Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION II. Bioenergetics & the Metabolism of Carbohydrates & Lipids
Chapter 16. Overview of Metabolism & the Provision of Metabolic Fuels
David A. Bender, PhD & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Explain what is meant by anabolic, catabolic and amphibolic metabolic pathways.
Explain what is meant by anabolic, catabolic and amphibolic metabolic pathways.
![]() Describe in outline the metabolism of carbohydrates, lipids and amino acids at the level of tissues and organs, and at the subcellular level, and how metabolic fuels are interconvertible.
Describe in outline the metabolism of carbohydrates, lipids and amino acids at the level of tissues and organs, and at the subcellular level, and how metabolic fuels are interconvertible.
![]() Describe the ways in which flux of metabolites through metabolic pathways is regulated.
Describe the ways in which flux of metabolites through metabolic pathways is regulated.
![]() Describe how a supple of metabolic fuels is provided in both the fed and fasting states; the formation of metabolic fuels reserves in the fed state and their mobilization in fasting.
Describe how a supple of metabolic fuels is provided in both the fed and fasting states; the formation of metabolic fuels reserves in the fed state and their mobilization in fasting.
BIOMEDICAL IMPORTANCE
Metabolism is the term used to describe the interconversion of chemical compounds in the body, the pathways taken by individual molecules, their interrelationships, and the mechanisms that regulate the flow of metabolites through the pathways. Metabolic pathways fall into three categories. (1) Anabolic pathways, which are those involved in the synthesis of larger and more complex compounds from smaller precursors—for example, the synthesis of protein from amino acids and the synthesis of reserves of triacylglycerol and glycogen. Anabolic pathways are endothermic. (2) Catabolic pathways, which are involved in the breakdown of larger molecules, commonly involving oxidative reactions; they are exothermic, producing reducing equivalents, and, mainly via the respiratory chain, ATP. (3) Amphibolic pathways, which occur at the “crossroads” of metabolism, acting as links between the anabolic and catabolic pathways, for example, the citric acid cycle.
Knowledge of normal metabolism is essential for an understanding of abnormalities underlying disease. Normal metabolism includes adaptation to periods of starvation, exercise, pregnancy, and lactation. Abnormal metabolism may result from nutritional deficiency, enzyme deficiency, abnormal secretion of hormones, or the actions of drugs and toxins.
A 70-kg adult human being requires about 8-12 MJ (1920-2900 kcal) from metabolic fuels each day, depending on the physical activity. Larger animals require less, and smaller animals more, per kg body weight, and growing children and animals have a proportionally higher requirement to allow for the energy cost of growth. For human beings, this requirement is met from carbohydrates (40-60%), lipids (mainly triacylglycerol, 30-40%), and protein (10-15%), as well as alcohol. The mix of carbohydrate, lipid, and protein being oxidized varies, depending on whether the subject is in the fed or fasting state, and on the duration and intensity of physical work.
The requirement for metabolic fuels is relatively constant throughout the day, since the average physical activity increases metabolic rate only by about 40-50% over the basal or resting metabolic rate. However, most people consume their daily intake of metabolic fuels in two or three meals, so there is a need to form reserves of carbohydrate (glycogen in liver and muscle) and lipid (triacylglycerol in adipose tissue) in the period following a meal, for use during the intervening time when there is no intake of food.
If the intake of metabolic fuels is consistently greater than energy expenditure, the surplus is stored, largely as triacylglycerol in adipose tissue, leading to the development of obesity and its associated health hazards. By contrast, if the intake of metabolic fuels is consistently lower than energy expenditure, there are negligible reserves of fat and carbohydrate, and amino acids arising from protein turnover are used for energy-yielding metabolism rather than replacement protein synthesis, leading to emaciation, wasting, and, eventually, death (see Chapter 43).
In the fed state, after a meal, there is an ample supply of carbohydrate, and the metabolic fuel for most tissues is glucose. In the fasting state glucose must be spared for use by the central nervous system (which is largely dependent on glucose) and the red blood cells (which are wholly reliant on glucose). Therefore, tissues that can use fuels other than glucose do so; muscle and liver oxidize fatty acids and the liver synthesizes ketone bodies from fatty acids to export to muscle and other tissues. As glycogen reserves become depleted, amino acids arising from protein turnover are used for gluconeogenesis.
The formation and utilization of reserves of triacylglycerol and glycogen, and the extent to which tissues take up and oxidize glucose, are largely controlled by the hormones insulin and glucagon. In diabetes mellitus, there is either impaired synthesis and secretion of insulin (type I diabetes, sometimes called juvenile onset, or insulin-dependent diabetes) or impaired sensitivity of tissues to insulin action (type II diabetes, sometimes called adult onset or noninsulin-dependent diabetes), leading to severe metabolic derangement. In cattle, the demands of heavy lactation can lead to ketosis, as can the demands of twin pregnancy in sheep.
PATHWAYS THAT PROCESS THE MAJOR PRODUCTS OF DIGESTION
The nature of the diet sets the basic pattern of metabolism. There is a need to process the products of digestion of dietary carbohydrate, lipid, and protein. These are mainly glucose, fatty acids and glycerol, and amino acids, respectively. In ruminants (and, to a lesser extent, other herbivores), dietary cellulose is fermented by symbiotic microorganisms to short-chain fatty acids (acetic, propionic, butyric), and metabolism in these animals is adapted to use these fatty acids as major substrates. All the products of digestion are metabolized to a common product, acetyl-CoA, which is then oxidized by the citric acid cycle (Figure 16–1).
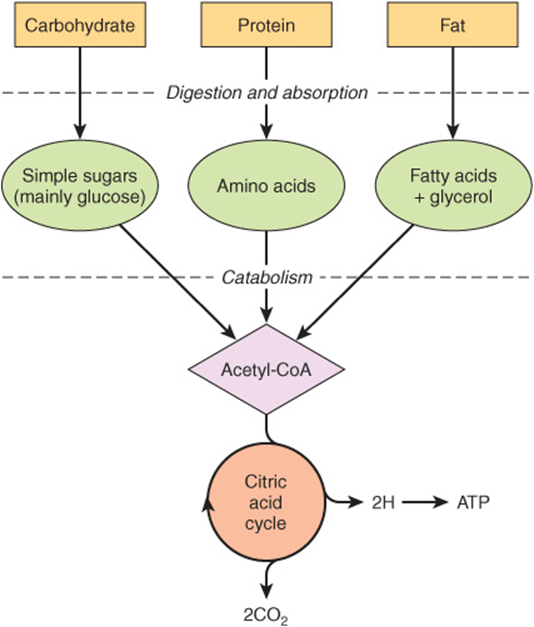
FIGURE 16–1 Outline of the pathways for the catabolism of dietary carbohydrate, protein, and fat. All the pathways lead to the production of acetyl-CoA, which is oxidized in the citric acid cycle, ultimately yielding ATP by the process of oxidative phosphorylation.
Carbohydrate Metabolism Is Centered on the Provision & Fate of Glucose
Glucose is the major fuel of most tissues (Figure 16–2). It is metabolized to pyruvate by the pathway of glycolysis. Aerobic tissues metabolize pyruvate to acetyl-CoA, which can enter the citric acid cycle for complete oxidation to CO2 and H2O, linked to the formation of ATP in the process of oxidative phosphorylation (Figure 13–2). Glycolysis can also occur anaerobically (in the absence of oxygen) when the end product is lactate.
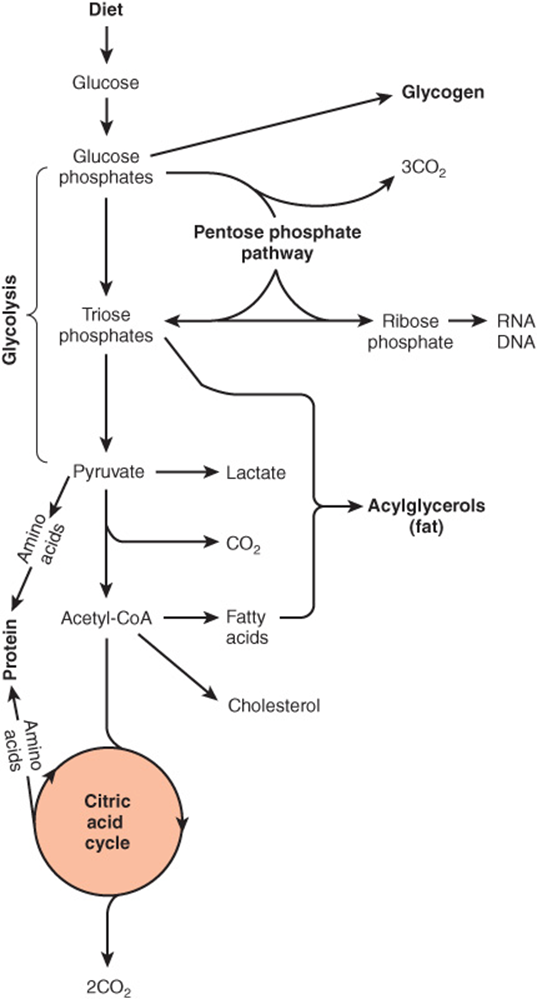
FIGURE 16–2 Overview of carbohydrate metabolism showing the major pathways and end products. Gluconeogenesis is not shown.
