Chemistry for Dummies
Part II. Blessed Be the Bonds That Tie
Chapter 9. Electrochemistry: Batteries to Teapots
In This Chapter
· Finding out about redox reactions
· Figuring out how to balance redox equations
· Taking a look at electrochemical cells
· Checking out electroplating
· Discovering how burning fuels and burning foods are similar
Many of the things we deal with in real life are related either directly or indirectly to electrochemical reactions. Think of all the things around you that contain batteries — flashlights, watches, automobiles, calculators, PDAs, pacemakers, cell phones, toys, garage door openers, and so on.
Do you drink from an aluminum can? The aluminum was extracted by an electrochemical reaction. Do you have a car with a chrome bumper? That chrome is electroplated onto the bumper, just like the silver on Grandmother Grace’s tea service or the gold on that five-dollar gold chain. Do you watch television, use electric lights or an electric blender, or have a desktop computer? There’s a good chance that the electricity you use for these things is generated from the combustion of some fossil fuel. Combustion is a redox reaction. So are respiration, photosynthesis, and many other biochemical processes that we depend upon for life. Electrochemical and redox reactions surround us.
In this chapter, I explain redox reactions, go through the balancing of this type of equation, and then show you some applications of redox reactions in an area of chemistry called electrochemistry.
There Go Those Pesky Electrons: Redox Reactions
Redox reactions — reactions in which there’s a simultaneous transfer of electrons from one chemical species to another — are really composed of two different reactions: oxidation (a loss of electrons) and reduction (a gain of electrons). These reactions are coupled, as the electrons that are lost in the oxidation reaction are the same electrons that are gained in the reduction reaction. In fact, these two reactions (reduction and oxidation) are commonly called half-reactions, because it takes these two halves to make a whole reaction, and the overall reaction is called a redox (reduction/oxidation) reaction. In Chapter 8, I describe a redox reaction that occurs between zinc metal and the cupric (Cu2+) ion. The zinc metal loses electrons and the cupric ion gains them.
Now where did I put those electrons? Oxidation
There are three definitions you can use for oxidation:
ü The loss of electrons
ü The gain of oxygen
ü The loss of hydrogen
Because I typically deal with electrochemical cells, I normally use the definition that describes the loss of the electrons. The other definitions are useful in processes such as combustion and photosynthesis.
Loss of electrons
One way to define oxidation is with the reaction in which a chemical substance loses electrons in going from reactant to product. For example, when sodium metal reacts with chlorine gas to form sodium chloride (NaCl), the sodium metal loses an electron, which is then gained by chlorine. The following equation shows sodium losing the electron:
![]()
When it loses the electron, chemists say that the sodium metal has been oxidized to the sodium cation. (A cation is an ion with a positive charge due to the loss of electrons — see Chapter 6.)
Reactions of this type are quite common in electrochemical reactions, reactions that produce or use electricity. (For more info about electrochemical reactions, flip to the section, “Power On the Go: Electrochemical Cells,” later in this chapter.)
Gain of oxygen
Sometimes, in certain oxidation reactions, it’s obvious that oxygen has been gained in going from reactant to product. Reactions where the gain of oxygen is more obvious than the gain of electrons include combustion reactions (burning) and the rusting of iron. Here are two examples:
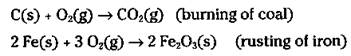
In these cases, chemists say that the carbon and the iron metal have been oxidized to carbon dioxide and rust, respectively.
Loss of hydrogen
In other reactions, oxidation can best be seen as the loss of hydrogen. Methyl alcohol (wood alcohol) can be oxidized to formaldehyde:
![]()
In going from methanol to formaldehyde, the compound went from having four hydrogen atoms to having two hydrogen atoms.
Look what I found! Reduction
Like oxidation, there are three definitions you can use to describe reduction:
ü The gain of electrons
ü The loss of oxygen
ü The gain of hydrogen
Gain of electrons
Reduction is often seen as the gain of electrons. In the process of electroplating silver onto a teapot (see the section, “Five Dollars for a Gold Chain? Electroplating,” later in this chapter), for example, the silver cation is reduced to silver metal by the gain of an electron. The following equation shows the silver cation gaining the electron:
![]()
When it gains the electron, chemists say that the silver cation has been reduced to silver metal.
