Chemistry for Dummies
Part III. The Mole: The Chemist's Best Friend
Chapter 11. Mixing Matter Up: Solutions
In This Chapter
· Finding out about solutes, solvents, and solutions
· Working with the different kinds of solution concentration units
· Checking out the colligative properties of solutions
· Figuring out colloids
You encounter solutions all the time in everyday life. The air you breathe is a solution. That sports drink you use to replenish your electrolytes is a solution. That soft drink and that hard drink are both solutions. Your tap water is most likely a solution, too. In this chapter, I show you some of the properties of solutions. I introduce you to the different ways chemists represent a solution’s concentration, and I tell you about the colligative properties of solutions and relate them to ice cream making and antifreeze. So sit back, sip on your solution of choice, and read all about solutions.
Solutes, Solvents, and Solutions
A solution is a homogeneous mixture, meaning that it is the same throughout. If you dissolve sugar in water and mix it really well, for example, your mixture is basically the same no matter where you sample it.
A solution is composed of a solvent and one or more solutes. The solvent is the substance that’s present in the largest amount, and the solute is the substance that’s present in the lesser amount. These definitions work most of the time, but there are a few cases of extremely soluble salts, such as lithium chloride, in which more than 5 grams of salt can be dissolved in 5 milliliters of water. However, water is still considered the solvent, because it’s the species that has not changed state. In addition, there can be more than one solute in a solution. You can dissolve salt in water to make a brine solution, and then you can dissolve some sugar in the same solution. You then have two solutes, salt and sugar, but you still have only one solvent — water.
When I talk about solutions, most people think of liquids. But there can also be solutions of gases. Our atmosphere, for example, is a solution. Because air is almost 79 percent nitrogen, it’s considered the solvent, and the oxygen, carbon dioxide, and other gases are considered the solutes. There are also solid solutions. Alloys, for example, are solutions of one metal in another metal. Brass is a solution of zinc in copper.
A discussion of dissolving
Why do some things dissolve in one solvent and not another? For example, oil and water will not mix to form a solution, but oil will dissolve in gasoline. There’s a general rule of solubility that says like-dissolves-like in regards to polarity of both the solvent and solutes. Water, for example, is a polar material; it’s composed of polar covalent bonds with a positive and negative end of the molecule. (For a rousing discussion of water and its polar covalent bonds, see Chapter 7.) Water will dissolve polar solutes, such as salts and alcohols. Oil, however, is composed of largely nonpolar bonds. So water will not act as a suitable solvent for oil.
You know from your own experiences, I’m sure, that there’s a limit to how much solute can be dissolved in a given amount of solvent. Most of us have been guilty of putting far too much sugar in iced tea. No matter how much you stir, there’s some undissolved sugar at the bottom of the glass. The reason is that the sugar has reached its maximum solubility in water at that temperature. Solubility is the maximum amount of solute that will dissolve in a given amount of a solvent at a specified temperature. Solubility normally has the units of grams solute per 100 milliliters of solvent (g/100 mL).
If you heat that iced tea, the sugar at the bottom will readily dissolve. The solubility is related to the temperature of the solvent. For solids dissolving in liquids, solubility normally increases with increasing temperature. However, for gases dissolving in liquids, such as oxygen dissolving in lake water, the solubility goes down as the temperature increases. This is the basis of thermal pollution, the addition of heat to water that decreases the solubility of the oxygen and affects the aquatic life.
Saturated facts
A saturated solution contains the maximum amount of dissolved solute possible at a given temperature. If it has less than this amount, it’s called an unsaturated solution. Sometimes, under unusual circumstances, the solvent may actually dissolve more than its maximum amount and become supersaturated. This supersaturated solution is unstable, though, and sooner or later solute will precipitate (form a solid) until the saturation point has been reached.