Glucose and its metabolites also take part in other processes, eg. (1) Synthesis of the storage polymer glycogen in skeletal muscle and liver. (2) The pentose phosphate pathway, an alternative to part of the pathway of glycolysis. It is a source of reducing equivalents (NADPH) for fatty acid synthesis and the source of ribose for nucleotide and nucleic acid synthesis. (3) Triose phosphates give rise to the glycerol moiety of triacylglycerols. (4) Pyruvate and intermediates of the citric acid cycle provide the carbon skeletons for the synthesis of nonessential or dispensable amino acids, and acetyl-CoA is the precursor of fatty acids and cholesterol (and hence of all steroids synthesized in the body). Gluconeogenesis is the process of forming glucose from noncarbohydrate precursors, for example, lactate, amino acids, and glycerol.
Lipid Metabolism Is Concerned Mainly with Fatty Acids & Cholesterol
The source of long-chain fatty acids is either dietary lipid or de novo synthesis from acetyl-CoA derived from carbohydrate or amino acids. Fatty acids may be oxidized to acetyl-CoA (β-oxidation) or esterified with glycerol, forming triacylglycerol (fat) as the body’s main fuel reserve.
Acetyl-CoA formed by β-oxidation may undergo three fates (Figure 16–3).
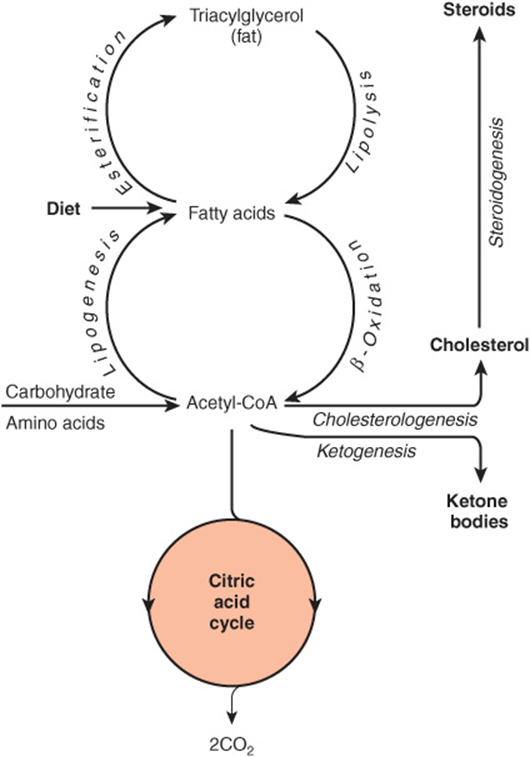
FIGURE 1–3 Overview of fatty acid metabolism showing the major pathways and end products. The ketone bodies are acetoacetate, 3-hydroxybutyrate, and acetone.
1. As with acetyl-CoA arising from glycolysis, it is oxidized to CO2 + H2O via the citric acid cycle.
2. It is the precursor for synthesis of cholesterol and other steroids.
3. In the liver, it is used to form ketone bodies (acetoacetate and 3-hydroxybutyrate), which are important fuels in prolonged fasting and starvation.
Much of Amino Acid Metabolism Involves Transamination
The amino acids are required for protein synthesis (Figure 16–4). Some must be supplied in the diet (the essential or indispensable amino acids), since they cannot be synthesized in the body. The remainder are nonessential or dispensable amino acids, which are supplied in the diet, but can also be formed from metabolic intermediates by transamination using the amino group from other amino acids. After deamination, amino nitrogen is excreted as urea, and the carbon skeletons that remain after transamination may (1) be oxidized to CO2 via the citric acid cycle, (2) be used to synthesize glucose (gluconeogenesis), or (3) form ketone bodies or acetyl CoA, which may be oxidized or be used for synthesis of fatty acids.
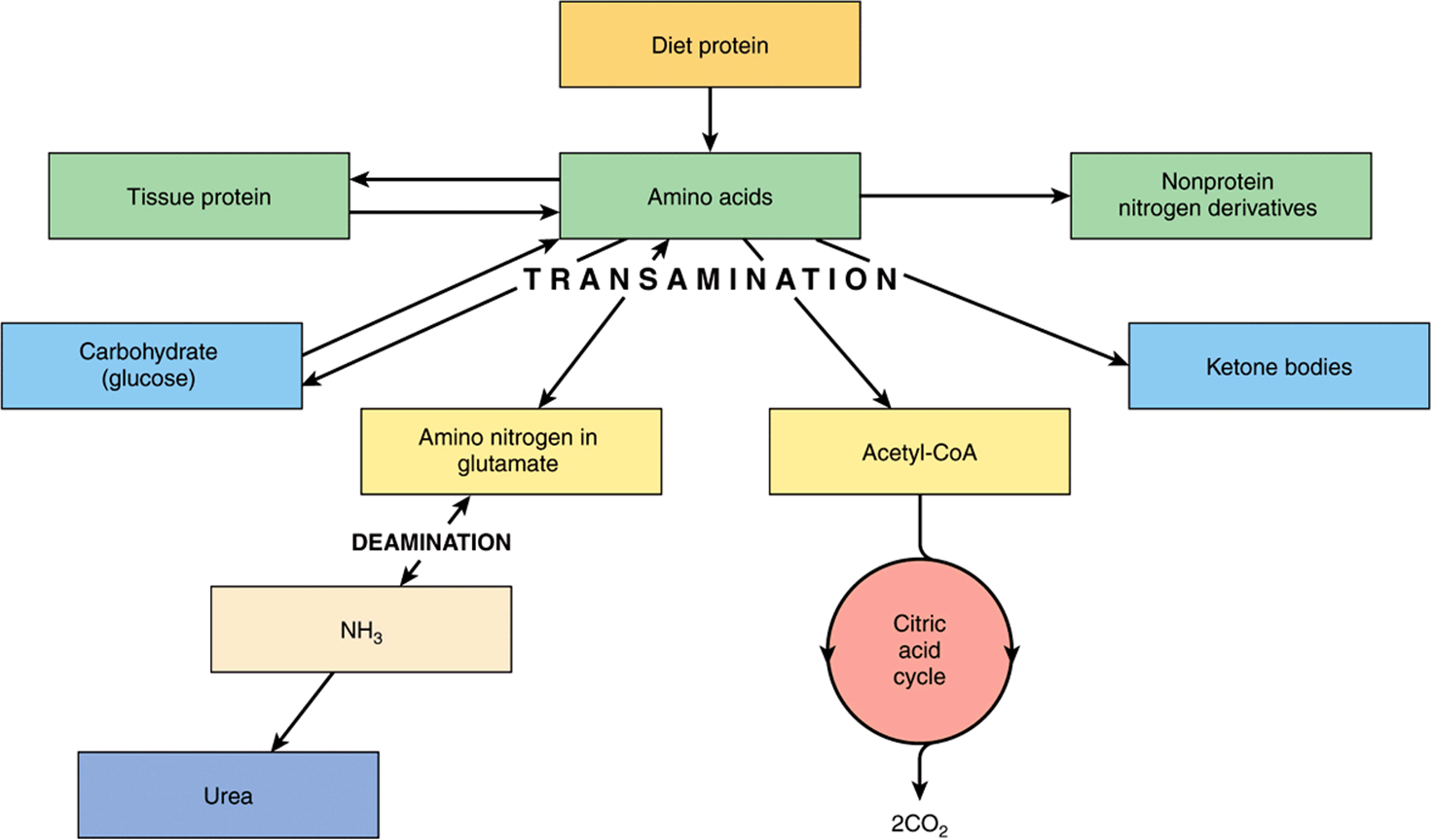
FIGURE 16–4 Overview of amino acid metabolism showing the major pathways and end products.
Several amino acids are also the precursors of other compounds, for example, purines, pyrimidines, hormones such as epinephrine and thyroxine, and neurotransmitters.
METABOLIC PATHWAYS MAY BE STUDIED AT DIFFERENT LEVELS OF ORGANIZATION
In addition to studies in the whole organism, the location and integration of metabolic pathways is revealed by studies at several levels of organization. (1) At the tissue and organ level the nature of the substrates entering and metabolites leaving tissues and organs is defined. (2) At the subcellular level each cell organelle (eg, the mitochondrion) or compartment (eg, the cytosol) has specific roles that form part of a subcellular pattern of metabolic pathways.
