The Handy Chemistry Answer Book (2014)
ANALYTICAL CHEMISTRY
MEASURE TWICE
What is accuracy?
Accuracy is used to refer to how close a measured value is to the true value of the quantity. This is pretty simple to understand. For example, if the true temperature in the room is 20 °C and a thermometer reads 20 °C, the measurement is pretty accurate. If the thermometer read 0 °C, the measurement is not very accurate.
What is precision?
Precision describes how reproducible a measurement is, regardless of whether the observed value matches the true value. Measurements can be very precise even if they are not perfectly accurate. For example, if you weighed yourself on a scale that was offset by 5 kg, you would always be off by the same amount, but you would not be measuring your real weight. So despite not being accurate, the value you obtain could still be very precise. This will generally be true in situations involving a systematic error that is simply a constant offset from the actual value.
What are some common units of concentration for a species in solution?
The most common unit of concentration in solution-phase chemistry is molarity, or the number of moles of a species present per liter of solution. Recall that the number of moles of a species is equal to the number of molecules of that species divided by Avogadro’s number (see “History of Chemistry” for information on Avogadro’s number).
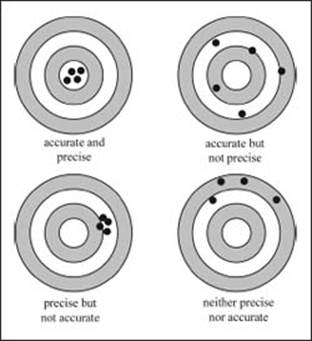
There is a difference between being precise and being accurate, as the bullet holes in these targets demonstrate.
In analytical chemistry, it can often be more convenient to discuss concentrations in terms of other units. This is particularly true when the species being studied is present in very small concentrations. Some other commonly used units are parts per million (ppm), parts per billion, or parts per trillion. These units refer to the mass fraction of the analyte in question to total mass of the sample. One part per million means 1 millionth (10−6) of a gram of the species in question, or analyte, per gram of the sample present. Similarly, one part per billion means 1 billionth (10−9) of a gram of analyte per gram of sample, and one part per trillion means 1 trillionth (10−12) of a gram of analyte per gram of sample. It is also possible to use each of these definitions based on a per unit volume basis, rather than a per unit mass basis (though this is more common in the gas phase). For example, 1 ppm by volume of an analyte means the analyte occupies 1 millionth (10−6) of the total volume of a sample.

A meniscus is the curve that forms on the surface of a liquid in a container. It can make measuring liquids by eye somewhat of a challenge.
What is a meniscus, and how should it be read?
A meniscus is the curve in the surface of a liquid contained within a (usually narrow) container, such as a graduated cylinder. Take a look at the graphic to the right.
The numerical value we read for the volume of liquid contained in this cylinder will depend on what part of the meniscus we choose to look at. Graduated cylinders and other containers are calibrated such that we should get the most accurate reading by looking at the center of the meniscus from eye level. If we don’t look at the meniscus from eye level, something called parallax error will occur that will distort our perception of level of the meniscus.
What is a titration?
A titration is a laboratory technique used to determine the concentration of a species in a solution. This is achieved by adding a second species of a known concentration (the titrant) that will react with the species whose concentration is to be determined (the analyte). There must be some indication of when the titration is complete, or, in other words, there must be some indication of when the unknown species has reacted completely with the titrant. A common example is the titration of a base by an acid. In these cases, a small amount of a pH-sensitive colored indicator can be added, and the end of the titration can be determined by observing a sudden large change in pH via a change in the color of the solution.
What is an aqueous solution?
An aqueous solution is any solution in which water is the solvent. This term is used pretty frequently in chemistry, so it’s good to be familiar with it.
What is a “standard state”?
A standard state is a set of reference conditions with respect to which properties can be described/calculated under other conditions. For example, the properties at standard-state conditions of a gas may be defined by its properties at 293.15 K and 1 bar of pressure. Knowledge of the properties of a gas under these standard conditions can be used to calculate the properties under other conditions.
What is a chemical indicator?
As we mentioned, for a titration to be successful there must be some indication of when it has finished. Chemical indicators are a common way of determining whether a titration is complete. In acid–base titrations, chemical indicators are typically small molecules whose colors change depending on the pH of the solution (or, in other words, on their protonation state). In other cases, the indicator may change color based on whether it is coordinated to another species in a solution. It is also possible to use a device like a pH-sensitive electrode to determine when the titration has ended; this doesn’t rely on you (or another person) being able to notice a visible change in the color of the solution. Such a device can be more accurate, since it doesn’t rely on a qualitative interpretation of a color change.
How do you use pH paper?
