The Handy Chemistry Answer Book (2014)
BIOCHEMISTRY
MOLECULES OF LIFE
What is biochemistry?
Biochemistry is the field focused on elucidating and explaining the complex chemical processes that take place in biological systems. It’s a diverse field, drawing on aspects from virtually every other subfield of chemistry to explain the molecular processes that drive living things. Often, biochemists study complex reaction sequences and catalytic processes involving molecules much larger than those routinely encountered in other subfields of chemistry. A few topics commonly encountered in biochemistry include studying how our cells obtain energy, understanding how our genetic material (DNA) dictates who we are, and explaining how our body regulates and stores nutrients from the foods we eat.
In what professions is knowledge of biochemistry important?
Biochemistry is important for people interested in careers in human medicine, veterinary medicine, dentistry, pharmacy, and food science, as well as research in almost any physical or biological science and many subfields of engineering as well. While this list isn’t all-inclusive, you can see that biochemistry is used by people working in many different areas!
Where are biomolecules found?
Biomolecules are molecules that are specifically found inside of living things and have some function related to life. This includes molecules found in plants, animals, insects, bacteria, or even viruses (which are often considered not to be “alive” in a technical sense). Biomolecules span a wide range of sizes, some weighing only about 50 atomic mass units (amu), while others weigh millions of amu (see “Atoms and Molecules” to review the definition of amu). They are in our hair, skin, tissues, organs, and just about everywhere else in our bodies too.
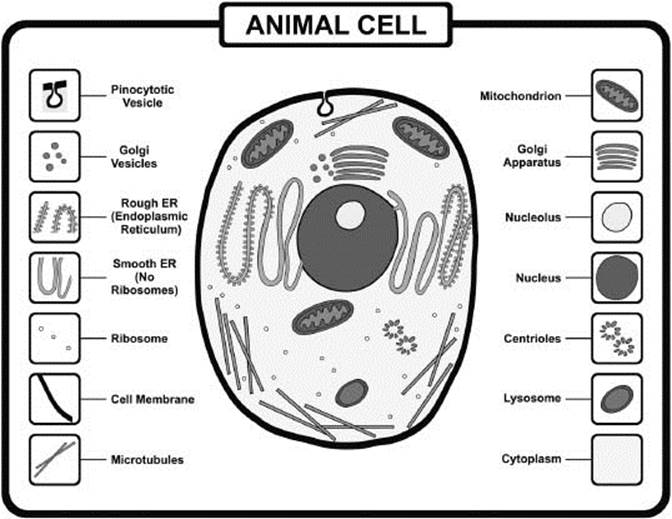
Cells contain a variety of organelles, which carry out specific functions for the cell to survive. Lipid bilayers separate the organelles from the rest of the cell.
What is a cell?
Cells are basic structures that make up all living things. The smallest organisms, like bacteria, can be made up of only a single cell; these are known as single-celled organisms. Other organisms, like animals or humans, are made up of many, many cells. The size of a typical cell is very small, usually on the scale of 10−4 or 10−5 meters. This is small enough that we can’t see it with our eyes, but only under a microscope where cells are readily visible.
How many cells are there in your body?
An adult human is made up of approximately fifty trillion cells!
What is a cellular organelle?
An organelle is a compartmentalized subunit with a living cell that carries out a specific function. Organelles are separated from the rest of the cell by a lipid bilayer membrane that allows it to maintain different concentrations of solutes from the rest of the cell.
What are the main classes of biomolecules?
The four main classes of biomolecules are proteins, carbohydrates, lipids, and nucleic acids. Proteins are some of the most diverse biomolecules, and they can perform functions such as protecting the body from pathogens or acting as catalysts for chemical reactions. Carbohydrates can be a source of energy when your body needs it. Lipids include fat molecules and are used for energy storage as well as to form the membranes that surround each cell. Nucleic acids are what make up our genetic material and store the information that determines a lot about who we are. We’ll go into more details about each of these types of biomolecules in the upcoming pages.
What is the basic structure of an amino acid?
The basic structure of an amino acid is shown below.
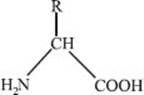
Each amino acid has an amine (NH2) group, a central carbon atom known as the alpha carbon, a side chain (denoted R), and a carboxylic acid (COOH) group. The amine groups and carboxylic acid groups of amino acids can be joined in a chain to form polymers of amino acids, known as peptides. Two amino acids form a dipeptide, three form a tripeptide, and chains of four or more amino acids are commonly referred to as polypeptides. Long polypeptides fold into specific conformations, which is what makes up a protein.
How many amino acids are found in humans?
There are twenty amino acids commonly found in humans. They are classified into four groups based on the identity of their side chain. The four groups are polar, nonpolar, acidic, and basic. These twenty amino acids are what make up all of the proteins and enzymes in your body. The interactions between the individual amino acids determine the overall conformation of the protein, though the relationship between the sequence of amino acids and the structure adopted by the protein is very difficult to predict.
Is there a reason all amino acids in biological systems share the same chirality?