At the Tissue & Organ Level, the Blood Circulation Integrates Metabolism
Amino acids resulting from the digestion of dietary protein and glucose resulting from the digestion of carbohydrates are absorbed via the hepatic portal vein. The liver has the role of regulating the blood concentration of these water-soluble metabolites (Figure 16–5). In the case of glucose, this is achieved by taking up glucose in excess of immediate requirements and using it to synthesize glycogen (glycogenesis, Chapter 19) or to fatty acids (lipogenesis, Chapter 23). Between meals, the liver acts to maintain the blood glucose concentration by breaking down glycogen (glycogenolysis, Chapter 19) and, together with the kidney, by converting non-carbohydrate metabolites such as lactate, glycerol, and amino acids to glucose (gluconeogenesis, Chapter 20). The maintenance of an adequate concentration of blood glucose is vital for those tissues for which it is the major fuel (the brain) or the only fuel (erythrocytes). The liver also synthesizes the major plasma proteins (eg, albumin) and deaminates amino acids that are in excess of requirements, synthesizing urea, which is transported to the kidney and excreted (Chapter 28).
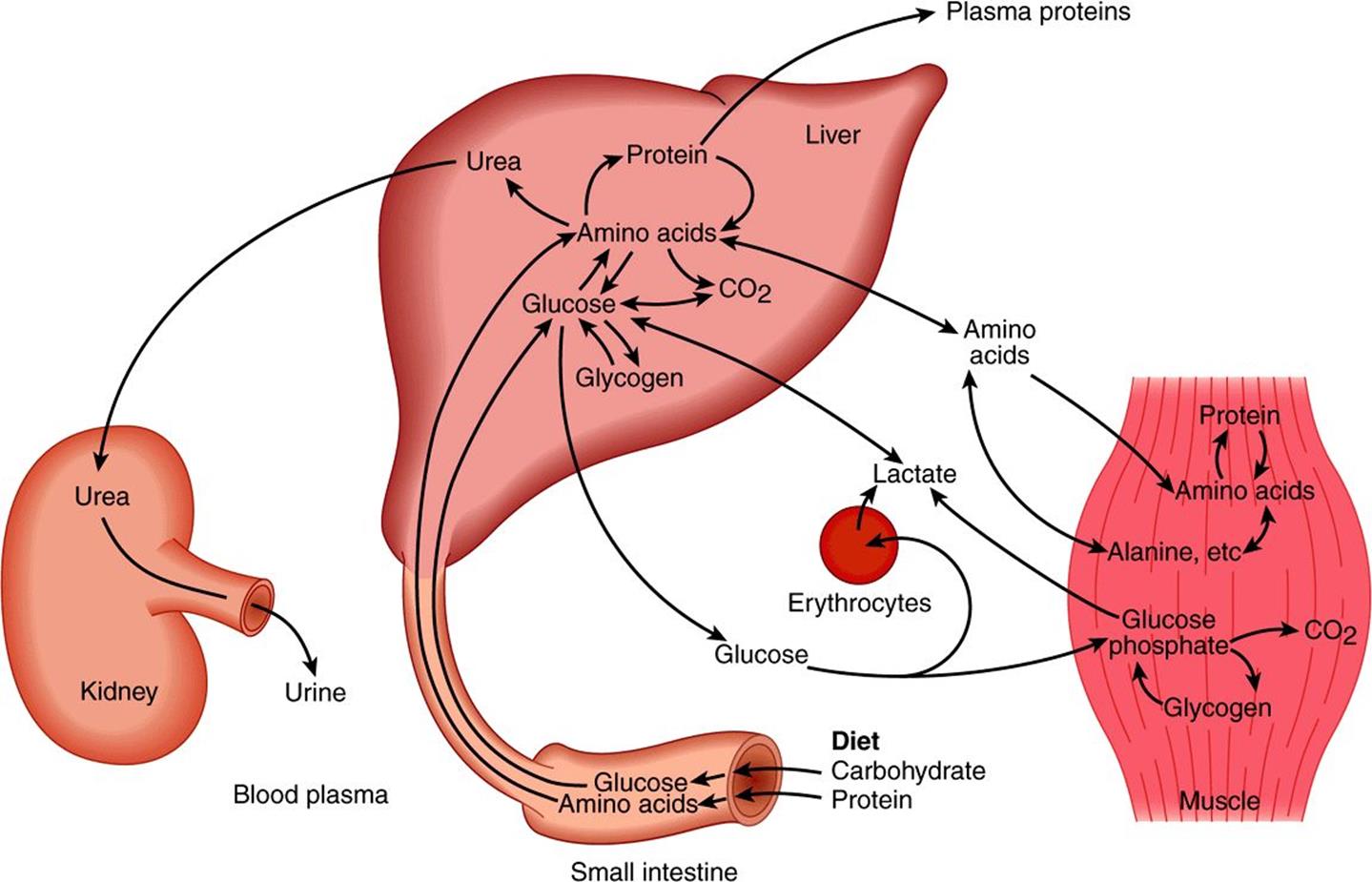
FIGURE 16–5 Transport and fate of major carbohydrate and amino acid substrates and metabolites. Note that there is little free glucose in muscle, since it is rapidly phosphorylated upon entry.
Skeletal muscle utilizes glucose as a fuel, both aerobically, forming CO2, and anaerobically, forming lactate. It stores glycogen as a fuel for use in muscle contraction and synthesizes muscle protein from plasma amino acids. Muscle accounts for approximately 50% of body mass and consequently represents a considerable store of protein that can be drawn upon to supply amino acids for gluconeogenesis in starvation (Chapter 20).
Lipids in the diet (Figure 16–6) are mainly triacylglycerol, and are hydrolyzed to monoacylglycerols and fatty acids in the gut, then re-esterified in the intestinal mucosa. Here they are packaged with protein and secreted into the lymphatic system and thence into the bloodstream as chylomicrons, the largest of the plasma lipoproteins. Chylomicrons also contain other lipid-soluble nutrients. Unlike glucose and amino acids, chylomicron triacylglycerol is not taken up directly by the liver. It is first metabolized by tissues that have lipoprotein lipase, which hydrolyzes the triacylglycerol, releasing fatty acids that are incorporated into tissue lipids or oxidized as fuel. The chylomicron remnants are cleared by the liver. The other major source of long-chain fatty acids is synthesis (lipogenesis) from carbohydrate, in adipose tissue and the liver.
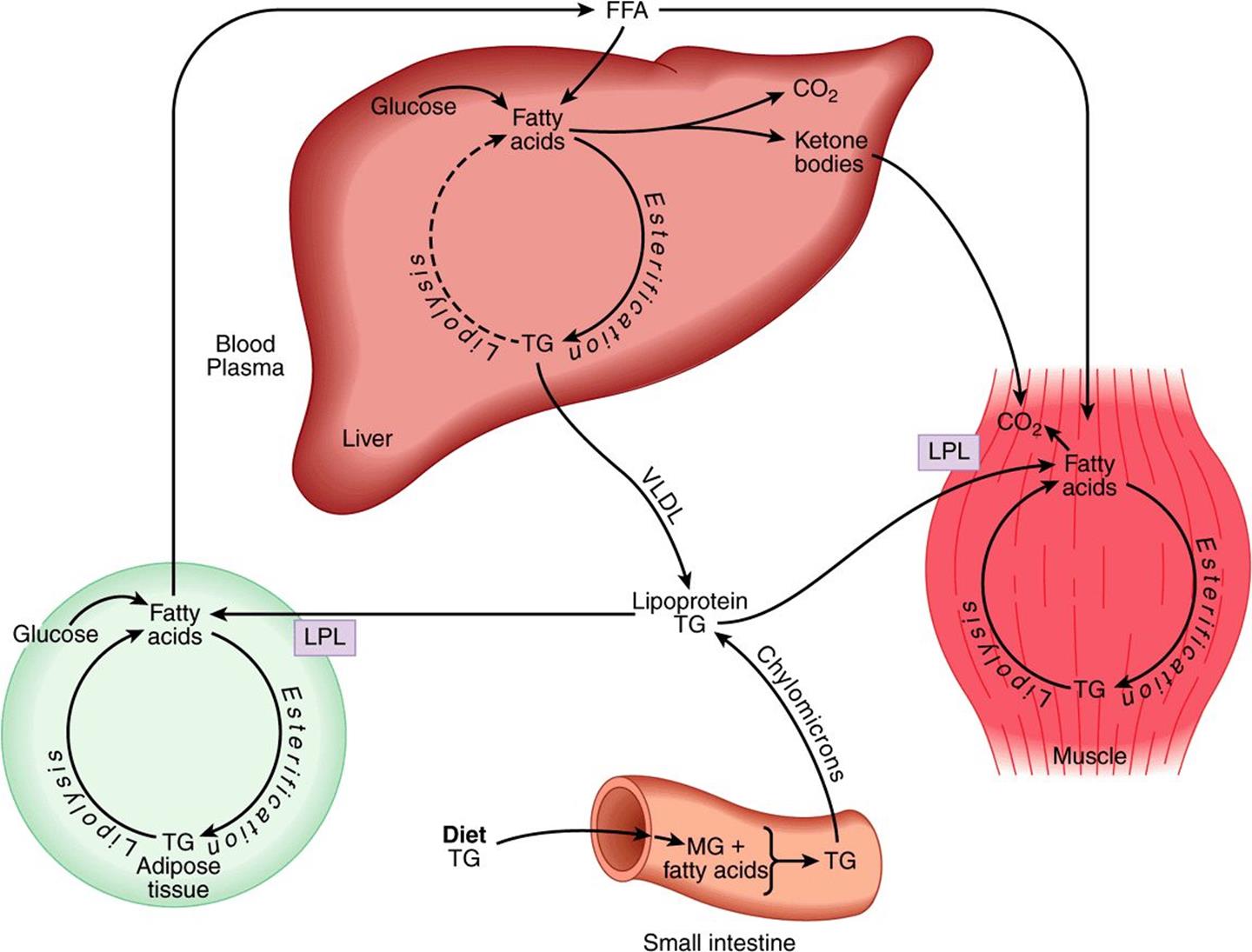
FIGURE 16–6 Transport and fate of major lipid substrates and metabolites. (FFA, free fatty acids; LPL, lipoprotein lipase; MG, monoacylglycerol; TG, triacylglycerol; VLDL, very low density lipoprotein.)