Loss of oxygen
In other reactions, it’s easier to see reduction as the loss of oxygen in going from reactant to product. For example, iron ore (primarily rust, Fe2O3) is reduced to iron metal in a blast furnace by a reaction with carbon monoxide:
![]()
The iron has lost oxygen, so chemists say that the iron ion has been reduced to iron metal.
Gain of hydrogen
In certain cases, a reduction can also be described as the gain of hydrogen atoms in going from reactant to product. For example, carbon monoxide and hydrogen gas can be reduced to methyl alcohol:
![]()
In this reduction process, the CO has gained the hydrogen atoms.
One's loss is the other's gain
Neither oxidation nor reduction can take place without the other. When those electrons are lost, something has to gain them.
Consider, for example, the net-ionic equation (the equation showing just the chemical substances that are changed during a reaction — see Chapter 8) for a reaction with zinc metal and an aqueous copper(II) sulfate solution:
![]()
This overall reaction is really composed of two half-reactions:
![]() (oxidation half-reaction — the loss of electrons)
(oxidation half-reaction — the loss of electrons)
![]() (reduction half-reaction — the gain of electrons)
(reduction half-reaction — the gain of electrons)
TIP. To help yourself remember which is oxidation and which is reduction in terms of electrons, memorize the phrase “LEO goes GER” (lose Electrons Oxidation; Gain Electrons Reduction).
Zinc loses two electrons; the copper(II) cation gains those same two electrons. Zn is being oxidized. But without Cu2+ present, nothing will happen. That copper cation is the oxidizing agent. It’s a necessary agent for the oxidation process to proceed. The oxidizing agent accepts the electrons from the chemical species that is being oxidized.
Cu2+ is reduced as it gains electrons. The species that furnishes the electrons is called the reducing agent. In this case, the reducing agent is zinc metal.
TIP. The oxidizing agent is the species that’s being reduced, and the reducing agent is the species that’s being oxidized. Both the oxidizing and reducing agents are on the left (reactant) side of the redox equation.
Playing the numbers: Oxidation numbers, that is
Oxidation numbers are bookkeeping numbers. They allow chemists to do things such as balance redox equations. Oxidation numbers are positive or negative numbers, but don’t confuse them with charges on ions or valences. Oxidation numbers are assigned to elements using these rules:
ü Rule 1: The oxidation number of an element in its free (uncombined) state is zero (for example, Al(s) or Zn(s)). This is also true for elements found in nature as diatomic (two-atom) elements (H2, O2, N2, F2, Cl2, Br2, or I2) and for sulfur, found as S8.
ü Rule 2: The oxidation number of a monatomic (one-atom) ion is the same as the charge on the ion (for example, Na+ = +1, S2- = -2).
ü Rule 3: The sum of all oxidation numbers in a neutral compound is zero. The sum of all oxidation numbers in a polyatomic (many-atom) ion is equal to the charge on the ion. This rule often allows chemists to calculate the oxidation number of an atom that may have multiple oxidation states, if the other atoms in the ion have known oxidation numbers. (See Chapter 6 for examples of atoms with multiple oxidation states.)
ü Rule 4: The oxidation number of an alkali metal (IA family) in a compound is +1; the oxidation number of an alkaline earth metal (IIA family) in a compound is +2.
ü Rule 5: The oxidation number of oxygen in a compound is usually -2. If, however, the oxygen is in a class of compounds called peroxides (for example, hydrogen peroxide, or H2O2), then the oxygen has an oxidation number of -1. If the oxygen is bonded to fluorine, the number is +1.
ü Rule 6: The oxidation state of hydrogen in a compound is usually +1. If the hydrogen is part of a binary metal hydride (compound of hydrogen and some meted), then the oxidation state of hydrogen is -1.
ü Rule 7: The oxidation number of fluorine is always -1. Chlorine, bromine, and iodine usually have an oxidation number of -1, unless they’re in combination with an oxygen or fluorine. (For example, in CIO-, the oxidation number of oxygen is -2 and the oxidation number of chlorine is +1; remember that the sum of all the oxidation numbers in CIO- have to equal -1.)
These rules give you another way to define oxidation and reduction — in terms of oxidation numbers. For example, consider this reaction, which shows oxidation by the loss of electrons:
![]()
Notice that the zinc metal (the reactant) has an oxidation number of zero (rule 1), and the zinc cation (the product) has an oxidation number of +2 (rule 2). In general, you can say that a substance is oxidized when there’s an increase in its oxidation number.