If a solution is unsaturated, then the amount of solute that is dissolved may vary over a wide range. A couple of rather nebulous terms describe the relative amount of solute and solvent that you can use:
ü You can say that the solution is dilute, meaning that, relatively speaking, there’s very little solute per given amount of solvent. If you dissolve 0.01 grams of sodium chloride in a liter of water, for example, the solution is dilute. I once asked some students to give me an example of a dilute solution, and one replied “A $1 margarita.” She was right — a lot of solvent (water) and a very little solute (tequila) are used in her example.
ü A solution may be concentrated, containing a large amount of solute per the given amount of solvent. If you dissolve 200 grams of sodium chloride in a liter of water, for example, the solution is concentrated.
But suppose you dissolve 25 grams or 50 grams of sodium chloride in a liter of water? Is the solution dilute or concentrated? These terms don’t hold up very well for most cases. And consider the case of IV solutions — they must have a very precise amount of solute in them, or the patient will be in danger. So you must have a quantitative method to describe the relative amount of solute and solvent in a solution. Such a method exists — solution concentration units.
Solution Concentration Units
You can use a variety of solution concentration units to quantitatively describe the relative amounts of the solute(s) and the solvent. In everyday life, percentage is commonly used. In chemistry, molarity (the moles of solute per liter of solution) is the solution concentration unit of choice. In certain circumstances, though, another unit, molality (the moles of solute per kilogram of solvent), is used. And I use parts-per-million or parts-per- billion when I discuss pollution control. The following sections cover some of these concentration units.
Percent composition
Most of us have looked at a bottle of vinegar and seen “5% acetic acid,” a bottle of hydrogen peroxide and seen “3% hydrogen peroxide,” or a bottle of bleach and seen “5% sodium hypochlorite.” Those percentages are expressing the concentration of that particular solute in each solution. Percentage is the amount per one hundred. Depending on the way you choose to express the percentage, the units of amount per one hundred vary. Three different percentages are commonly used:
ü Weight/weight (w/w) percentage
ü Weight/volume (w/v) percentage
ü Volume/volume (v/v) percentage
Unfortunately, although the percentage of solute is often listed, the method (w/w, w/v, v/v) is not. In this case, I normally assume that the method is weight/weight, but I’m sure you know about assumptions.
Most of the solutions I talk about in the following examples of these percentages are aqueous solutions, solutions in which water is the solvent.
Weiqht/weiqht percentage
In weight/weight percentage, or weight percentage, the weight of the solute is divided by the weight of the solution and then multiplied by 100 to get the percentage. Normally the weight unit is grams. Mathematically, it looks like this:
![]()
If, for example, you dissolve 5.0 grams of sodium chloride in 45 grams of water, the weight percent is
![]()
Therefore, the solution is a 10 percent (w/w) solution.
Suppose that you want to make 350.0 grams of a 5 percent (w/w) sucrose, or table sugar, solution. You know that 5 percent of the weight of the solution is sugar, so you can multiply the 350.0 grams by 0.05 to get the weight of the sugar:
350.0 grams x 0.05 = 17.5 grams of sugar
The rest of the solution (350.0 grams - 17.5 grams = 332.5 grams) is water. You can simply weigh out 17.5 grams of sugar and add it to 332.5 grams of water to get your 5 percent (w/w) solution.
Weight percentage is the easiest percentage solution to make, but sometimes you may need to know the volume of the solution. In this case, you can use the weight/volume percentage.
Weight/volume percentage
Weight/volume percentage is very similar to weight/weight percentage, but instead of using grams of solution in the denominator, it uses milliliters of solution:
![]()
Proof reading
When it comes to ethyl alcohol solutions, another concentration unit; called proof, is commonly used to measure the relative amount of alcohol and water. The proof is simply twice the percentage. A 50 percent ethyl alcohol solution is 100 proof. Pure ethyl alcohol (100 percent) is 200 proof. This term dates back to earlier times, when the production of ethyl alcohol for human consumption was a cottage industry. (In the part of North Carolina where I grew up, it still is a cottage industry.) There was no quality control back then, so the buyer had to be sure that the alcohol he was buying was concentrated enough (or "strong" enough) for the desired purpose. Some of the alcohol solution was poured over gunpowder and then lit. If there was enough alcohol present, the gunpowder would ignite, giving "proof" that the solution was strong enough.
