CHEMISTRY THE CENTRAL SCIENCE
2 ATOMS, MOLECULES, AND IONS
2.7 IONS AND IONIC COMPOUNDS
The nucleus of an atom is unchanged by chemical processes, but some atoms can readily gain or lose electrons. If electrons are removed from or added to an atom, a charged particle called an ion is formed. An ion with a positive charge is a cation (pronounced CAT-ion); a negatively charged ion is an anion (AN-ion).
To see how ions form, consider the sodium atom, which has 11 protons and 11 electrons. This atom easily loses one electron. The resulting cation has 11 protons and 10 electrons, which means it has a net charge of 1+.
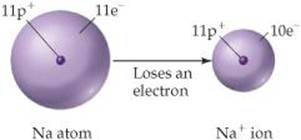
The net charge on an ion is represented by a superscript. The superscripts +, 2+, and 3+, for instance, mean a net charge resulting from the loss of one, two, and three electrons, respectively. The superscripts –, 2–, and 3– represent net charges resulting from the gain of one, two, and three electrons, respectively. Chlorine, with 17 protons and 17 electrons, for example, can gain an electron in chemical reactions, producing the Cl– ion:
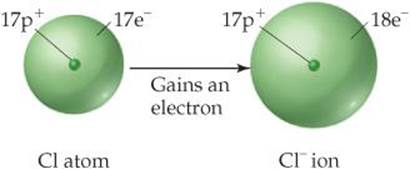
In general, metal atoms tend to lose electrons to form cations and nonmetal atoms tend to gain electrons to form anions. Thus, ionic compounds tend to be composed of metals bonded with nonmetals, as in NaCl.
SAMPLE EXERCISE 2.7 Writing Chemical Symbols for Ions
Give the chemical symbol, including superscript indicating mass number, for (a) the ion with 22 protons, 26 neutrons, and 19 electrons; (b) the ion of sulfur that has 16 neutrons and 18 electrons.
SOLUTION
(a) The number of protons is the atomic number of the element. A periodic table or list of elements tells us that the element with atomic number 22 is titanium (Ti). The mass number (protons plus neutrons) of this isotope of titanium is 22 + 26 = 48. Because the ion has three more protons than electrons, it has a net charge of 3+: 48Ti3+.
(b) The periodic table tells us that sulfur (S) has an atomic number of 16. Thus, each atom or ion of sulfur contains 16 protons. We are told that the ion also has 16 neutrons, meaning the mass number is 16 + 16 = 32. Because the ion has 16 protons and 18 electrons, its net charge is 2– and the ion symbol is 32S2–.
In general, we will focus on the net charges of ions and ignore their mass numbers unless the circumstances dictate that we specify a certain isotope.
PRACTICE EXERCISE
How many protons, neutrons, and electrons does the 79Se2– ion possess?
Answer: 34 protons, 45 neutrons, and 36 electrons
In addition to simple ions such as Na+ and Cl–, there are polyatomic ions, such as NH4+ (ammonium ion) and SO42– (sulfate ion). These latter ions consist of atoms joined as in a molecule, but they have a net positive or negative charge. We consider polyatomic ions in Section 2.8.
It is important to realize that the chemical properties of ions are very different from the chemical properties of the atoms from which the ions are derived. Although a given atom and its ion may be essentially the same (plus or minus a few electrons), the behavior of the ion is very different from that of its associated atom.
Predicting Ionic Charges
Many atoms gain or lose electrons to end up with the same number of electrons as the noble gas closest to them in the periodic table. Noble-gas elements are chemically non-reactive and form very few compounds. We might deduce that this is because their electron arrangements are very stable. Nearby elements can obtain these same stable arrangements by losing or gaining electrons. For example, the loss of one electron from an atom of sodium leaves it with the same number of electrons as in a neon atom (10). Similarly, when chlorine gains an electron, it ends up with 18, the same number of electrons as in argon. We will use this simple observation to explain the formation of ions until Chapter 8, where we discuss chemical bonding.