Reduction works the same way. Consider this reaction:
![]()
The copper is going from an oxidation number of +2 to zero. A substance is reduced if there’s a decrease in its oxidation number.
Balancing redox equations
Redox equations are often so complex that the inspection method (the fiddling with coefficients method) of balancing chemical equations doesn’t work well with them (see Chapter 8 for a discussion of this balancing method). So chemists have developed two different methods of balancing redox equations. One method is called the oxidation number method. It’s based on the changes in oxidation numbers that take place during the reaction. Personally, I don’t think this method works nearly as well as the second method, the ion-electron (half-reaction) method, because it’s sometimes difficult to determine the exact change in the numerical value of the oxidation numbers. So I’m just going to show you the second method.
Here’s an overview of the ion-electron method: The unbalanced redox equation is converted to the ionic equation and then broken down into two halfreactions — oxidation and reduction. Each of these half-reactions is balanced separately and then combined to give the balanced ionic equation. Finally, the spectator ions are put into the balanced ionic equation, converting the reaction back to the molecular form. (Buzzword-o-rama, eh? For a discussion of molecular, ionic, and net-ionic equations, see Chapter 8.) It’s important to follow the steps precisely and in the order listed. Otherwise, you may not be successful in balancing redox equations.
Now how about an example? I’m going to show you how to balance this redox equation with the ion-electron method:
![]()
Follow these steps:
1. Convert the imbalanced redox reaction to the ionic form.
In this reaction, you show the nitric acid in the ionic form, because it’s a strong acid (for a discussion of strong acids, see Chapter 12). Copper(ff) nitrate is soluble (indicated by (aqj), so it’s shown in its ionic form (see Chapter 8). Because NO(g) and water are molecular compounds, they remain shown in the molecular form:
![]()
2. If necessary, assign oxidation numbers and then write two half-reactions (oxidation and reduction) showing the chemical species that have had their oxidation numbers changed.
In some cases, it’s easy to tell what has been oxidized and reduced; but in other cases, it isn’t as easy. Start by going through the example reaction and assigning oxidation numbers. You can then use the chemical species that have had their oxidation numbers changed to write your unbalanced half-reactions:
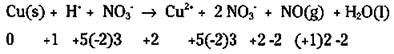
Look closely. Copper changed its oxidation number (from 0 to 2) and so has nitrogen (from -2 to +2). Your unbalanced half-reactions are
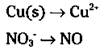
3. Balance all atoms, with the exception of oxygen and hydrogen.
It’s a good idea to wait until the end to balance hydrogen and oxygen atoms, so always balance the other atoms first. You can balance them by inspection — fiddling with the coefficients. (You can’t change subscripts; you can only add coefficients.) However, in this particular case, both the copper and nitrogen atoms already balance, with one each on both sides:
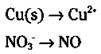
4. Balance the oxygen atoms.
How you balance these atoms depends on whether you’re dealing with acid or basic solutions:
• In acid solutions, take the number of oxygen atoms needed and add that same number of water molecules to the side that needs oxygen.
• In basic solutions, add 2 OH- to the side that needs oxygen for every oxygen atom that is needed. Then, to the other side of the equation, add half as many water molecules as OH" anions used.
An acidic solution will have some acid or H+ shown; a basic solution will have an OH' present. The example equation is in acidic conditions (nitric acid, HNO3, which, in ionic form, is H+ + NO3-). There’s nothing to do on the half-reaction involving the copper, because there are no oxygen atoms present. But you do need to balance the oxygen atoms in the second half-reaction:
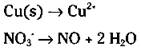
5. Balance the hydrogen atoms.
Again, how you balance these atoms depends on whether you’re dealing with acid or basic solutions:
• In acid solutions, take the number of hydrogen atoms needed and add that same number of H+ to the side that needs hydrogen.
• In basic solutions, add one water molecule to the side that needs hydrogen for every hydrogen atom that’s needed. Then, to the other side of the equation, add as many OH' anions as water molecules used.
The example equation is in acidic conditions. You need to balance the hydrogen atoms in the second half-reaction:
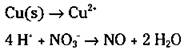
6. Balance the ionic charge on each half-reaction by adding electrons.
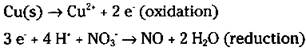
The electrons should end up on opposite sides of the equation in the two half-reactions. Remember that you’re using ionic charge, not oxidation numbers.