Suppose that you want to make 100 milliliters of a 15 percent (w/v) potassium nitrate solution. Because you’re making 100 milliliters, you already know that you’re going to weigh out 15 grams of potassium nitrate (commonly called saltpeter — KNO3). Now, here comes something that’s a little different: You dissolve the 15 grams of KNO3 in a little bit of water and dilute it to exactly 100 milliliters in a volumetric flask. In other words, you dissolve and dilute 15 grams of KNO3 to 100 milliliters. (I tend to abbreviate dissolve and dilute by writing d&d, but sometimes it gets confused with Dungeons & Dragons. Yes, chemists are really, really nerds.) You won’t know exactly how much water you put in, but it’s not important as long as the final volume is 100 milliliters.
You can also use the percentage and volume to calculate the grams of solute present. You may want to know how many grams of sodium hypochlorite are in 500 milliliters of a 5 percent (w/v) solution of household bleach. You can set up the problem like this:
![]()
You now know that you have 25 grams of sodium hypochlorite in the 500 milliliters of solution.
Sometimes both the solute and solvent are liquids. In this case, it’s convenient to use a volume/volume percentage.
Volume/volume percentage
With volume/volume percentages, both the solute and solution are expressed in milliliters:
![]()
Ethyl alcohol (the drinking alcohol) solutions are commonly made using volume/volume percentages. If you want to make 100 milliliters of a 50 percent ethyl alcohol solution, you take 50 milliliters of ethyl alcohol and dilute it to 100 milliliters with water. Again, it’s a case of dissolving and diluting to the required volume. You can’t simply add 50 milliliters of alcohol to 50 milliliters of water — you’d get less than 100 milliliters of solution. The polar water molecules will attract the polar alcohol molecules. This tends to fill in the open framework of water molecules and prevents the volumes from simply being added together.
It's number one! Molarity
Molarity is the concentration unit most often used by chemists, because it utilizes moles. The mole concept is central to chemistry, and molarity lets chemists easily work solutions into reaction stoichiometry. (If you’re cussing me out right now because you have no idea what burrowing, insect-eating mammals have to do with chemistry, let alone what stoichiometry is, just flip to Chapter 10 for the scoop. Your mother would probably recommend washing your mouth out with soap first.)
Molarity (M) is defined as the moles of solute per liter of solution.
Mathematically, it looks like this:
![]()
For example, you can take 1 mole (abbreviated as mol) of KCl (formula weight of 74.55 g/mol — you can get the scoop on formula and molecular weights in Chapter 10, too) and dissolve and dilute the 74.55 grams to 1 liter of solution in a volumetric flask. You then have a 1-molar solution of KCl. You can label that solution as 1 M KCl. You don’t add the 74.55 grams to 1 liter of water. You want to end up with a final volume of 1 liter. When preparing molar solutions, always dissolve and dilute to the required volume. This process is shown in Figure 11-1.
Here’s another example: If 25.0 grams of KCl are dissolved and diluted to 350.0 milliliters, how would you calculate the molarity of the solution? You know that molarity is moles of solute per liter of solution. So you can take the grams, convert them to moles using the formula weight of KCl (74.55 g/mol), and divide them by 0.350 liters (350.0 milliliters). You can set up the equation like this:
![]()
Now suppose that you want to prepare 2.00 liters of a 0.550 M KCl solution. The first thing you do is calculate how much KCl you need to weigh:
![]()
You then take that 82.0 grams of KCl and dissolve and dilute it to 2.00 liters.