SAMPLE EXERCISE 2.8 Predicting Ionic Charge
Predict the charge expected for the most stable ion of barium and the most stable ion of oxygen.
SOLUTION
We will assume that these elements form ions that have the same number of electrons as the nearest noble-gas atom. From the periodic table, we see that barium has atomic number 56. The nearest noble gas is xenon, atomic number 54. Barium can attain a stable arrangement of 54 electrons by losing two electrons, forming the Ba2+ cation.
Oxygen has atomic number 8. The nearest noble gas is neon, atomic number 10. Oxygen can attain this stable electron arrangement by gaining two electrons, forming the O2– anion.
PRACTICE EXERCISE
Predict the charge expected for the most stable ion of (a) aluminum and (b) fluorine.
Answer: (a) 3+, (b) 1–
The periodic table is very useful for remembering ionic charges, especially those of elements on the left and right sides of the table. As ![]() FIGURE 2.20 shows, the charges of these ions relate in a simple way to their positions in the table: The group 1A elements (alkali metals) form 1+ ions, the group 2A elements (alkaline earths) form 2+ ions, the group 7A elements (halogens) form 1– ions, and the group 6A elements form 2– ions. (Many of the other groups do not lend themselves to such simple rules.)
FIGURE 2.20 shows, the charges of these ions relate in a simple way to their positions in the table: The group 1A elements (alkali metals) form 1+ ions, the group 2A elements (alkaline earths) form 2+ ions, the group 7A elements (halogens) form 1– ions, and the group 6A elements form 2– ions. (Many of the other groups do not lend themselves to such simple rules.)
Ionic Compounds
A great deal of chemical activity involves the transfer of electrons from one substance to another. ![]() FIGURE 2.21 shows that when elemental sodium is allowed to react with elemental chlorine, an electron transfers from a sodium atom to a chlorine atom, forming a Na+ ion and a Cl– ion. Because objects of opposite charge attract, the Na+ and the Cl– ions bind together to form the compound sodium chloride (NaCl). Sodium chloride, which we know better as common table salt, is an example of an ionic compound, a compound made up of cations and anions.
FIGURE 2.21 shows that when elemental sodium is allowed to react with elemental chlorine, an electron transfers from a sodium atom to a chlorine atom, forming a Na+ ion and a Cl– ion. Because objects of opposite charge attract, the Na+ and the Cl– ions bind together to form the compound sodium chloride (NaCl). Sodium chloride, which we know better as common table salt, is an example of an ionic compound, a compound made up of cations and anions.
We can often tell whether a compound is ionic (consisting of ions) or molecular (consisting of molecules) from its composition. In general, cations are metal ions and anions are nonmetal ions. Consequently, ionic compounds are generally combinations of metals and nonmetals, as in NaCl. In contrast, molecular compounds are generally composed of nonmetals only, as in H2O.
![]() GO FIGURE
GO FIGURE
The most common ions for silver, zinc, and scandium are Ag+, Zn2+, and Sc3+. Locate the boxes in which you would place these ions in this table. Which of these ions have the same number of electrons as a noble-gas element?
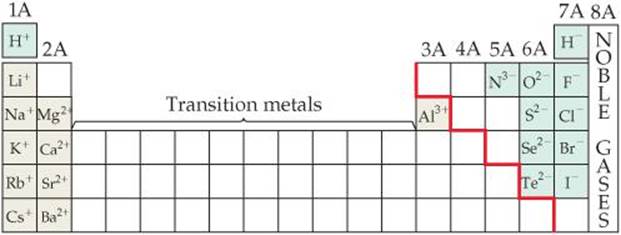
![]() Figure 2.20 Predictable charges of some common ions. Notice that the red stepped line that divides metals from nonmetals also separates cations from anions. Hydrogen forms both 1 + and 1 – ions.
