRNA (miRNA) determines the final outcome.] RISC = RNA-induced silencing complex.
1) RNA-interference–based therapeutics: Modulation of gene expression by providing siRNA to trigger RNAi has enormous therapeutic potential. The first clinical trial of RNAi-based therapy involved patients with the neovascular form of age-related macular degeneration (AMD), a leading form of adult blindness. Neovascular AMD is triggered by overproduction of vascular endothelial growth factor (VEGF), leading to the sprouting of excess blood vessels behind the retina. The vessels leak, clouding and often entirely destroying vision (therefore, neovascular AMD is also referred to as “wet” macular degeneration). An siRNA designed to target the mRNA of VEGF and promote its degradation went to clinical trials. Although considerable effort and resources have been expended to develop RNAi-based therapeutics, especially for the treatment of cancer, no products have gone from trials to the market. Development has been hindered by the problems of targeted delivery and stability. The use of nano-sized vectors such as liposomes may eliminate these issues. The research applications of RNAi, however, have grown rapidly.
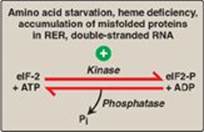
4. Translation of messenger RNA: Regulation of gene expression can also occur at the level of translation. One mechanism by which translation is regulated is through phosphorylation of the eukaryotic translation initiation factor, eIF-2 (Figure 32.16). Phosphorylation of eIF-2 inhibits its function and so inhibits translation at the initiation step (see p. 443). [Note: Phosphorylation of eIF-2 prevents its reactivation by inhibiting GDP–GTP exchange.] Phosphorylation is catalyzed by kinases that are activated in response to environmental conditions, such as amino acid starvation, heme deficiency in erythroblasts, the presence of double-stranded RNA (signaling viral infection), and the accumulation of misfolded proteins in the rough endoplasmic reticulum.
Figure 32.16 Regulation of translation initiation in eukaryotes by phosphorylation of eukaryotic translation initiation factor, eIF-2. RER = rough endoplasmic reticulum; ADP = adenosine diphosphate; Pi = inorganic phosphate.
D. Regulation through modifications to DNA
Gene expression in eukaryotes is also influenced by the availability of DNA to the transcriptional apparatus, the amount of DNA, and the arrangement of DNA. [Note: Localized transitions between the B and Z forms of DNA (see p. 398) can also affect gene expression.]
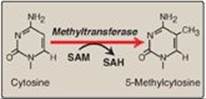
1. Access to DNA: In eukaryotes, DNA is found complexed with histone and nonhistone proteins to form chromatin (see p. 409). Transcriptionally active, decondensed chromatin (euchromatin) differs from the more condensed, inactive form (heterochromatin) in a number of ways. Active chromatin contains histone proteins that have been covalently modified at their amino terminal ends by acetylation or phosphorylation (see p. 422 for a discussion of histone acetylation/deacetylation by the histone acetyltransferase and histone deacetylase enzymes). Such modifications decrease the positive charge of these basic proteins, thereby decreasing the strength of their association with negatively charged DNA. This relaxes the nucleosome (see p. 409), allowing transcription factors access to specific regions on the DNA. Nucleosomes can also be repositioned, an ATP-requiring process called chromatin remodeling. Another difference between transcriptionally active and inactive chromatin is the extent of methylation of cytosine bases in CG-rich regions (CpG islands) in the promoter region of many genes. Methylation is by methyltransferases that use S-adenosylmethionine as the methyl donor (Figure 32.17). Transcriptionally active genes are less methylated (hypomethylated) than their inactive counterparts, suggesting that DNA hypermethylation silences gene expression. [Note: Modification of histones and methylation of DNA are epigenetic. They are heritable changes in DNA that alter gene expression without altering the base sequence.]
Figure 32.17 The methylation of cytosine in eukaryotic DNA. SAM = S-adenosylmethionine; SAH = S-adenosylhomocysteine.
2. Amount of DNA: A change up or down in the number of copies of a gene can affect the amount of gene product produced. An increase in copy number (gene amplification) has contributed to increased genomic complexity and is still a normal developmental process in certain nonmammalian species. In mammals, however, gene amplification is seen with some diseases and in response to particular chemotherapeutic drugs such as methotrexate, an inhibitor of the enzyme dihydrofolate reductase (DHFR), required for the synthesis of thymidine triphosphate (TTP) in the pyrimidine biosynthetic pathway (see p. 304). TTP is essential for DNA synthesis. Gene amplification results in an increase in the number of DHFR genes and resistance to the drug, allowing TTP to be made.
Figure 32.18 DNA rearrangements in the generation of immunoglobulins. V= variable; D = diversity; J = joining.
