CHEMISTRY THE CENTRAL SCIENCE
3 STOICHIOMETRY: CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATIONS
3.2 SOME SIMPLE PATTERNS OF CHEMICAL REACTIVITY
In this section we examine three types of reactions that we see frequently throughout this chapter: combination reactions, decomposition reactions, and combustion reactions. Our first reason for examining these reactions is to become better acquainted with chemical reactions and their balanced equations. Our second reason is to consider how we might predict the products of some of these reactions knowing only their reactants. The key to predicting the products formed by a given combination of reactants is recognizing general patterns of chemical reactivity. Recognizing a pattern of reactivity for a class of substances gives you a broader understanding than merely memorizing a large number of unrelated reactions.
Combination and Decomposition Reactions
In combination reactions two or more substances react to form one product (![]() TABLE 3.1). For example, magnesium metal burns brilliantly in air to produce magnesium oxide (
TABLE 3.1). For example, magnesium metal burns brilliantly in air to produce magnesium oxide (![]() FIGURE 3.6):
FIGURE 3.6):
![]()
This reaction is used to produce the bright flame generated by flares and some fireworks.
A combination reaction between a metal and a nonmetal, as in Equation 3.6, produces an ionic solid. Recall that the formula of an ionic compound can be determined from the charges of its ions.![]() (Section 2.7) When magnesium reacts with oxygen, the magnesium loses electrons and forms the magnesium ion, Mg2+. The oxygen gains electrons and forms the oxide ion, O2−. Thus, the reaction product is MgO.
(Section 2.7) When magnesium reacts with oxygen, the magnesium loses electrons and forms the magnesium ion, Mg2+. The oxygen gains electrons and forms the oxide ion, O2−. Thus, the reaction product is MgO.
You should be able to recognize when a reaction is a combination reaction and to predict the products when the reactants are a metal and a nonmetal.
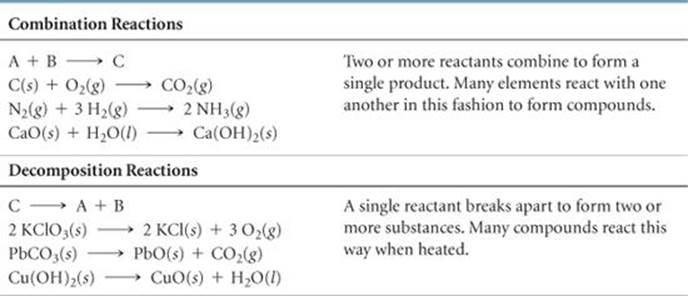
TABLE 3.1 • Combination and Decomposition Reactions
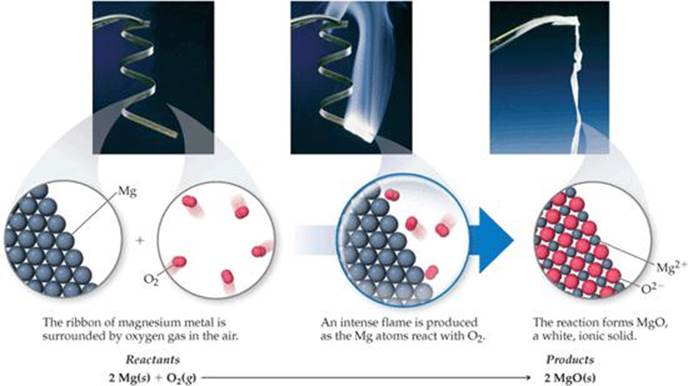
![]() FIGURE 3.6 Combustion of magnesium metal in air, a combination reaction.
FIGURE 3.6 Combustion of magnesium metal in air, a combination reaction.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
When Na and S undergo a combination reaction, what is the chemical formula of the product?
In a decomposition reaction one substance undergoes a reaction to produce two or more other substances (Table 3.1). For example, many metal carbonates decompose to form metal oxides and carbon dioxide when heated:
![]()
Decomposition of CaCO3 is an important commercial process. Limestone or seashells, which are both primarily CaCO3, are heated to prepare CaO, known as lime or quicklime. About 2 × 1010 kg (20 million tons) of CaO is used in the United States each year, principally in making glass, in obtaining iron from its ores, and in making mortar to bind bricks.
The decomposition of sodium azide (NaN3) rapidly releases N2(g), so this reaction is used to inflate safety air bags in automobiles (![]() FIGURE 3.7):
FIGURE 3.7):
![]()
The system is designed so that an impact ignites a detonator cap, which in turn causes NaN3 to decompose explosively. A small quantity of NaN3 (about 100 g) forms a large quantity of gas (about 50 L).

![]() FIGURE 3.7 Decomposition of sodium azide, NaN3(s), is used to inflate automobile air bags.
FIGURE 3.7 Decomposition of sodium azide, NaN3(s), is used to inflate automobile air bags.