Adipose tissue triacylglycerol is the main fuel reserve of the body. It is hydrolyzed (lipolysis) and glycerol and free fatty acids are released into the circulation. Glycerol is a substrate for gluconeogenesis. The fatty acids are transported bound to serum albumin; they are taken up by most tissues (but not brain or erythrocytes) and either esterified to triacylglycerols for storage or oxidized as a fuel. In the liver, newly synthesized triacylglycerol and triacylglycerol from chylomicron remnants (see Figure 25–3) is secreted into the circulation in very low density lipoprotein (VLDL). This triacylglycerol undergoes a fate similar to that of chylomicrons. Partial oxidation of fatty acids in the liver leads to ketone body production (ketogenesis, Chapter 22). Ketone bodies are exported to extrahepatic tissues, where they act as a fuel in prolonged fasting and starvation.
At the Subcellular Level, Glycolysis Occurs in the Cytosol & the Citric Acid Cycle in the Mitochondria
Compartmentation of pathways in separate subcellular compartments or organelles permits integration and regulation of metabolism. Not all pathways are of equal importance in all cells. Figure 16–7 depicts the subcellular compartmentation of metabolic pathways in a liver parenchymal cell.
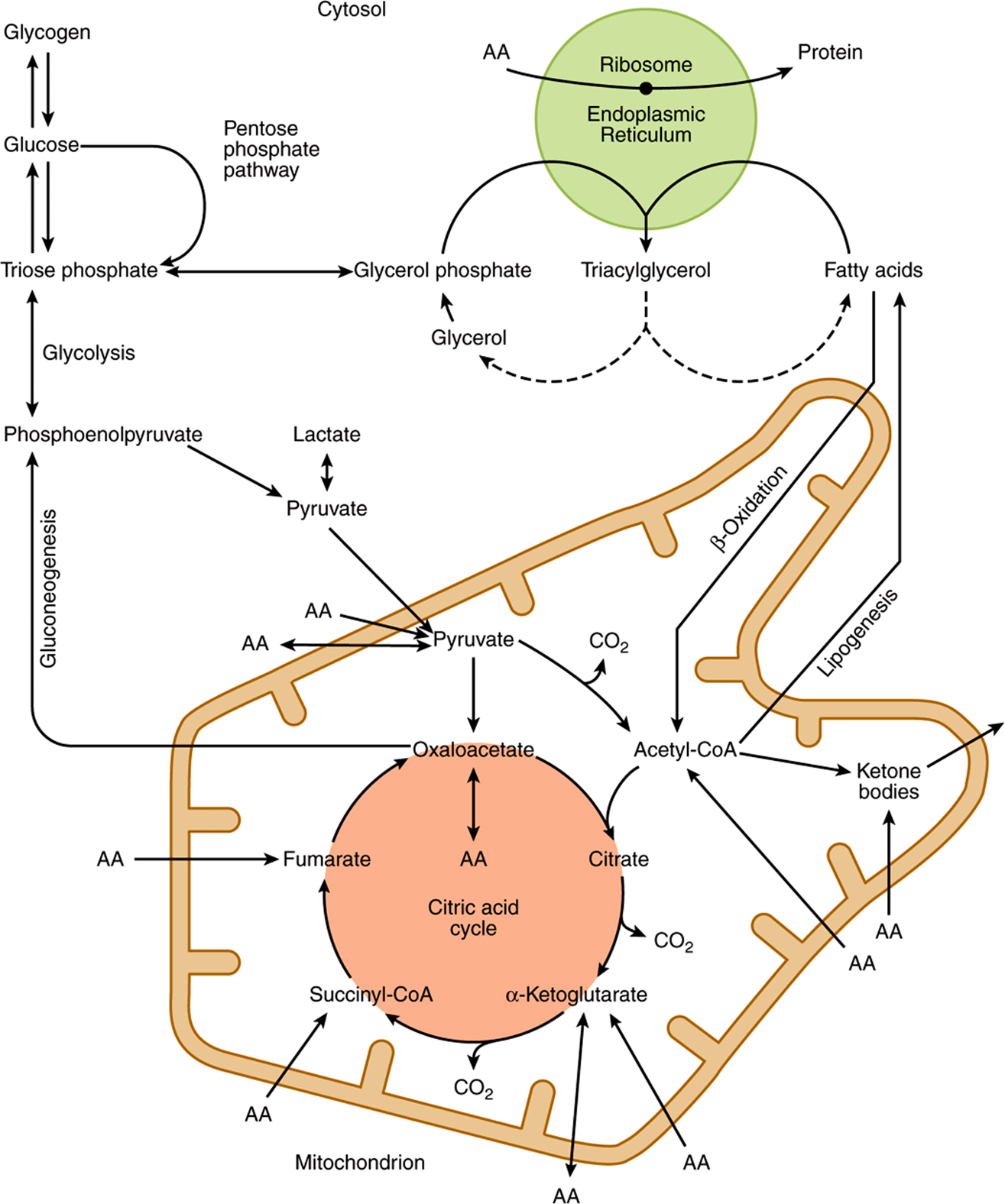
FIGURE 16–7 Intracellular location and overview of major metabolic pathways in a liver parenchymal cell. (AA →, metabolism of one or more essential amino acids; AA ↔, metabolism of one or more nonessential amino acids.)
The central role of the mitochondrion is immediately apparent, since it acts as the focus of carbohydrate, lipid, and amino acid metabolism. It contains the enzymes of the citric acid cycle (Chapter 17), β-oxidation of fatty acids and keto-genesis (Chapter 22), as well as the respiratory chain and ATP synthase (Chapter 13).
Glycolysis (Chapter 18), the pentose phosphate pathway (Chapter 21), and fatty acid synthesis (Chapter 23) all occur in the cytosol. In gluconeogenesis (Chapter 20), substrates such as lactate and pyruvate, which are formed in the cytosol, enter the mitochondrion to yield oxaloacetate as a precursor for the synthesis of glucose in the cytosol.
The membranes of the endoplasmic reticulum contain the enzyme system for triacylglycerol synthesis (Chapter 24), and the ribosomes are responsible for protein synthesis (Chapter 37).
THE FLUX OF METABOLITES THROUGH METABOLIC PATHWAYS MUST BE REGULATED IN A CONCERTED MANNER
Regulation of the overall flux through a pathway is important to ensure an appropriate supply of the products of that pathway. It is achieved by control of one or more key reactions in the pathway, catalyzed by regulatory enzymes.The physicochemical factors that control the rate of an enzyme-catalyzed reaction, such as substrate concentration, are of primary importance in the control of the overall rate of a metabolic pathway (Chapter 9).
Nonequilibrium Reactions Are Potential Control Points
In a reaction at equilibrium, the forward and reverse reactions occur at equal rates, and there is therefore no net flux in either direction.