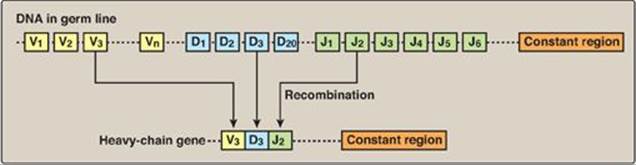
3. Arrangement of DNA: The process by which immunoglobulins (antibodies) are produced by B lymphocytes involves permanent rearrangements of the DNA in these cells. The immunoglobulins (for example, IgG) consist of two light and two heavy chains, with each chain containing regions of variable and constant amino acid sequence. The variable region is the result of somatic recombination of segments within both the light- and the heavy-chain genes. During B-lymphocyte development, single variable (V), diversity (D), and joining (J) gene segments are brought together through gene rearrangement to form a unique variable region (Figure 32.18). This process allows the generation of 109–1011 different immunoglobulins from a single gene, providing the diversity needed for the recognition of an enormous number of antigens. [Note: The shift from the membrane-bound form to the secreted form of immunoglobulins involves poly-A site choice (see p. 426).]
4. Mobile DNA elements: Transposons (Tns) are mobile segments of DNA that move in an essentially random manner from one site to another on the same or a different chromosome. Movement is mediated by transposase, an enzyme encoded by the Tn itself. Movement can be direct, in which transposase cuts out and then inserts the Tn at a new site, or replicative, in which the Tn is copied and the copy inserted elsewhere while the original remains in place. In eukaryotes, including humans, replicative transposition frequently involves an RNA intermediate, in which case the Tn is called a retrotransposon (see p. 408). Transposition has contributed to structural variation in the genome but also has the potential to alter gene expression and even to cause disease. Although the vast majority of retrotransposons in the human genome have lost the ability to move, some are still active. Their transposition is thought to be the basis for some rare cases of hemophilia A and Duchenne muscular dystrophy. [Note: The growing problem of antibiotic-resistant bacteria is a consequence, at least in part, of the exchange of plasmids among bacterial cells. If the plasmids contain Tns carrying antibiotic resistance genes, the recipient bacteria gain resistance to one or more antimicrobial drugs.]
V. CHAPTER SUMMARY
Gene expression results in the production of a functional gene product (either RNA or protein) through the processes of replication, transcription, and translation (Figure 32.19). Genes can be either constitutive (always expressed, housekeeping genes) or regulated (expressed only under certain conditions in all cells or in a subset of cells). The ability to appropriately express (positive regulation) or repress (negative regulation) genes is essential in all organisms. Regulation of gene expression occurs primarily at the level of transcription in both prokaryotes and eukaryotes and is mediated through the binding of trans-acting proteins to cis-acting regulatory elements on the DNA. In eukaryotes, regulation also occurs through modifications to the DNA as well as through posttranscriptional and posttranslational events. In prokaryotes, such as E. coli, the coordinate regulation of genes whose protein products are required for a particular process is achieved through operons (groups of genes sequentially arranged on the chromosome along with the regulatory elements that determine their transcription). The lac operon contains the Z, Y, and A structural genes, the protein products of which are needed for the catabolism of lactose. It is subject to negative and positive regulation. When glucose is available, the operon is repressed by the binding of the repressor protein (the product of the lacI gene) to the operator, thus preventing transcription. When only lactose is present, the operon is induced by an isomer of lactose (allolactose) that binds the repressor protein, preventing it from binding to the operator. In addition, cyclic AMP binds the catabolite-activator protein (CAP), and the complex binds the DNA at the CAP site. This increases promoter efficiency and results in the expression of the structural genes through the production of a polycistronic messenger RNA (mRNA). When both glucose and lactose are present, glucose prevents formation of cAMP and transcription of these genes is negligible. The trp operon contains genes needed for the synthesis of tryptophan (Trp), and, like the lac operon, it is regulated by negative control. Unlike the lac operon, it is also regulated by attenuation, in which mRNA synthesis that escaped repression by Trp is terminated before completion. Transcription of ribosomal RNA and transfer RNA is selectively inhibited in prokaryotes by the stringent response to amino acid starvation. Translation is also a site of prokaryotic gene regulation: Excess ribosomal proteins bind the Shine-Dalgarno sequence on their own polycistronic mRNA, preventing ribosomes from binding. Gene regulation is more complex in eukaryotes. Operons typically are not present, but coordinate regulation of the transcription of genes located on different chromosomes can be achieved through the binding of trans-acting proteins to cis-acting elements. In multicellular organisms, hormones can cause coordinated regulation, either through the binding of the hormone receptor–hormone complex to the DNA (as with steroid hormones) or through the binding of a protein that is activated in response to a second messenger (as with glucagon). In each case, binding to DNA is mediated through structural motifs such as the zinc finger. Co- and posttranscriptional regulation is also seen in eukaryotes and includes splice-site choice, polyA-site choice, mRNA editing, and variations in mRNA stability as seen with transferrin receptor synthesis and with RNA interference. Regulation at the translational level can be caused by the phosphorylation and inhibition of eukaryotic initiation factor, eIF-2. Gene expression in eukaryotes is also influenced by availability of DNA to the transcriptional apparatus, the amount of DNA, and the arrangement of the DNA. Epigenetic changes to histone proteins and DNA also influence gene expression.
Study Questions
Choose the one best answer.