Another way of measuring the pH of a solution is with pH paper. This is a paper strip that contains a chemical indicator that changes color depending on the pH of a solution, and it can be capable of determining a very wide range of pH values. To properly use pH paper, it should not be dipped directly into the solution, but rather a drop of the solution should be placed on the pH paper. It can then be held next to a gradient color scale to obtain a pH value. Since this relies on visible color changes, it isn’t the most accurate method of determining pH, but it can be a useful tool in the lab for getting a quick estimate of the pH of a solution.
How long does it take a chemical reaction to reach completion?
The length of time it takes a reaction to run to completion can range from a fraction of a second to thousands of years. It all depends on how large the energy barrier is for reactants to convert to products. If it’s a simple acid-base reaction, like the addition of an HCl solution to water, the reaction will proceed almost instantly. Other reactions can be slower, such as the rusting of a car door. What you first notice as a small spot of rust could take years to spread over the rest of the door. Other reactions can be even slower yet. For example, the slowest biologically relevant reaction known in humans is estimated to take about one trillion years in the absence of an enzyme to catalyze it. That’s longer than scientists believe the universe has even existed! Fortunately, enzymes have evolved that allow this reaction to take place in only a few milliseconds.
What are some different types of titrations?
We already considered the titration of a base by an acid. Certainly acids can also be titrated by bases, but there are also many other types of titrations, so let’s look at just two more.
Complexometric titrations—This type of titration involves using a titrant that forms a complex with the analyte. In this case, the species of unknown concentration is often a metal ion, and the indicator may be a dye molecule that forms a weak complex with the metal ions. The indicator-ion complex is bound more weakly than the titrant-ion complex, such that the titrant can displace the indicator from the metal ions. Thus when the titrant has bound to a significant fraction of analyte molecules, the indicator molecules are displaced and the solution will change color.
Redox titrations—A redox titration is a titration that involves the use of a reducing agent or an oxidizing agent to determine the concentration of the unknown species. In this case, the indicator can again be sensitive to the presence of excess titrant, giving a color change when the titrant becomes present in excess.

In a titration, two solutions—one of a known concentration and one of an unknown concentration— are slowly combined until a reaction occurs. When that happens, the concentration of the second solution can be determined.
What is a buffer solution?
A buffer solution is a solution that has a tendency to resist changes in pH, even when an acid or base is added to the solution. Buffer solutions consist of a mixture of either a weak acid and its conjugate base, or a weak base and its conjugate acid, dissolved in water. When an acid is added, the base present in the solution tends to bind to the H+ from the acid, serving to “buffer” the change in pH and preventing the concentration of H3O+ from changing very much. Similarly, when a base is added, the acid in the solution tends to neutralize the base, again serving to buffer the change in pH by preventing the concentration of H3O+ from changing significantly.
How are buffer solutions important in the human body?
One very important buffer solution is human blood! An equilibrium between carbonic acid (H2CO3) and its conjugate base bicarbonate (HCO3−) helps blood to maintain a relatively constant pH of around 7.4. The carbonic acid buffer system is created by carbon dioxide (CO2) dissolved in blood; carbon dioxide reacts with water (H2O) to form carbonic acid. Since the amount of carbon dioxide in the blood depends on the rate at which you breathe, your blood pH is influenced by your breathing rate. Your body can actually tell when your blood pH gets too high or too low and adjusts your respiratory rate accordingly!
What is precipitation?
In chemistry, precipitation means something different from when the weather forecaster on the news predicts rain. A precipitate is a solid substance that does not dissolve in a solution, and precipitation describes the process of the precipitate forming. This generally takes place when one or more soluble reactants in a solution undergo a reaction to form one or more insoluble products. The result is that a solid substance will form in a solution, either depositing on the bottom of the container or sometimes floating around in the solution. Depending on the circumstances, precipitation can be a desirable or undesirable result.
What is gravimetric analysis?
Gravimetric analysis refers to any method for quantifying the amount of an analyte based on mass. For analytes dissolved in a solution, this may require first reacting the analyte with another species to cause it to precipitate out of the solution. The solid product can then be filtered and weighed, and the mass of the analyte initially dissolved in the solution can be determined. Other situations may require different methods of preparing the analyte in an appropriate form for weighing, but the general idea is always that the composition of the weighed species is known, so it’s possible to figure out the mass of the analyte originally present.
What is the difference between mass and weight?
Mass and weight are similar terms, but they have slightly different meanings. Weight tells us how much of something there is by telling us how much gravity is pulling down on it. Mass, on the other hand, just tells us how much of something there is, independent of the force of gravity on the object. One might reasonably ask, why does this distinction even matter? The reason it matters is that if we went to the Moon, for example, the weight of an object would change (because the force of gravity on the Moon is different from that on Earth), but the mass would be the same, since the object is made of the same amount of stuff; we just need to make sure everyone is talking about the same thing.
We should point out that people commonly use scales to measure either weight or mass, but when we’re using them to measure mass, the measurement relies on the fact that the scale has been calibrated based on the strength of gravity on the Earth, so it “knows” how to figure out the mass of an object from measuring its weight.
What is spectrophotometry?
