Chemistry Essentials for Dummies
Chapter 11. Acids and Bases
The Bronsted-Lowry Acid-Base Theory
REMEMBER. Here’s how the Bronsted-Lowry theory defines acids and bases:
✓ An acid is a proton (H+) donor
✓ A base is a proton (H+) acceptor
The base accepts the H+ by furnishing a lone pair of electrons for a coordinate-covalent bond, which is a covalent bond (shared pair of electrons) in which one atom furnishes both of the electrons for the bond. Normally, one atom furnishes one electron for the bond and the other atom furnishes the second electron (see Chapter 6). In the coordinate-covalent bond, one atom furnishes both bonding electrons.
Figure 11-1 shows the NH3/HCl reaction using the electron-dot structures of the reactants and products. (I cover electron-dot structures in Chapter 7.)
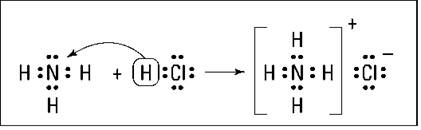
Figure 11-1: Reaction of NH3 with HCl.
HCl is the proton donor, and the acid and ammonia are the proton acceptor, or the base. Ammonia has a lone pair of nonbonding electrons that it can furnish for the coordinate-covalent bond.