![]()
In vivo, under “steady-state” conditions, there is a net flux from left to right because there is a continuous supply of A and removal of D. In practice, there are normally one or more non-equilibrium reactions in a metabolic pathway, where the reactants are present in concentrations that are far from equilibrium. In attempting to reach equilibrium, large losses of free energy occur, making this type of reaction essentially irreversible.
![]()
Such a pathway has both flow and direction. The enzymes catalyzing nonequilibrium reactions are usually present in low concentration and are subject to a variety of regulatory mechanisms. However, most reactions in metabolic pathways cannot be classified as equilibrium or nonequilibrium, but fall somewhere between the two extremes.
The Flux-Generating Reaction Is the First Reaction in a Pathway That Is Saturated with the Substrate
It may be identified as a nonequilibrium reaction in which the Km of the enzyme is considerably lower than the normal substrate concentration. The first reaction in glycolysis, catalyzed by hexokinase (Figure 18–2), is such a flux-generating step because its Km for glucose of 0.05 mmol/L is well below the normal blood glucose concentration of 5 mmol/L. Later reactions then control the rate of flux through the pathway.
ALLOSTERIC & HORMONAL MECHANISMS ARE IMPORTANT IN THE METABOLIC CONTROL OF ENZYME-CATALYZED REACTIONS
A hypothetical metabolic pathway is shown in Figure 16–8, in which reactions A ↔ B and C ↔ D are equilibrium reactions and B → C is a nonequilibrium reaction. The flux through such a pathway can be regulated by the availability of substrate A. This depends on its supply from the blood, which in turn depends on either food intake or key reactions that release substrates from tissue reserves into the bloodstream, for example, glycogen phosphorylase in liver (Figure 19–1) and hormone-sensitive lipase in adipose tissue (Figure 25–8). It also depends on the transport of substrate A into the cell. The flux is also determined by removal of the end product D and the availability of cosubstrates or cofactors represented by X and Y. Enzymes catalyzing nonequilibrium reactions are often allosteric proteins subject to the rapid actions of “feed-back” or “feed-forward” control by allosteric modifiers, in immediate response to the needs of the cell (Chapter 9). Frequently, the end product of a biosynthetic pathway inhibits the enzyme catalyzing the first reaction in the pathway. Other control mechanisms depend on the action of hormones responding to the needs of the body as a whole; they may act rapidly by altering the activity of existing enzyme molecules, or slowly by altering the rate of enzyme synthesis (see Chapter 42).
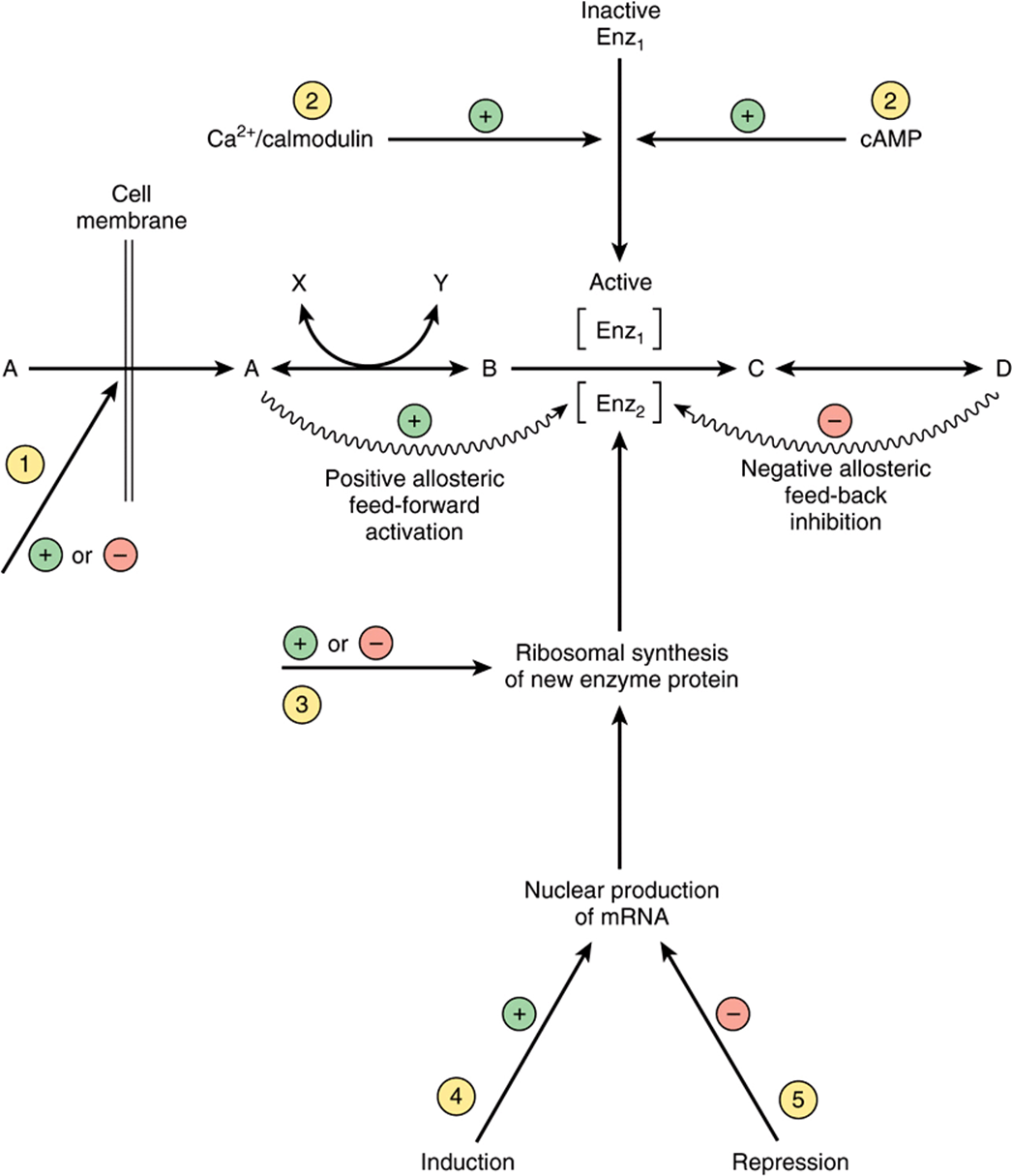
FIGURE 16–8 Mechanisms of control of an enzyme-catalyzed reaction. Circled numbers indicate possible sites of action of hormones: ![]() alteration of membrane permeability;
alteration of membrane permeability; ![]() conversion of an inactive to an active enzyme, usually involving phosphorylation/dephosphorylation reactions;
conversion of an inactive to an active enzyme, usually involving phosphorylation/dephosphorylation reactions; ![]() alteration of the rate translation of mRNA at the ribosomal level;
alteration of the rate translation of mRNA at the ribosomal level; ![]() induction of new mRNA formation; and
induction of new mRNA formation; and ![]() repression of mRNA formation.
repression of mRNA formation. ![]() and
and ![]() are rapid, whereas
are rapid, whereas ![]() through
through ![]() are slower ways of regulating enzyme activity.
are slower ways of regulating enzyme activity.
MANY METABOLIC FUELS ARE INTERCONVERTIBLE
Carbohydrate in excess of requirements for immediate energy-yielding metabolism and formation of glycogen reserves in muscle and liver can readily be used for synthesis of fatty acids, and hence triacylglycerol in both adipose tissue and liver (whence it is exported in very low-density lipoprotein). The importance of lipogenesis in humans is unclear; in Western countries dietary fat provides 35-45% of energy intake, while in less-developed countries, where carbohydrate may provide 60-75% of energy intake, the total intake of food is so low that there is little surplus for lipogenesis anyway. A high intake of fat inhibits lipogenesis in the adipose tissue and liver.
Fatty acids (and ketone bodies formed from them) cannot be used for the synthesis of glucose. The reaction of pyruvate dehydrogenase, forming acetyl-CoA, is irreversible, and for every two-carbon unit from acetyl-CoA that enters the citric acid cycle, there is a loss of two carbon atoms as carbon dioxide before oxaloacetate is reformed. This means that acetyl-CoA (and hence any substrates that yield acetyl-CoA) can never be used for gluconeogenesis. The (relatively rare) fatty acids with an odd number of carbon atoms yield propionyl CoA as the product of the final cycle of β oxidation, and this can be a substrate for gluconeogenesis, as can the glycerol released by lipolysis of adipose tissue triacylglycerol reserves.