32.1 Which of the following mutations is most likely to result in reduced expression of the lac operon?
A. cya– (no adenylyl cyclase made)
B. i– (no repressor protein made)
C. Oc (operator cannot bind repressor protein)
D. One resulting in functionally impaired glucose transport
Correct answer = A. In the absence of glucose, adenylyl cyclase makes cyclic AMP, which forms a complex with the catabolite activator protein (CAP). The cAMP–CAP complex binds the CAP site on the DNA, causing RNA polymerase to bind more efficiently to the lac operon promoter, thereby increasing expression of the operon. With cya– mutations, adenylyl cyclase is not made, and so the operon is unable to be turned on even when glucose is absent and lactose is present. The absence of a repressor protein or decreased ability of the repressor to bind the operator results in constitutive (constant) expression of the lac operon.
Figure 32.19 Summary of key concepts for the regulation of gene expression. GRE = glucocorticoid-response element; ppGpp = polyphosphorylated guanosine; r-protein = ribosomal protein; SD sequence = Shine-Dalgarno sequence; RNAi = RNA interference; eIF-2 = eukaryotic initiation factor 2.
32.2 Which of the following is best described as cis acting?
A. Cyclic AMP response element-binding protein
B. Operator
C. Repressor protein
D. Thyroid hormone nuclear receptor
Correct answer = B. The operator is part of the DNA itself, and so is cis acting. The cAMP response element-binding protein, repressor protein, and thyroid hormone nuclear receptor protein are molecules that transit to the DNA, bind, and affect the expression of that DNA and so are trans acting.
32.3 Which of the following is the basis for the intestine-specific expression of apolipoprotein B-48?
A. DNA rearrangement and loss
B. DNA transposition
C. RNA alternative splicing
D. RNA editing
E. RNA interference
Correct answer = D. The production of apolipoprotein (apo) B-48 in the intestine and apoB-100 in liver is the result of RNA editing in the intestine, where a sense codon is changed to a nonsense codon by posttranscriptional deamination of cytosine to uracil. DNA rearrangement and transposition, as well as RNA interference and alternate splicing, do alter gene expression but are not the basis of apoB-48 tissue-specific production.
32.4 Which of the following is most likely to be true in hemochromatosis, a disease of iron accumulation?
A. The mRNA for the transferrin receptor (TfR) is stabilized by the binding of iron regulatory proteins to 3 iron-responsive elements.
B. The mRNA for the transferrin receptor is not bound by iron regulatory proteins and is degraded.
C. The mRNA for apoferritin is not bound by iron regulatory proteins at its 5 iron-responsive element and is translated.
D. The mRNA for apoferritin is bound by iron regulatory proteins and is not translated.
E. Both B and C are correct.
Correct answer = E. When iron levels in the body are high, as is seen with hemochromatosis, there is increased synthesis of the iron-storage molecule, apoferritin, and decreased synthesis of the transferrin receptor (TfR) that mediates iron uptake by cells. These effects are the result of trans-acting iron regulatory proteins binding cis-acting iron-responsive elements, resulting in degradation of the mRNA for TfR, and increased translation of the mRNA for apoferritin.
32.5 Patients with estrogen receptor–positive (hormone responsive) breast cancer may be treated with the drug tamoxifen, which binds the estrogen nuclear receptor without activating it. Which of the following is the most logical outcome of tamoxifen use?
A. Increased acetylation of estrogen-responsive genes
B. Increased growth of estrogen receptor–positive breast cancer cells
C. Increased production of cyclic AMP
D. Inhibition of the estrogen operon
E. Inhibition of transcription of estrogen-responsive genes
Correct answer = E. Tamoxifen competes with estrogen for binding to the estrogen nuclear receptor. Tamoxifen fails to activate the receptor, preventing its binding to DNA sequences that upregulate expression of estrogen-responsive genes. Tamoxifen, then, blocks the growth-promoting effects of these genes and results in growth inhibition of estrogen-dependent breast cancer cells. Acetylation increases transcription by relaxing the nucleosome. Cyclic AMP is a regulatory signal mediated by cell-surface rather than nuclear receptors. Mammalian cells do not have operons.
32.6 The ZYA region of the lac operon will be maximally expressed if:
A. cyclic AMP levels are low.
B. glucose and lactose are both available.
C. the attenuation stem–loop is able to form.
D. the CAP site is occupied.
Correct answer = D. It is only when glucose is gone, cyclic AMP levels are increased, the cAMP–catabolite activator protein (CAP) complex is bound to the CAP site, and lactose is available that the operon is maximally expressed (induced). If glucose is present, the operon is off as a result of catabolite repression. The lac operon is not regulated by attenuation, a mechanism for decreasing transcription in some operons such as the tryptophan operon.
32.7 X chromosome inactivation is a process by which one of two X chromosomes in human females is condensed and inactivated to prevent overexpression of X-linked genes. What would most likely be true about the degree of DNA methylation and histone acetylation on the inactivated X chromosome?
Cytosines in CG islands would be hypermethylated and histone proteins would be deacetylated. Both conditions are associated with decreased gene expression, and both are important in maintaining X inactivation.