Spectrophotometry is a technique that involves passing light through a sample to measure the fraction of light transmitted or reflected by the sample. This can be useful for determining the concentration of an analyte in a solution or for determining the identity of an analyte present.
A law known as Beer’s law (see also “Physical and Theoretical Chemistry”) is particularly important in spectrophotometry. Beer’s law tells us that the concentration of a species is directly proportional to its absorbance in a solution. This means that if we know the absorbance of a solution of our analyte at a known concentration, we can determine the concentration of that analyte in any other solution.
What is an electrolyte?
An electrolyte is a substance that contains ions (charged particles) and is capable of conducting electricity. Most often this is a solution containing dissolved ions, like an aqueous solution of sodium chloride (which contains Na+ and Cl− ions). The presence of ions in the solution is what makes it capable of conducting electricity. It’s for this reason that it can be dangerous to get the electrical outlet wet when drying your hair in the bathroom. Pure water itself doesn’t conduct electricity, but in practice, there are almost always some ions present, making tap water an electrolyte.
Another place electrolytes are important is in living things. Fluids in the human body, and in other organisms, contain free ions and are thus electrolytes. Gradients in ion concentrations in cellular fluids are often important for regulating cellular processes and bodily functions such as muscle and nerve activity.
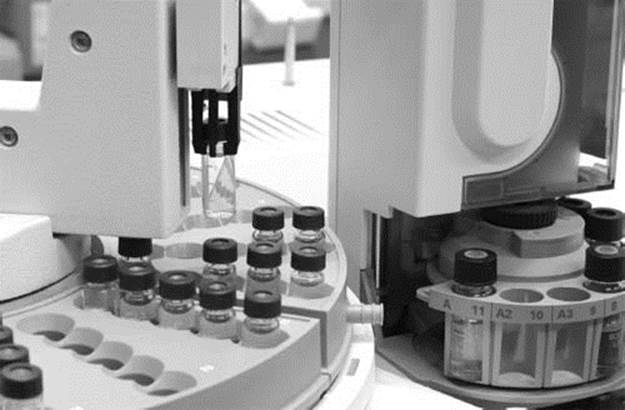
An autosampler selects a sample to be separated by gas chromatography, one of several types of chromatography techniques.
What is chromatography?
Chromatography describes a series of techniques used for separating the components of a mixture of chemicals. The most common type of chromatography is column chromatography. This involves dissolving the mixture to be separated into a solution and flowing it over a column of stationary material that interacts to a different extent with each component of the mixture. As the liquid flows over the stationary phase, the components that interact most strongly will be held behind longer while those that interact weakly will be eluted from the column first. The liquid is collected into a series of small vials or test tubes, which will, hopefully, each only contain one component of the mixture. The solvent can then be evaporated from each vial to recover the individual components of the mixture. A wide variety of chromatographic techniques exist, and each generally follows the same basic principles as the column chromatography described here.
What do the terms miscible and immiscible mean?
Miscible and immiscible are terms that refer to whether two liquids that come into contact will mix to form a single uniform solution. Two miscible liquids are able to mix together to form a uniform solution, while two immiscible liquids will separate into two layers. If the liquids are immiscible, the liquid with the lower density will stay on top and the liquid with the higher density will be on the bottom.
What is a liquid-liquid extraction?
Extractions are techniques used to separate the components of a mixture based on their solubilities in different media. A liquid-liquid extraction specifically uses two immiscible liquids to separate the components of a mixture. Two liquids are placed in the same container and shaken vigorously to allow the dissolved components to equilibrate between the two liquid phases. One liquid can then be drained from the container, and the solvents can then be evaporated to recover the separated components of the mixture.
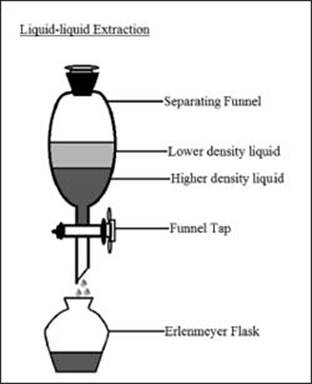
An apparatus for liquid-liquid extractions.
What is aqua regia and what is it used for?
Aqua regia translates to “royal water” in Latin, and it consists of a mixture of concentrated nitric and hydrochloric acids, typically in a 1 to 3 ratio, respectively. Since these are both strong acids, aqua regia can be quite dangerous and must be handled with extreme caution. This mixture is capable of dissolving several types of metals and has found many applications in chemistry, including the refinement of very high purity gold!
What is an oxidation/reduction reaction?
Oxidation and reduction reactions are chemical processes that involve the transfer of an electron between two species. Oxidation is the process of taking an electron away from a species, so if a molecule loses an electron, it has been oxidized. Reduction is the process of giving an additional electron to a species, so if a molecule gains an electron, it has been reduced. Oxidation and reduction reactions are particularly important in the field of electrochemistry.