The Handy Chemistry Answer Book (2014)
ANALYTICAL CHEMISTRY
ANALYTICAL CHEMISTRY IN OUR LIVES
How is analytical chemistry used in medicine?
Analytical chemistry techniques are routinely used in many areas of medicine. When your blood is drawn, for example, the doctors use techniques borrowed from analytical chemistry to determine levels of several analytes in your body, including cholesterol, vitamins, glucose, and white blood cells. Analysis of tissue samples and other biological fluids is also based on analytical techniques. Other examples of medically related applications include tests for illegal drugs and steroids, glucose sensors used by diabetic patients, or to check for toxic substances in your body if you are exposed to hazardous chemicals.
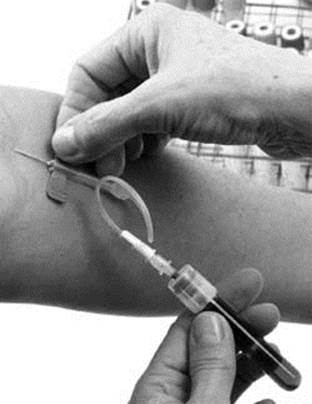
Analytical chemistry is commonly used in medicine, such as when your blood is drawn for tests to see how much glucose, cholesterol, or other chemicals are present.
What analytical techniques are used in quality control for pharmaceuticals?
Some of the most commonly used analytical techniques in pharmaceutical quality control include infrared spectroscopy, UV/Visible spectrophotometry, melting point determination, reactions based on color changes, and various types of chromatography, including thin-layer chromatography, high-performance liquid chromatography, and gas chromatography.

The numbers on gas pump indicate octane ratings, which relate to the amount of compression a fuel can undergo before combusting.
What do the different octane ratings mean at the gas pump?
The octane number provides a measure of the amount of compression a fuel can undergo before it combusts. The name “octane rating” comes from the fact that isooctane is used as the standard against which a fuel’s compression characteristics are measured. A fuel with an octane rating of 89 means that it has the same ability to withstand compression as a mixture of 89% isooctane and 11% heptane. In reality, gasoline contains many other components. Fuels with higher octane ratings are typically used in higher performance automobiles. Since fuels may be able to withstand compression better than isooctane, it’s entirely possible to achieve octane ratings of over 100.
How is the presence of illegal narcotics identified?
Analytical chemists have developed simple chemical tests that can identify the presence of illegal drugs within seconds. Law enforcement officers routinely use these when they are unsure whether someone is in possession of an illegal narcotic. A common field test for cocaine, for example, uses a cobalt thiocyanate complex that turns blue in the presence of cocaine.
How can the presence of blood be detected by forensic experts?
An ultraviolet light source can be used to detect a blood stain that has been cleaned up. It’s also possible to use a chemical substance like luminol or phenolphthalein to detect the presence of hemoglobin, revealing its presence even after the visible stain has been washed away.
How does a breathalyzer work?
When a person blows into a breathalyzer device, the air they exhale enters a chamber containing two glass vials. One is a mixture of aqueous sulfuric acid, silver nitrate (a catalyst), and potassium dichromate. The air is bubbled through this mixture and the sulfuric acid and potassium dichromate react with the alcohol, producing chromium sulfate and other products. The chromium ion of chromium sulfate is green in the solution, whereas the initially present dichromate ion was red/orange in color. The second vial contains the same reactants but doesn’t interact with the air sample, thus serving as a standard for comparison. The color change in the sample exposed to the air is monitored by a photocell (light sensor) system that is then used to determine the alcohol content of the air sample.

Calories in a serving of food can be calculated by the amount of protein, fat, and carbohydrates in the food item.
How are the quantities on food nutrition labels determined?
The calorie content of food can be determined using a simple calorimetry experiment. The food just needs to be placed inside a calorimeter and burned to determine its calorie content. These days, however, the calorie content of a food can also be determined by simply using standard values of calories in each gram of protein (4 calories/gram), fat (9 calories/gram), and carbohydrates (4 calories/gram).
The trick is just to accurately determine the quantities of protein, fat, and carbohydrates in the food, and that’s where analytical chemistry comes in particularly handy. The basic idea is that the fat, protein, or carbohydrates need to be extracted from the food. This can be done by choosing an appropriate solvent in which the fats/proteins/carbohydrates are soluble, and then using spectrophotometry, or one of a variety of other methods, to determine the content of each species in a solution.
Is a calorie in food the same as the unit of energy used by scientists?
The calories listed on food labels are actually kilocalories when converted to standard units for energy. So if the package for your snack says it contains 100 calories, that’s really 100,000 calories in the units of energy used by scientists.
What is smog?
The word smog was originally derived from a combination of the words smoke and fog, and today it describes air pollution that, once in the atmosphere, reacts with sunlight to form additional pollutants. The main sources of this pollution are commonly motor vehicles, factories, power plants, and burning of fuels. The main chemical components of smog are ozone, nitric oxide, nitric dioxide, and volatile organic compounds. Smog is present in pretty much every large city around the world. It can be hazardous to human health and has been blamed for health problems and even deaths.
How does a pregnancy test work?
A pregnancy test works by detecting a hormone called human chorionic gonadotropin (hCG) that is produced in the placenta after fertilization. The hCG hormone will be present in the blood and urine about a week after conception, and it’s detected by an indicator molecule on the pregnancy test that changes color upon binding to hCG. The tests you can buy in the store are actually pretty similar to those used at a doctor’s office; both are based on hCG binding to a chemical indicator that changes color. The main difference is that the doctor’s office has technicians who are more familiar with using the tests, so there’s less chance of a mistake with using or interpreting the test.