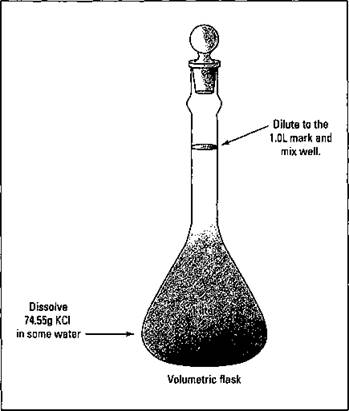
Figure 11-1: Making a 1-molar KCl solution.
There’s one more way to prepare solutions — the dilution of a more concentrated solution to a less-concentrated one. For example, you can buy hydrochloric acid from the manufacturer as a concentrated solution of 12.0 M. Suppose that you want to prepare 500 milliliters of 2.0 M HCl. You can dilute some of the 12.0 M to 2.0 M, but how much of the 12.0 M HCl is needed? You can easily figure the volume (V) you need by using the following formula:
![]()
In the preceding equation, Vold is the old volume, or the volume of the original solution, Mold is the molarity of the original solution, Vnew is the volume of the new solution, and Mnew is the molarity of the new solution. After substituting the values, you have
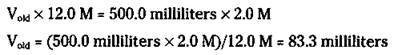
You then take 83.3 milliliters of the 12.0 M HCl solution and dilute it to exactly 500.0 milliliters.
WARNING! If you’re actually doing a dilution of concentrated acids, be sure to add the acid to the water instead of the other way around! If the water is added to the concentrated acid, then so much heat will be generated that the solution will quite likely splatter all over you.
So to be safe, you should take about 400 milliliters of water, slowly add the 83.3 milliliters of the concentrated HCl as you stir, and then dilute to the final 500 milliliters with water.
The usefulness of the molarity concentration unit is readily apparent when dealing with reaction stoichiometry. For example, suppose that you want to know how many milliliters of 2.50 M sulfuric acid it takes to neutralize a solution containing 100.0 grams of sodium hydroxide. The first thing you must do is write the balanced chemical equation for the reaction:
![]()
You know that you have to neutralize 100.0 grams of NaOH. You can convert the weight to moles (using the formula weight of NaOH, 40.00 g/mol) and then convert from moles of NaOH to moles of H2SO4. Then you can use the molarity of the acid solution to get the volume:
![]()
It takes 500.0 milliliters of the 2.50 M H2SO4 solution to completely react with the solution that contains 100. grams of NaOH.
Molality: Another use for the mote
Molality is another concentration term that involves moles of solute. It isn’t used very much, but I want to tell you a little about it, just in case you happen to run across it.
Molality (m) is defined as the moles of solute per kilogram of solvent. It’s one of the few concentration units that doesn’t use the solution’s weight or volume. Mathematically, it looks like this:
![]()
Suppose, for example, you want to dissolve 15.0 grams of NaCl in 50.0 grams of water. You can calculate the molality like this (you must convert the 50.0 grams to kilograms before you use it in the equation):
![]()
Parts per million: The pollution unit
Percentage and molarity, and even molality, are convenient units for the solutions that chemists routinely make in the lab or the solutions that are commonly found in nature. However, if you begin to examine the concentrations of certain pollutants in the environment, you’ll find that those concentrations are very, very small. Percentage and molarity will work when you’re measuring solutions found in the environment, but they’re not very convenient. In order to express the concentrations of very dilute solutions, scientists have developed another concentration unit — parts per million.
Percentage is parts per hundred, or grams solute per 100 grams of solution. Parts per million (ppm) is grams solute per one million grams of solution. It’s most commonly expressed as milligrams solute per kilogram solution, which is the same ratio. The reason it’s expressed this way is that chemists can easily weigh out milligrams or even tenths of milligrams, and, if you’re talking about aqueous solutions, a kilogram of solution is the same as a liter of solution. (The density of water is 1 gram per milliliter, or 1 kilogram per liter. The weight of the solute in these solutions is so very small that it’s negligible when converting from the mass of the solution to the volume.)