Most of the amino acids in excess of requirements for protein synthesis (arising from the diet or from tissue protein turnover) yield pyruvate, or four- and five-carbon intermediates of the citric acid cycle (Chapter 29). Pyruvate can be carboxylated to oxaloacetate, which is the primary substrate for gluconeogenesis, and the other intermediates of the cycle also result in a net increase in the formation of oxaloacetate, which is then available for gluconeogenesis. These amino acids are classified as glucogenic. Two amino acids (lysine and leucine) yield only acetyl-CoA on oxidation, and hence cannot be used for gluconeogenesis, and four others (ie, phenylalanine, tyrosine, tryptophan, and isoleucine) give rise to both acetyl-CoA and intermediates that can be used for gluconeogenesis. Those amino acids that give rise to acetyl-CoA are referred to as ketogenic, because in prolonged fasting and starvation much of the acetyl-CoA is used for synthesis of ketone bodies in the liver.
A SUPPLY OF METABOLIC FUELS IS PROVIDED IN BOTH THE FED & FASTING STATES
Glucose Is Always Required by the Central Nervous System and Erythrocytes
Erythrocytes lack mitochondria and hence are wholly reliant on (anaerobic) glycolysis and the pentose phosphate pathway at all times. The brain can metabolize ketone bodies to meet about 20% of its energy requirements; the remainder must be supplied by glucose. The metabolic changes that occur in the fasting state and starvation are the consequences of the need to preserve glucose and the limited reserves of glycogen in liver and muscle for use by the brain and red blood cells, and to ensure the provision of alternative metabolic fuels for other tissues. In pregnancy, the fetus requires a significant amount of glucose, as does the synthesis of lactose in lactation (Figure 16–9).
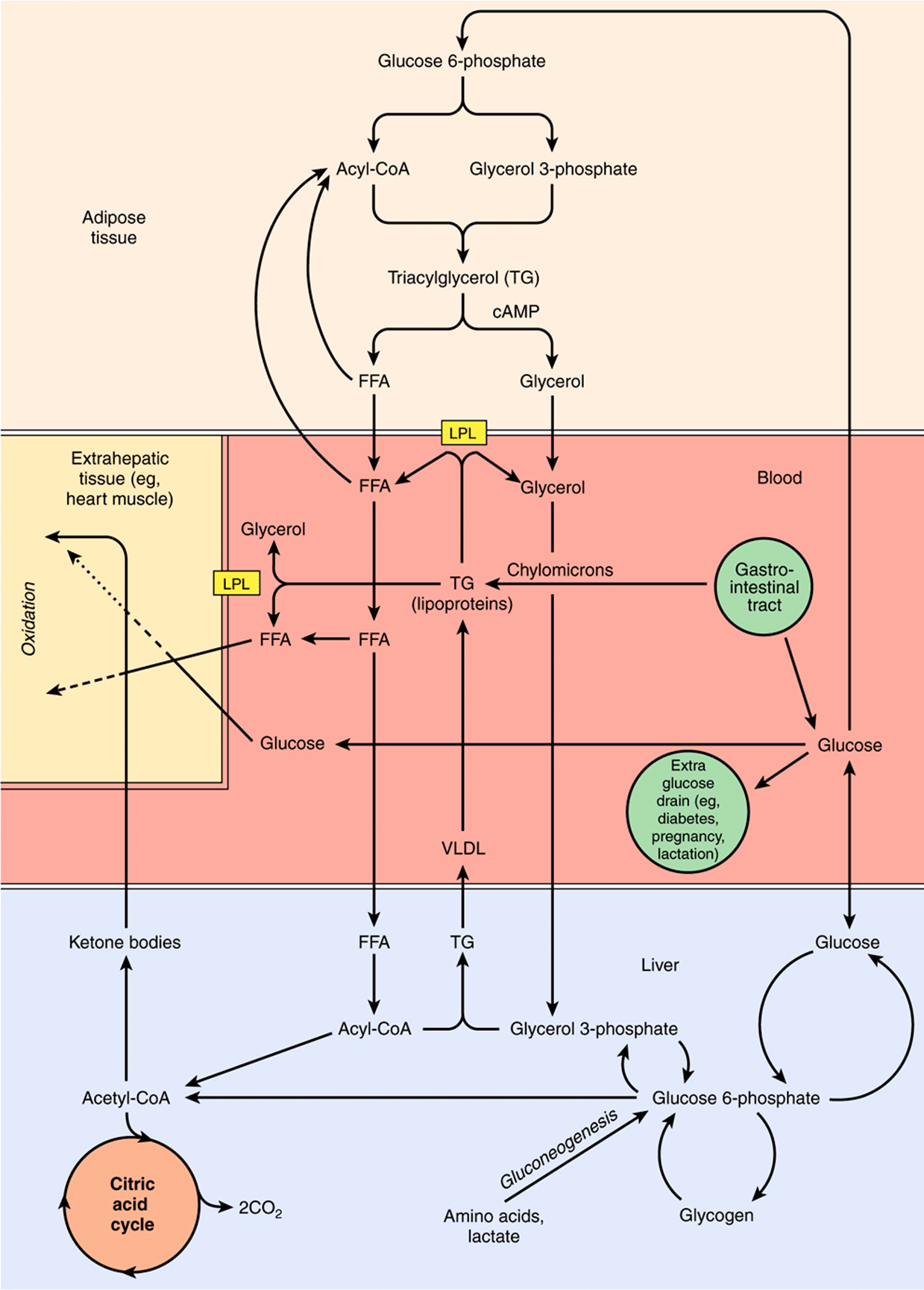
FIGURE 16–9 Metabolic interrelationships among adipose tissue, the liver, and extrahepatic tissues. In tissues such as heart, metabolic fuels are oxidized in the following order of preference: ketone bodies > fatty acids > glucose. (FFA, free fatty acids; LPL, lipoprotein lipase; VLDL, very low density lipoproteins.)
In the Fed State, Metabolic Fuel Reserves Are Laid Down
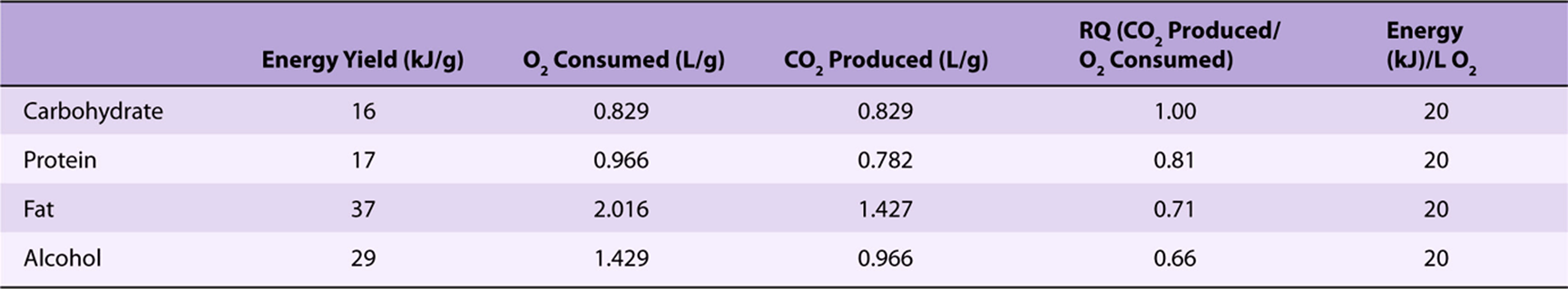
For several hours after a meal, while the products of digestion are being absorbed, there is an abundant supply of metabolic fuels. Under these conditions, glucose is the major fuel for oxidation in most tissues; this is observed as an increase in the respiratory quotient (the ratio of carbon dioxide produced/oxygen consumed) from about 0.8 in the fasting state to near 1 (Table 16-1).
TABLE 16–1 Energy Yields, Oxygen Consumption, and Carbon Dioxide Production in the Oxidation of Metabolic Fuels
Glucose uptake into muscle and adipose tissue is controlled by insulin, which is secreted by the β-islet cells of the pancreas in response to an increased concentration of glucose in the portal blood. In the fasting state, the glucose transporter of muscle and adipose tissue (GLUT-4) is in intracellular vesicles. An early response to insulin is the migration of these vesicles to the cell surface, where they fuse with the plasma membrane, exposing active glucose transporters. These insulin sensitive tissues only take up glucose from the bloodstream to any significant extent in the presence of the hormone. As insulin secretion falls in the fasting state, so the receptors are internalized again, reducing glucose uptake. However, in skeletal muscle, the increase in cytoplasmic calcium ion concentration in response to nerve stimulation stimulates the migration of the vesicles to the cell surface and exposure of active glucose transporters whether or not there is significant insulin stimulation.