7. Balance electron loss with electron gain between the two halfreactions.
The electrons that are lost in the oxidation half-reaction are the same electrons that are gained in the reduction half-reaction. The number of electrons lost and gained must be the same. But Step 6 shows a loss of 2 electrons and a gain of 3. So you must adjust the numbers using appropriate multipliers for both half-reactions. In this case, you have to find the lowest common denominator between 2 and 3. It’s 6, so multiply the first half-reaction by 3 and the second half-reaction by 2.
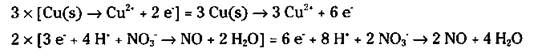
8. Add the two half-reactions together and cancel anything common to both sides. The electrons should always cancel (the number of electrons should be the same on both sides).
![]()
9. Convert the equation back to the molecular form by adding the spectator ions.
If it’s necessary to add spectator ions to one side of the equation, add the same number to the other side of the equation. For example, there are 8 H+ on the left side of the equation. In the original equation, the H+ was in the molecular form of HNO3. You need to add the NO3- spectator ions back to it. You already have 2 on the left, so you simply add 6 more. You then add 6 NO3- to the right-hand side to keep things balanced. Those are the spectator ions that you need for the Cu2+ cation to convert it back to the molecular form that you want.
![]()
10. Check to make sure that all the atoms are balanced, all the charges are balanced (if working with an ionic equation at the beginning), and all the coefficients are in the lowest whole-number ratio.
That’s how it’s done. Reactions that take place in base are just as easy, as long as you follow the rules.
Pouter On the Go: Electrochemical Cells
In the section, “One’s loss is the other’s gain,” I discuss a reaction in which I put a piece of zinc metal into a copper(II) sulfate solution. The copper metal begins spontaneously plating out on the surface of the zinc. The equation for this reaction is
![]()
This is an example of direct electron transfer. Zinc gives up two electrons (becomes oxidized) to the Cu2+ ion that accepts the electrons (reducing it to copper metal). In Chapter 8, I show you that nothing happens if you place a piece of copper meted into a solution containing Zn2+, because zinc gives up electrons more easily than copper. I also show you the activity series of metals that allows you to predict whether or not a displacement (redox) reaction will take place.
Now this is a useful reaction if you want to plate out copper onto zinc. However, not many of us have a burning desire to do this! But if you were able to separate those two half-reactions so that when the zinc is oxidized, the electrons it releases are forced to travel through a wire to get to the Cu2+, you’d have something useful. You’d have a galvanic or voltaic cell, a redox reaction that produces electricity. In this section, I show you how that Zn/Cu2+ reaction may be separated out so that you have an indirect electron transfer and can produce some useable electricity.
TECHNICAL STUFF. Galvanic cells are commonly called batteries, but sometimes this name is somewhat incorrect. A battery is composed of two or more cells connected together. You put a battery in your car, but you put a cell into your flashlight.
Nice cell there, Daniell
Take a look at Figure 9-1, which shows a Daniell cell that uses the Zn/Cu2+ reaction to produce electricity. (This cell is named after John Frederic Daniell, the British chemist who invented it in 1836.)
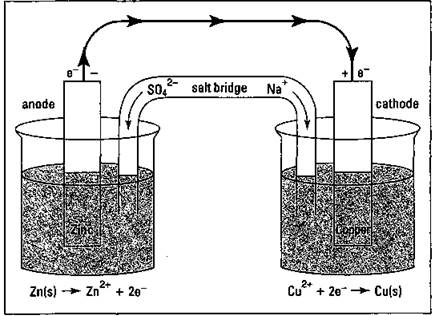
Figure 9-1: A Daniell cell.
In the Daniell cell, a piece of zinc metal is placed in a solution of zinc sulfate in one container, and a piece of copper metal is placed in a solution of copperQI) sulfate in another container. These strips of metal are called the cell’s electrodes. They act as a terminal, or a holding place, for electrons. A wire connects the electrodes, but nothing happens until you put a salt bridge between the two containers. The salt bridge, normally a U-shaped hollow tube filled with a concentrated salt solution, provides a way for ions to move from one container to the other to keep the solutions electrically neutral. It’s like running only one wire up to a ceiling light; the light won’t work unless you put in a second wire to complete the circuit.