By law, the maximum contamination level of lead in drinking water is 0.05 ppm. This number corresponds to 0.05 milligrams of lead per liter of water. That’s pretty dilute. But mercury is regulated at the 0.002 ppm level. Sometimes, even this unit isn’t sensitive enough, so environmentalists have resorted to the parts per billion (ppb) or parts per trillion (ppt) concentration units. Some neurotoxins are deadly at the parts per billion level.
Colligative Properties of Solutions
Some properties of solutions depend on the specific nature of the solute.
In other words, an effect you can record about the solution depends on the specific nature of the solute. For example, salt solutions taste salty, while sugar solutions taste sweet. Salt solutions conduct electricity (they’re electrolytes — see Chapter 6), while sugar solutions don’t (they’re nonelectrolytes). Solutions containing the nickel cation are commonly green, while those containing the copper cation are blue.
There’s also a group of solution properties that doesn’t depend on the specific type of solute — just the number of solute particles. These properties are called colligative properties — properties that simply depend on the relative number of solute particles. The effect you can record about the solution depends on the number of solute particles present. These colligative properties — these effects — include
ü Vapor pressure lowering
ü Boiling point elevation
ü Freezing point depression
ü Osmotic pressure
Vapor pressure lowering
If a liquid is contained in a closed container, the liquid eventually evaporates, and the gaseous molecules contribute to the pressure above the liquid. The pressure due to the gaseous molecules of the evaporated liquid is called the liquid’s vapor pressure.
If you take that same liquid and make it the solvent in a solution, the vapor pressure due to the solvent evaporation will be lower. This is because the solute particles in the liquid take up space at the surface and so the solvent can’t evaporate as easily. And many times there may be an attraction between the solute and solvent that also makes it more difficult for the solvent to evaporate. And that lowering is independent of what kind of solute you use. Instead, it depends on the number of solute particles.
In other words, if you add one mole of sucrose to a liter of water and add one mole of dextrose to another liter of water, the amount that the pressure lowers will be the same, because you’re adding the same number of solute particles. If, however, you add a mole of sodium chloride, the vapor pressure will be lowered by twice the amount of sucrose or glucose. The reasons is that the sodium chloride breaks apart into two ions, so adding a mole of sodium chloride yields two moles of particles (ions).
This lowering of vapor pressure partially explains why the Great Salt Lake has a lower evaporation rate than you may expect. The salt concentration is so high that the vapor pressure (and evaporation) has been significantly lowered.
Why use antifreeze in the summer? Boiling point elevation
Each individual liquid has a specific temperature at which it boils (at a given atmospheric pressure). This temperature is the liquid’s boiling point If you use a particular liquid as a solvent in a solution, you find that the boiling point of the solution is always higher than the pure liquid. This is called the boiling point elevation.
It explains why you don’t replace your antifreeze with pure water in the summer. You want the coolant to boil at a higher temperature so that it will absorb as much engine heat as possible without boiling. You also use a pressure cap on your radiator, because the higher the pressure, the higher the boiling point. It also explains why a pinch of salt in the cooking water will cause foods to cook a little faster. The salt raises the boiling point so that more energy can be transferred to cooking the food during a given amount of time.
TECHNICAL STUFF. As an FYI, you can actually calculate the amount of boiling point elevation by using this formula:
![]()
∆Tb is the increase in the boiling point, Kb is the boiling point elevation constant (0.512°C kg/mol for water), and m is the molality of particles. (For molecular substances, the molality of particles is the same as the molality of the substance; for ionic compounds, you have to take into consideration the formation of ions and calculate the molality of the ion particles.) Solvents other than water have a different boiling point elevation constant (Kb).
Making ice cream: Freezing point depression
Each individual liquid has a specific temperature at which it freezes. If you use a particular liquid as a solvent in a solution, though, you find that the freezing point of the solution is always lower than the pure liquid. This is called the freezing point depression, and it’s a colligative property of a solution.