SAMPLE EXERCISE 3.3 Writing Balanced Equations for Combination and Decomposition Reactions
Write a balanced equation for (a) the combination reaction between lithium metal and fluorine gas and (b) the decomposition reaction that occurs when solid barium carbonate is heated (two products form, a solid and a gas).
SOLUTION
(a) With the exception of mercury, all metals are solids at room temperature. Fluorine occurs as a diatomic molecule. Thus, the reactants are Li(s) and F2(g). The product will be composed of a metal and a nonmetal, so we expect it to be an ionic solid. Lithium ions have a 1+ charge, Li+, whereas fluoride ions have a 1– charge, F−. Thus, the chemical formula for the product is LiF. The balanced chemical equation is
2 Li(s) + F2(g) → 2 LiF(s)
(b) The chemical formula for barium carbonate is BaCO3. As noted in the text, many metal carbonates decompose to metal oxides and carbon dioxide when heated. In Equation 3.7, for example, CaCO3 decomposes to form CaO and CO2. Thus, we expect BaCO3 to decompose to BaO and CO2. Barium and calcium are both in group 2A in the periodic table, which further suggests they react in the same way:
BaCO3(s) → BaO(s) + CO2(g)
PRACTICE EXERCISE
Write a balanced equation for (a) solid mercury(II) sulfide decomposing into its component elements when heated and (b) aluminum metal combining with oxygen in the air.
Answers: (a) HgS(s) → Hg(l) + S(s), (b) 4 Al(i) + 3 O2(g) → 2 Al2O3(s)
Combustion Reactions
Combustion reactions are rapid reactions that produce a flame. Most combustion reactions we observe involve O2 from air as a reactant. Equation 3.5 illustrates a general class of reactions involving the burning, or combustion, of hydrocarbons (compounds that contain only carbon and hydrogen, such as CH4 and C2H4). ![]() (Section 2.9)
(Section 2.9)
Hydrocarbons combusted in air react with O2 to form CO2 and H2O.* The number of molecules of O2 required and the number of molecules of CO2 and H2O formed depend on the composition of the hydrocarbon, which acts as the fuel in the reaction. For example, the combustion of propane (C3H8, ![]() FIGURE 3.8), a gas used for cooking and home heating, is described by the equation
FIGURE 3.8), a gas used for cooking and home heating, is described by the equation
![]()
![]() GO FIGURE
GO FIGURE
In what ways are the reactions depicted in Figures 3.4 and 3.8 alike?

![]() FIGURE 3.8 Propane burning in air. Liquid propane in the tank, C3H8, vaporizes and mixes with air as it escapes through the nozzle. The combustion reaction of C3H8 and O2 produces a blue flame.
FIGURE 3.8 Propane burning in air. Liquid propane in the tank, C3H8, vaporizes and mixes with air as it escapes through the nozzle. The combustion reaction of C3H8 and O2 produces a blue flame.
The state of the water in this reaction, H2O(g) or H2O(l), depends on the reaction conditions. Water vapor, H2O(g), is formed at high temperature in an open container.
Combustion of oxygen-containing derivatives of hydrocarbons, such as CH3OH, also produces CO2 and H2O. The rule that hydrocarbons and their oxygen-containing derivatives form CO2 and H2O when they burn in air summarizes the behavior of about 3 million compounds. Many substances that our bodies use as energy sources, such as the sugar glucose (C6H12O6), react with O2 to form CO2 and H2O. In our bodies, however, the reactions take place in a series of intermediate steps that occur at body temperature. These reactions that involve intermediate steps are described as oxidation reactions instead of combustion reactions.
SAMPLE EXERCISE 3.4 Writing Balanced Equations for Combustion Reactions
Write the balanced equation for the reaction that occurs when methanol, CH3OH(l), is burned in air.
SOLUTION
When any compound containing C, H, and O is combusted, it reacts with the O2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is
CH3OH(l) + O2(g) → CO2(g) + H2O(g)
The C atoms are balanced, one on each side of the arrow. Because CH3OH has four H atoms, we place the coefficient 2 in front of H2O to balance the H atoms:
CH3OH(l) + O2(g) → CO2(g) + 2 H2O(g)
Adding this coefficient balances H but gives four O atoms in the products. Because there are only three O atoms in the reactants, we are not finished. We can place the coefficient ![]() in front of O2 to give four O atoms in the reactants (
in front of O2 to give four O atoms in the reactants (![]() × 2 = 3 O atoms in
× 2 = 3 O atoms in ![]() O2):
O2):
![]()
Although this equation is balanced, it is not in its most conventional form because it contains a fractional coefficient. However, multiplying through by 2 removes the fraction and keeps the equation balanced:
2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(g)
PRACTICE EXERCISE
Write the balanced equation for the reaction that occurs when ethanol, C2H5OH(l), burns in air.
Answer: C2H5OH(l) + 3 O2(g) → 2 CO2(g) + 3 H2O(g)