With the salt bridge in place, electrons can start to flow. It’s the same basic redox reaction as the one I show you at the beginning of this section. Zinc is being oxidized, releasing electrons that flow through the wire to the copper electrode, where they’re available for the Cu2+ ions to use in forming copper metal. Copper ions from the copper(II) sulfate solution are being plated out on the copper electrode, while the zinc electrode is being consumed. The cations in the salt bridge migrate to the container containing the copper electrode to replace the copper ions being consumed, while the anions in the salt bridge migrate toward the zinc side, where they keep the solution containing the newly formed Zn2+ cations electrically neutral.
The zinc electrode is called the anode, the electrode at which oxidation takes place, and is labeled with a sign. The copper electrode is called the cathode, the electrode at which reduction takes place, and is labeled with a “+” sign.
This cell will produce a little over one volt. You can get just a little more voltage if you make the solutions that the electrodes are in very concentrated. But what can you do if you want, for example, two volts? You have a couple of choices. You can hook two of these cells up together and produce two volts, or you can choose two different metals from the activity series chart in Chapter 8 that are farther apart than zinc and copper. The farther apart the metals are on the activity series, the more voltage the cell will produce.
Let the light shine: Flashlight cells
The common flashlight cell (see Figure 9-2), a dry cell (it’s not in a solution like a Daniell cell), is contained in a zinc housing that acts as the anode. The other electrode, the cathode, is a graphite rod in the middle of the cell. A layer of manganese oxide and carbon black (one of the many forms of carbon) surrounds the graphite rod, and a thick paste of ammonium chloride and zinc chloride serves as the electrolyte. The cell reactions are
![]() (anode reaction/oxidation)
(anode reaction/oxidation)
![]() (cathode reaction/reduction)
(cathode reaction/reduction)
Note that the case is actually one of the electrodes; it’s being used up in the reaction. If there’s a thin spot in the case, a hole could form, and the cell could leak the corrosive contents. In addition, the ammonium chloride tends to corrode the metal case, again allowing for the possibility of leakage.
In the alkaline dry cell (alkaline battery), the acidic ammonium chloride of the regular dry cell is replaced by basic (alkaline) potassium hydroxide. With this chemical, corrosion of the zinc case is greatly reduced.
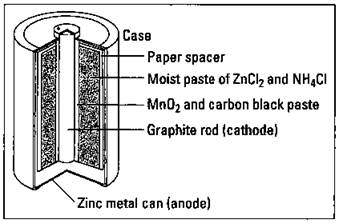
Figure 9-2: A dry cell.
Another cell with the same basic construction is the small mercury battery commonly used in watches, pacemakers, and so on. With this battery, the anode is zinc, as in the regular dry cell, but the cathode is steel. Mercury(ir) oxide (HgO) and some alkaline paste form the electrolyte. You should dispose of this type of battery carefully, to keep the mercury from being released into the environment.
All these galvanic cells produce electricity until they run out of a reactant. Then they must be discarded. However, there are cells (batteries) that can be recharged, as the redox reaction can be reversed to regenerate the original reactants. Nickel-cadmium (Ni-Cad) and lithium batteries fall into this category. The most familiar type of rechargeable battery is probably the automobile battery.
Gentlemen, start your engines: Automobile batteries
The ordinary automobile battery, or lead storage battery, consists of six cells connected in series (see Figure 9-3). The anode of each cell is lead, while the cathode is lead dioxide (PbO2). The electrodes are immersed in a sulfuric acid (H2SO4) solution. When you start your car, the following cell reactions take place:
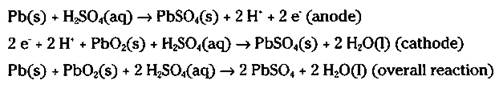
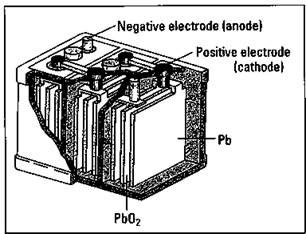
Figure 9-3: The lead storage battery.
When this reaction takes place, both electrodes become coated with solid lead (II) sulfate, and the sulfuric acid is used up.
After the automobile has been started, the alternator or generator takes over the job of producing electricity (for spark plugs, lights, and so on) and also recharges the battery. The alternator reverses both the flow of electrons into the battery and the original redox reactions, and regenerates the lead and lead dioxide:
![]()
The lead storage battery can be discharged and charged many times. But the shock of running over bumps in the road (or dead armadillos in Texas) or into the curb flakes off a little of the lead (II) sulfate and eventually causes the battery to fail.