Figure 2.20 Predictable charges of some common ions. Notice that the red stepped line that divides metals from nonmetals also separates cations from anions. Hydrogen forms both 1 + and 1 – ions.
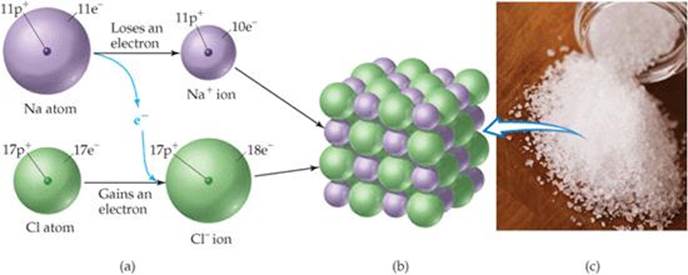
![]() Figure 2.21 Formation of an ionic compound. (a) The transfer of an electron from a Na atom to a Cl atom leads to the formation of a Na+ ion and a Cl– ion. (b) Arrangement of these ions in solid sodium chloride, NaCl. (c) A sample of sodium chloride crystals.
Figure 2.21 Formation of an ionic compound. (a) The transfer of an electron from a Na atom to a Cl atom leads to the formation of a Na+ ion and a Cl– ion. (b) Arrangement of these ions in solid sodium chloride, NaCl. (c) A sample of sodium chloride crystals.
SAMPLE EXERCISE 2.9 Identifying Ionic and Molecular Compounds
Which of these compounds would you expect to be ionic: N2O, Na2O, CaCl2, SF4?
SOLUTION
We predict that Na2O and CaCl2 are ionic compounds because they are composed of a metal combined with a nonmetal. We predict (correctly) that N2O and SF4 are molecular compounds because they are composed entirely of nonmetals.
PRACTICE EXERCISE
Which of these compounds are molecular: CBr4, FeS, P4O6, PbF2?
Answer: CBr4 and P4O6
The ions in ionic compounds are arranged in three-dimensional structures, as Figure 2.21(b) shows for NaCl. Because there is no discrete “molecule” of NaCl, we are able to write only an empirical formula for this substance. This is true for most other ionic compounds.
We can write the empirical formula for an ionic compound if we know the charges of the ions. This is true because chemical compounds are always electrically neutral. Consequently, the ions in an ionic compound always occur in such a ratio that the total positive charge equals the total negative charge. Thus, there is one Na+ to one Cl– (giving NaCl), one Ba2+ to two Cl– (giving BaCl2), and so forth.
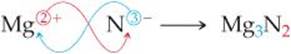
As you consider these and other examples, you will see that if the charges on the cation and anion are equal, the subscript on each ion is 1. If the charges are not equal, the charge on one ion (without its sign) will become the subscript on the other ion. For example, the ionic compound formed from Mg (which forms Mg2+ ions) and N (which forms N3– ions) is Mg3N2:
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Why don't we write the formula for the compound formed by Ca2+ and O2– as Ca2O2?
 CHEMISTRY AND LIFE
CHEMISTRY AND LIFE
ELEMENTS REQUIRED BY LIVING ORGANISMS
The colored regions of ![]() FIGURE 2.22 shows the elements essential to life. More than 97% of the mass of most organisms is made up of just six of these elements—oxygen, carbon, hydrogen, nitrogen, phosphorus, and sulfur. Water is the most common compound in living organisms, accounting for at least 70% of the mass of most cells. In the solid components of cells, carbon is the most prevalent element by mass. Carbon atoms are found in a vast variety of organic molecules, bonded either to other carbon atoms or to atoms of other elements. All proteins, for example, contain the group
FIGURE 2.22 shows the elements essential to life. More than 97% of the mass of most organisms is made up of just six of these elements—oxygen, carbon, hydrogen, nitrogen, phosphorus, and sulfur. Water is the most common compound in living organisms, accounting for at least 70% of the mass of most cells. In the solid components of cells, carbon is the most prevalent element by mass. Carbon atoms are found in a vast variety of organic molecules, bonded either to other carbon atoms or to atoms of other elements. All proteins, for example, contain the group
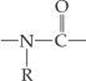
which occurs repeatedly in the molecules. (R is either an H atom or a combination of atoms, such as CH3.)