The uptake of glucose into the liver is independent of insulin, but liver has an isoenzyme of hexokinase (glucokinase) with a high Km, so that as the concentration of glucose entering the liver increases, so does the rate of synthesis of glucose 6-phosphate. This is in excess of the liver’s requirement for energy-yielding metabolism, and is used mainly for synthesis of glycogen. In both liver and skeletal muscle, insulin acts to stimulate glycogen synthetase and inhibit glycogen phosphorylase. Some of the additional glucose entering the liver may also be used for lipogenesis and hence triacylglycerol synthesis. In adipose tissue, insulin stimulates glucose uptake, its conversion to fatty acids, and their esterification to triacylglycerol. It inhibits intracellular lipolysis and the release of free fatty acids.
The products of lipid digestion enter the circulation as chylomicrons, the largest of the plasma lipoproteins, especially rich in triacylglycerol (see Chapter 25). In the adipose tissue and skeletal muscle, extracellular lipoprotein lipase is synthesized and activated in response to insulin; the resultant nonesterified fatty acids are largely taken up by the tissue and used for synthesis of triacylglycerol, while the glycerol remains in the bloodstream and is taken up by the liver and used for either gluconeogenesis and glycogen synthesis or lipogenesis. Fatty acids remaining in the bloodstream are taken up by the liver and reesterified. The lipid-depleted chylomicron remnants are cleared by the liver, and the remaining triacylglycerol is exported, together with that synthesized in the liver, in very low density lipoprotein.
Under normal conditions, the rate of tissue protein catabolism is more or less constant throughout the day; it is only in cachexia associated with advanced cancer and other diseases that there is an increased rate of protein catabolism. There is net protein catabolism in the fasting state, and net protein synthesis in the fed state, when the rate of synthesis increases by 20-25%. The increased rate of protein synthesis in response to increased availability of amino acids and metabolic fuel is again a response to insulin action. Protein synthesis is an energy expensive process; it may account for up to 20% of resting energy expenditure after a meal, but only 9% in the fasting state.
Metabolic Fuel Reserves Are Mobilized in the Fasting State
There is a small fall in plasma glucose in the fasting state, and then little change as fasting is prolonged into starvation. Plasma free fatty acids increase in fasting, but then rise little more in starvation; as fasting is prolonged, the plasma concentration of ketone bodies (acetoacetate and 3-hydroxybutyrate) increases markedly (Table 16-2, Figure 16–10).
TABLE 16–2 Plasma Concentrations of Metabolic Fuels (mmol/L) in the Fed and Fasting States
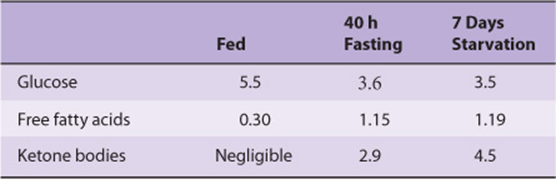
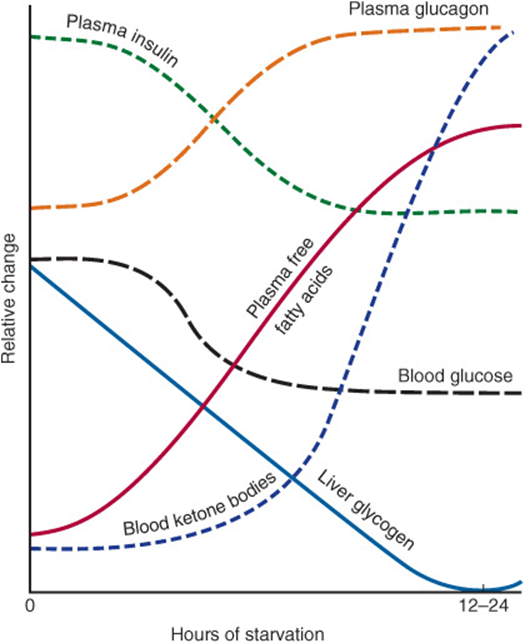
FIGURE 16–10 Relative changes in metabolic parameters during the onset of starvation.
In the fasting state, as the concentration of glucose in the portal blood falls, insulin secretion decreases, and skeletal muscle and adipose tissue take up less glucose. The increase in secretion of glucagon by α cells of the pancreas inhibits glycogen synthetase, and activates glycogen phosphorylase in the liver. The resulting glucose 6-phosphate is hydrolyzed by glucose 6-phosphatase, and glucose is released into the bloodstream for use by the brain and erythrocytes.
Muscle glycogen cannot contribute directly to plasma glucose, since muscle lacks glucose 6-phosphatase, and the primary purpose of muscle glycogen is to provide a source of glucose 6-phosphate for energy-yielding metabolism in the muscle itself. However, acetyl-CoA formed by oxidation of fatty acids in muscle inhibits pyruvate dehydrogenase, leading to an accumulation of pyruvate. Most of this is transaminated to alanine, at the expense of amino acids arising from breakdown of muscle protein. The alanine, and much of the keto acids resulting from this transamination are exported from muscle, and taken up by the liver, where the alanine is transaminated to yield pyruvate. The resultant amino acids are largely exported back to muscle, to provide amino groups for formation of more alanine, while the pyruvate is a major substrate for gluconeogenesis in the liver.
In adipose tissue, the decrease in insulin and increase in glucagon results in inhibition of lipogenesis, inactivation and internalization of lipoprotein lipase, and activation of intracellular hormone-sensitive lipase (Chapter 25). This leads to release from adipose tissue of increased amounts of glycerol (which is a substrate for gluconeogenesis in the liver) and free fatty acids, which are used by liver, heart, and skeletal muscle as their preferred metabolic fuel, therefore sparing glucose.
Although muscle preferentially takes up and metabolizes free fatty acids in the fasting state, it cannot meet all of its energy requirements by β-oxidation. By contrast, the liver has a greater capacity for β-oxidation than it requires to meet its own energy needs, and as fasting becomes more prolonged, it forms more acetyl-CoA than can be oxidized. This acetyl-CoA is used to synthesize the ketone bodies (Chapter 22), which are major metabolic fuels for skeletal and heart muscle and can meet up to 20% of the brain’s energy needs. In prolonged starvation, glucose may represent less than 10% of whole body energy-yielding metabolism.
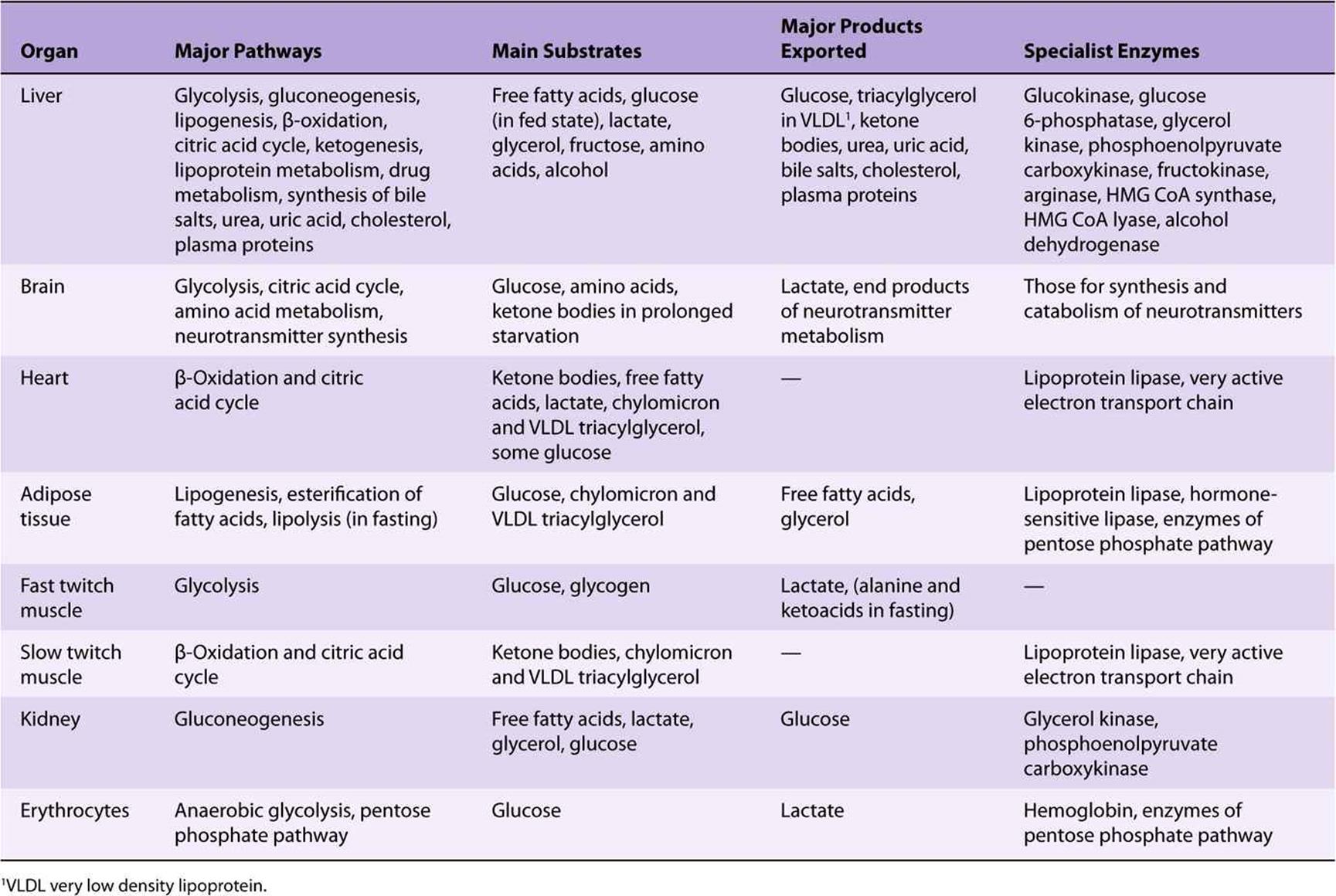
Were there no other source of glucose, liver and muscle glycogen would be exhausted after about 18 h fasting. As fasting becomes more prolonged, so an increasing amount of the amino acids released as a result of protein catabolism is utilized in the liver and kidneys for gluconeogenesis (Table 16-3).