The depression of the freezing point of a solution relative to the pure solvent explains why you put rock salt in the ice/water mix when making homemade ice cream. The rock salt forms a solution with a lower freezing point than water (or the ice cream mix that’s to be frozen). The freezing point depression effect also explains why a salt (normally calcium chloride, CaCl2) is spread on ice to melt it. The dissolving of calcium chloride is highly exothermic (it gives off a lot of heat). When the calcium chloride dissolves, it melts the ice. The salt solution that’s formed when the ice melts has a lowered freezing point that keeps the solution from refreezing. Freezing point depression also explains the use of antifreeze in your cooling system during the winter. The more you use (up to a concentration of 50/50), the lower the freezing point.
TECHNICAL STUFF. In case you’re interested, you can actually calculate the amount the freezing point will be depressed:
![]()
∆Tf is the amount the freezing point will be lowered, Kf is the freezing point depression constant (1.86°C kg/mol for water), and m is the molality of the particles.
Figure 11-2 shows the effect of a solute on both the freezing point and boiling point of a solvent.
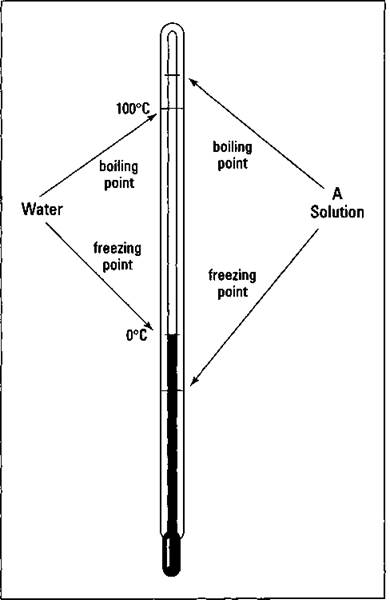
Figure 11-2: Boiling point elevation and freezing point depression of a solution.
Keeping blood cells alive and well: Osmotic pressure
Suppose that you take a container and divide it into two compartments with a thin membrane containing microscopic pores large enough to allow water molecules but not solute particles to pass through. This membrane type is called a semipermeable membrane; it lets some small particles pass through but not other, larger particles.
You then add a concentrated salt solution to one compartment and a more dilute salt solution to the other. Initially, the two solution levels start out the same. But after a while, you notice that the level on the more concentrated side has risen, and the level on the more dilute side has dropped. This change in levels is due to the passage of water molecules from the more dilute side to the more concentrated side through the semipermeable membrane. This process is called osmosis, the passage of a solvent through a semipermeable membrane into a solution of higher solute concentration. The pressure that you have to exert on the more concentrated side in order to stop this process is called osmotic pressure. This process is shown in Figure 11-3.
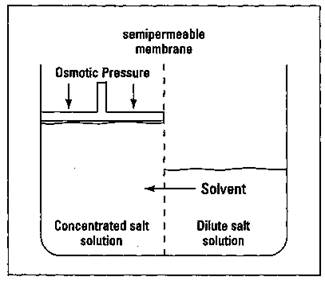
Figure 11-3: Osmotic pressure.
The solvent always flows through the semipermeable membrane from the more dilute side to the more concentrated side. In fact, you can have pure water on one side and any salt solution on the other, and water always goes from the pure-water side to the salt-solution side. The more concentrated the salt solution, the more pressure it takes to stop the osmosis (the higher the osmotic pressure).
But what if you apply more pressure than is necessary to stop the osmotic process, exceeding the osmotic pressure? Water is forced through the semipermeable membrane from the more concentrated side to the more dilute side, a process called reverse osmosis. Reverse osmosis is a good, relatively inexpensive way of purifying water. My local “water store” uses this process to purify drinking water (so-called “RO water”). There are many reverse osmosis plants in the world, extracting drinking water from seawater. Navy pilots even carry small reverse osmosis units with them in case they have to eject at sea.