In addition, 23 more elements have been found in various living organisms. Five are ions required by all organisms: Ca2+, Cl–, Mg2+, K+, and Na+. Calcium ions, for example, are necessary for the formation of bone and transmission of nervous system signals. Many other elements are needed in only very small quantities and consequently are called trace elements. For example, trace quantities of copper are required in the diet of humans to aid in the synthesis of hemoglobin.
RELATED EXERCISE: 2.96
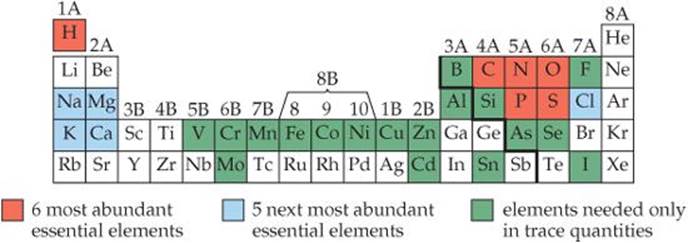
![]() Figure 2.22 Elements essential to life.
Figure 2.22 Elements essential to life.
 STRATEGIES IN CHEMISTRY
STRATEGIES IN CHEMISTRY
PATTERN RECOGNITION
Someone once said that drinking at the fountain of knowledge in a chemistry course is like drinking from a fire hydrant. Indeed, the pace can sometimes seem brisk. More to the point, however, we can drown in the facts if we do not see the general patterns. The value of recognizing patterns and learning rules and generalizations is that they free us from having to learn (or trying to memorize) many individual facts. The patterns, rules, and generalizations tie ideas together so that we do not get lost in the details.
Many students struggle with chemistry because they do not see how different topics relate to one another so they treat every idea and problem as being unique instead of as an example or application of a general rule, procedure, or relationship. You can avoid this pitfall by remembering the following.
Notice the structure of the topic you are studying. Pay attention to trends and rules given to summarize a large body of information. Notice, for example, how atomic structure helps us understand the existence of isotopes (as Table 2.2 shows) and how the periodic table helps us remember ionic charges (as Figure 2.20 shows).
You may surprise yourself by observing patterns that are not explicitly spelled out yet. Perhaps you have noticed certain trends in chemical formulas, for instance. Moving across the periodic table from element 11 (Na), we find that the elements form compounds with F having the following compositions: NaF, MgF2, and AlF3. Does this trend continue? Do SiF4, PF5, and SF6 exist? Indeed they do. If you have noticed trends like this from the scraps of information you have seen so far, then you are ahead of the game and have prepared yourself for some topics we will address in later chapters.
SAMPLE EXERCISE 2.10 Using Ionic Charge to Write Empirical Formulas for Ionic Compounds
Write the empirical formula of the compound formed by (a) Al3+ and Cl– ions, (b) Al3+ and O2– ions, (c) Mg2+ and NO3– ions.
SOLUTION
(a) Three Cl– ions are required to balance the charge of one Al3+ ion, making the formula AlCl3.
(b) Two Al3+ ions are required to balance the charge of three O2– ions. That is, the total positive charge is 6+, and the total negative charge is 6–. The formula is Al2O3.
(c) Two NO3– ions are needed to balance the charge of one Mg2+, yielding Mg(NO3)2. Note that the formula for the polyatomic ion, NO3–, must be enclosed in parentheses so that it is clear that the subscript 2 applies to all the atoms of that ion.
PRACTICE EXERCISE
Write the empirical formula for the compound formed by (a) Na+ and PO43–, (b) Zn2+ and SO42–, (c) Fe3+ and CO32–.
Answers: (a) Na3PO4, (b) ZnSO4, (c) Fe2(CO3)3