TABLE 16–3 Summary of the Major Metabolic Features of the Principal Organs
CLINICAL ASPECTS
In prolonged starvation, as adipose tissue reserves are depleted, there is a very considerable increase in the net rate of protein catabolism to provide amino acids, not only as substrates for gluconeogenesis, but also as the main metabolic fuel of all tissues. Death results when essential tissue proteins are catabolized and not replaced. In patients with cachexia as a result of release of cytokines in response to tumors and a number of other pathologic conditions, there is an increase in the rate of tissue protein catabolism, as well as a considerably increased metabolic rate, so they are in a state of advanced starvation. Again, death results when essential tissue proteins are catabolized and not replaced.
The high demand for glucose by the fetus, and for lactose synthesis in lactation, can lead to ketosis. This may be seen as mild ketosis with hypoglycemia in human beings; in lactating cattle and in ewes carrying a twin pregnancy, there may be very pronounced ketoacidosis and profound hypoglycemia.
In poorly controlled type 1 diabetes mellitus, patients may become hyperglycemic, partly as a result of lack of insulin to stimulate uptake and utilization of glucose, and partly because in the absence of insulin there is increased gluconeogenesis from amino acids in the liver. At the same time, the lack of insulin results in increased lipolysis in adipose tissue, and the resultant free fatty acids are substrates for ketogenesis in the liver.
Utilization of these ketone bodies in muscle (and other tissues) may be impaired because of the lack of oxaloacetate (all tissues have a requirement for some glucose metabolism to maintain an adequate amount of oxaloacetate for citric acid cycle activity). In uncontrolled diabetes, the ketosis may be severe enough to result in pronounced acidosis (ketoacidosis) since acetoacetate and 3-hydroxybutyrate are relatively strong acids. Coma results from both the acidosis and also the considerably increased osmolality of extracellular fluid (mainly as a result of the hyperglycemia, and diuresis resulting from the excretion of glucose and ketone bodies in the urine).
SUMMARY
![]() The products of digestion provide the tissues with the building blocks for the biosynthesis of complex molecules and also with the fuel for metabolic processes.
The products of digestion provide the tissues with the building blocks for the biosynthesis of complex molecules and also with the fuel for metabolic processes.
![]() Nearly all products of digestion of carbohydrate, fat, and protein are metabolized to a common metabolite, acetyl-CoA, before oxidation to CO2 in the citric acid cycle.
Nearly all products of digestion of carbohydrate, fat, and protein are metabolized to a common metabolite, acetyl-CoA, before oxidation to CO2 in the citric acid cycle.
![]() Acetyl-CoA is also the precursor for synthesis of long-chain fatty acids and steroids (including cholesterol) and ketone bodies.
Acetyl-CoA is also the precursor for synthesis of long-chain fatty acids and steroids (including cholesterol) and ketone bodies.
![]() Glucose provides carbon skeletons for the glycerol of triacylglycerols and nonessential amino acids.
Glucose provides carbon skeletons for the glycerol of triacylglycerols and nonessential amino acids.
![]() Water-soluble products of digestion are transported directly to the liver via the hepatic portal vein. The liver regulates the blood concentrations of glucose and amino acids. Lipids and lipid-soluble products of digestion enter the bloodstream from the lymphatic system, and the liver clears the remnants after extra-hepatic tissues have taken up fatty acids.
Water-soluble products of digestion are transported directly to the liver via the hepatic portal vein. The liver regulates the blood concentrations of glucose and amino acids. Lipids and lipid-soluble products of digestion enter the bloodstream from the lymphatic system, and the liver clears the remnants after extra-hepatic tissues have taken up fatty acids.
![]() Pathways are compartmentalized within the cell. Glycolysis, glycogenesis, glycogenolysis, the pentose phosphate pathway, and lipogenesis occur in the cytosol. The mitochondria contain the enzymes of the citric acid cycle, β-oxidation of fatty acids, and the respiratory chain and ATP synthase. The membranes of the endoplasmic reticulum contain the enzymes for a number of other processes, including triacylglycerol synthesis and drug metabolism.
Pathways are compartmentalized within the cell. Glycolysis, glycogenesis, glycogenolysis, the pentose phosphate pathway, and lipogenesis occur in the cytosol. The mitochondria contain the enzymes of the citric acid cycle, β-oxidation of fatty acids, and the respiratory chain and ATP synthase. The membranes of the endoplasmic reticulum contain the enzymes for a number of other processes, including triacylglycerol synthesis and drug metabolism.
![]() Metabolic pathways are regulated by rapid mechanisms affecting the activity of existing enzymes, that is, allosteric and covalent modification (often in response to hormone action) and slow mechanisms affecting the synthesis of enzymes.
Metabolic pathways are regulated by rapid mechanisms affecting the activity of existing enzymes, that is, allosteric and covalent modification (often in response to hormone action) and slow mechanisms affecting the synthesis of enzymes.
![]() Dietary carbohydrate and amino acids in excess of requirements can be used for fatty acid and hence triacylglycerol synthesis.
Dietary carbohydrate and amino acids in excess of requirements can be used for fatty acid and hence triacylglycerol synthesis.
![]() In fasting and starvation, glucose must be provided for the brain and red blood cells; in the early fasting state, this is supplied from glycogen reserves. In order to spare glucose, muscle and other tissues do not take up glucose when insulin secretion is low; they utilize fatty acids (and later ketone bodies) as their preferred fuel.
In fasting and starvation, glucose must be provided for the brain and red blood cells; in the early fasting state, this is supplied from glycogen reserves. In order to spare glucose, muscle and other tissues do not take up glucose when insulin secretion is low; they utilize fatty acids (and later ketone bodies) as their preferred fuel.
![]() Adipose tissue releases free fatty acids in the fasting state. In prolonged fasting and starvation these are used by the liver for synthesis of ketone bodies, which are exported to provide the major fuel for muscle.
Adipose tissue releases free fatty acids in the fasting state. In prolonged fasting and starvation these are used by the liver for synthesis of ketone bodies, which are exported to provide the major fuel for muscle.
![]() Most amino acids, arising from the diet or from tissue protein turnover, can be used for gluconeogenesis, as can the glycerol from triacylglycerol.
Most amino acids, arising from the diet or from tissue protein turnover, can be used for gluconeogenesis, as can the glycerol from triacylglycerol.
![]() Neither fatty acids, arising from the diet or from lipolysis of adipose tissue triacylglycerol, nor ketone bodies, formed from fatty acids in the fasting state, can provide substrates for gluconeogenesis.
Neither fatty acids, arising from the diet or from lipolysis of adipose tissue triacylglycerol, nor ketone bodies, formed from fatty acids in the fasting state, can provide substrates for gluconeogenesis.
REFERENCES
Bender DA: Introduction to Nutrition and Metabolism, 4th ed. CRC Press, 2007.
Brosnan JT: Comments on the metabolic needs for glucose and the role of gluconeogenesis. Eur J Clin Nutr 1999;53:S107-S111.
Frayn KN: Integration of substrate flow in vivo: some insights into metabolic control. Clin Nutr 1997;16:277-282.
Frayn KN: Metabolic Regulation: A Human Perspective, 3rd ed. Wiley-Blackwell, 2010.
Zierler K: Whole body metabolism of glucose. Am J Physiol 1999;276:E409-E426.