During charging, the automobile battery acts like a second type of electrochemical cell, an electrolytic cell, which uses electricity to produce a desired redox reaction. This reaction may be the recharging of a battery, or it may be involved in the plating of Grandmother Grace’s teapot.
Five Dollars for a Gold Chain? Electroplating
Electrolytic cells, cells that use electricity to produce a desired redox reaction, are used extensively in our society. Rechargeable batteries are a primary example of this type of cell, but there are many other applications. Ever wonder how the aluminum in that aluminum can is mined? Aluminum ore is primarily aluminum oxide (Al2O3). Aluminum metal is produced by reducing the aluminum oxide in a high temperature electrolytic cell using approximately 250,000 amps. That’s a lot of electricity. It’s far cheaper to take old aluminum cans, melt them down, and reform them into new cans than it is to extract the metal from the ore. That’s why the aluminum industry is strongly behind the recycling of aluminum. It’s just good business.
Water can be decomposed by the use of electricity in an electrolytic cell. This process of producing chemical changes by passing an electric current through an electrolytic cell is called electrolysis (yes, just like the permanent removal of hair). The overall cell reaction is
![]()
In a similar fashion, sodium metal and chlorine gas can be produced by the electrolysis of molten sodium chloride.
Electrolytic cells are also used in a process called electroplating. In electroplating, a more-expensive metal is plated (deposited in a thin layer) onto the surface of a cheaper metal by electrolysis. Back before plastic auto bumpers became popular, chromium metal was electroplated onto steel bumpers. Those five-dollar gold chains you can buy are really made of some cheap metal with an electroplated surface of gold. Figure 9-4 shows the electroplating of silver onto a teapot.
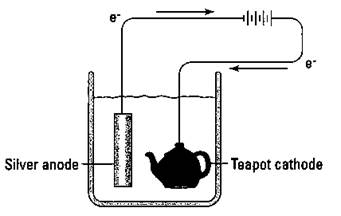
Figure 9-4: Electroplating silver onto a teapot.
A battery is commonly used to furnish the electricity for the process. The teapot acts as the cathode and a bar of silver acts as the anode. The silver bar furnishes the silver ions that are reduced onto the surface of the teapot. Many metals and even some alloys can be plated out in this fashion. Everybody loves those plated surfaces, especially without the high cost of the pure metal. (Reminds me of an Olympic athlete who was so proud of his gold metal that he had it bronzed!)
This Burns Me Up! Combustion of Fuels and Foods
Combustion reactions are types of redox reactions that are absolutely essential for life and civilization — because heat is the most important product of these reactions. The burning of coal, wood, natural gas, and petroleum heats our homes and provides the majority of our electricity. The combustion of gasoline, jet fuel, and diesel fuel powers our transportation systems. And the combustion of food powers our bodies.
Have you ever wondered how the energy content of a fuel or food is measured? An instrument called a bomb calorimeter is used to measure energy content. Figure 9-5 shows the major components of a bomb calorimeter.
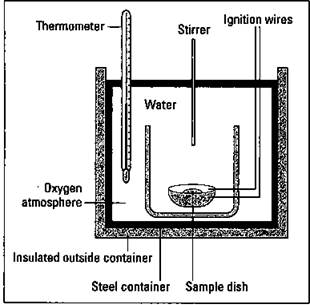
Figure 9-5: A bomb calorimeter.
To measure the energy content of fuels, a known mass of the material to be measured is placed into a sample cup and sealed. The air is removed from the sample cup and replaced with pure oxygen. The cup is then placed in the calorimeter with a known amount of water covering it. The initial temperature of the water is measured, and then the sample is ignited electrically. The rise in the temperature is measured, and the number of calories of energy that is released is calculated. A calorie is the amount of energy needed to raise the temperature of 1 gram of water 1 degree Celsius. The complete combustion of a large kitchen match, for example, gives you about one kilocalorie of heat. (See Chapter 2 for the basics of calories and measuring energy.)
The caloric content of foods can be determined in exactly the same fashion. Chemists report the results in calories or kilocalories, while nutritionists report the results in nutritional Calories. A nutritional Calorie is equal to a chemist’s kilocalorie (1,000 calories). A 300 Calorie candy bar produces 300,000 calories of energy. Unfortunately, not all that energy is required immediately, so some is stored in compounds such as fats. I’m carrying around the result of many candy bars.