The process of osmosis is important in biological systems. Cell walls often act as semipermeable membranes. Do you ever eat pickles? Cucumbers are soaked in a brine solution in order to make pickles. The concentration of the solution inside the cucumber is less than the concentration of the brine solution, so water migrates through the cell walls into the brine, causing the cucumber to shrink.
One of the most biologically important consequences of osmotic pressure involves the cells within our own body. You can look at red blood cells as an example. There’s an aqueous solution inside the blood cell and another aqueous solution outside the cell (intercellular fluid). When the solution outside the cell has the same osmotic pressure as the solution inside the cell, it’s said to be isotonic. Water can be exchanged in both directions, helping to keep the cell healthy. However, if the intercellular fluid becomes more concentrated and has a higher osmotic pressure (hypertonic), water flows primarily out of the blood cell, causing it to shrink and become irregular in shape. This is a process called crenation. The process may occur if the person becomes seriously dehydrated, and the crenated cells are not as efficient in carrying oxygen. If, on the other hand, the intercellular fluid is more dilute than the solution inside the cells and has a lower osmotic pressure (hypotonic), the water flows mostly into the cell. This process, called hemolysis, causes the cell to swell and eventually rupture. Figure 11-4 shows crenation and hemolysis.
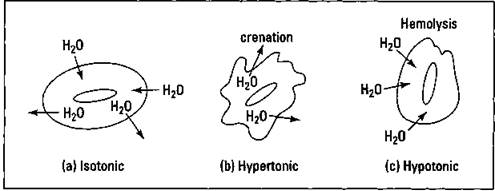
Figure 11-4: Crenation and hemolysis of red blood cells.
The processes of crenation and hemolysis explain why the concentration of IV solutions is so very critical. If they’re too dilute, then hemolysis can take place, and if they’re too concentrated, crenation is a possibility.
Smoke, Clouds, Whipped Cream, and Marshmallows: Colloids All
If you dissolve table salt in water, you form an aqueous solution. The solute particle size is very small — around 1 nanometer (nm), which is 1 x 10-9 meters. This solute doesn’t settle to the bottom of a glass, and it can’t be filtered out of the solution.
If, however, you go down to your local stream and dip out a glass of water, you’ll notice that there’s a lot of material in it. Many of the solute particles are larger than 1,000 nm. They quickly settle to the bottom of the glass and can be filtered out. In this case, you have a suspension and not a solution. Whether you have one or the other depends on the size of the solute particles.
But there’s also something in the intermediate range between solutions and suspensions. When the solute particle size is 1 to 1,000 nanometers, you have a colloid. Solutes in colloids don’t settle out like they do in suspensions. In fact, it’s sometimes difficult to distinguish colloids from true solutions. One of the few ways to distinguish between them is to shine a light through the suspected liquid. If it’s a true solution, with very small solute particles, the light beam will be invisible. If you have a colloid, however, you’ll be able to see the light beam as it reflects off the relatively large solute particles. This is called the Tyndall effect; and it’s shown in Figure 11-5.
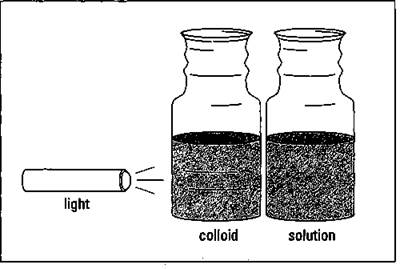
Figure 11-5: The Tyndall effect.
There are many types of colloids. Have you ever eaten a marshmallow? It’s a colloid of a gas in a solid. Whipped cream is a colloid of a gas in a liquid. Have you ever driven through the fog and seen your headlight beams? You were experiencing the Tyndall effect of a liquid-in-a-gas colloid. Smoke is a colloid of a solid (ash or soot) in a gas (air). Air pollution problems are often caused by the stability of this type of colloid.