While the answer to this question is uncertain, there have been some interesting developments in this area. It has been shown that life (as it exists today anyway) cannot arise from a racemic mixture of amino acids because the presence of chiral centers in many biomolecules is crucial for biological function. The self-replication of DNA relies on the presence of chiral centers and without a shared chirality, the error rate in DNA replication would cause severe problems for many longer-lived plants and animals. One hypothesis for the origin of chirality is that molecules from outer space reached Earth with a net chirality already present. Another is that the net chirality in amino acids was established on Earth in a very short period of time. It’s a pretty interesting thing to think about, and this topic is a subject of ongoing research and debate.
What is a protein?
Proteins consist of one or more long chains of amino acids that fold up into a specific arrangement, and each is presumably capable of some kind of biological function (though we don’t know for sure that every protein has a biological function—the function of each protein is one thing many biochemists are still working hard to figure out). Some proteins act to protect an organism from invading pathogens, some help to carry messages between different areas of the body, some are responsible for movement of muscles, and some provide structural support for cells. Many proteins also fall into a class called enzymes, which is a name for proteins that catalyze specific chemical reactions in biological systems.
What is a peptide bond?
A peptide bond, or peptide linkage, is the bond that links the individual amino acids in a peptide. Have a look at the figure of the dipeptide below.
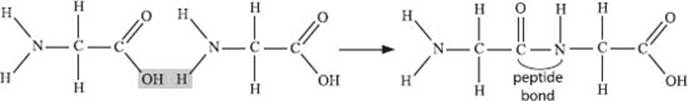
The formation of a peptide linkage is carried out by a structure called the ribosome, which is responsible for generating peptides and/or proteins based on the sequence of nucleotides present in an RNA molecule. We’ll get to the details of this process a little later.
What is an enzyme?
An enzyme is a protein whose function is to serve as a catalyst for a chemical reaction within a biological system. Some functions that enzymes perform are the synthesis of proteins and other biomolecules, digestion of fats and other molecules, and sometimes they are even put to use in industrial applications outside of their natural biological environment.
What is an active site?
Each enzyme has an area called the active site in which catalytic activity is carried out. The active site is shaped in a way that reduces the energy barrier to carrying out the chemical reaction it is meant to perform. It’s important to note that, while the reactant(s) must bind at the active site initially, they must also be released after the reaction has been carried out.
What is the native state of a protein?
Since there are so many atoms in a protein, there exist a very large number of possible conformations into which a protein can fold. The native state is the conformation in which the protein exists in its natural biological environment. This is most often the lowest energy conformation the protein can adopt. A protein in its native state is able to carry out its biological function, while a protein that is unable to reach its native state often will be unable to do so. If a protein is taken out of a cell, for example, the pH or other factors related to its environment may cause it to adopt a conformation other than its native state.
How many different conformations can a protein adopt?
A lot. For a typical protein consisting of 100 amino acids, there are approximately 3198 possible conformational states. This leads to something called Levinthal’s paradox, which has to do with how a protein goes about sampling each of these possible states. If a protein just randomly went through each of 3198 possible states, it would, on average, take longer than the age of the universe to get through all of them!
Fortunately proteins don’t sample each of the possible conformations randomly. Instead, the gradient (or slope) of the energy along the folding pathway, along with the formation of local interactions between the amino acids, guides the protein toward states of lower energy. This allows it to avoid sampling most of the unimportant conformations as it folds toward its native state.
What is a sugar?
Sugars are part of a class of biomolecules called carbohydrates that are made up of carbon, hydrogen, and oxygen atoms. The simplest sugars are called monosaccharides, but, like amino acids, they can polymerize to form disaccharides and oligosaccharides. Sugars can usually exist in either a ring form or an open chain form, as shown in the picture below. The sugar in this picture is called glucose, which is the sugar most commonly used for energy in the human body.
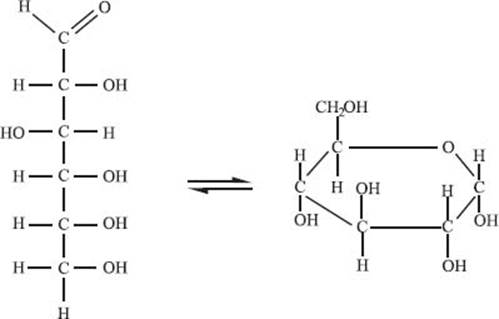
How are sugars stored in the body?
The body converts excess sugars into a branched polymer called glycogen for storage. In plants, sugars are instead stored as a polymer called starch.
How are glucose levels regulated?
In humans the amount of glucose in the blood at any given time is regulated very carefully. If your blood glucose level is too high, your body releases a chemical called insulin into the bloodstream, signaling that it’s time to convert some of the extra glucose floating around into glycogen. Similarly, if your blood glucose level gets too low, your body releases a chemical called glucagon into the bloodstream, telling your body to start breaking down that glycogen to release more glucose into the blood.
What is a glycosidic linkage?
A glycosidic linkage is the chemical bond that bonds a carbohydrate to another carbohydrate molecule (or to another species). Glycosidic linkages are what hold together all of the individual glucose monomers that make up glycogen or starch. The figure below shows a glycosidic linkage.
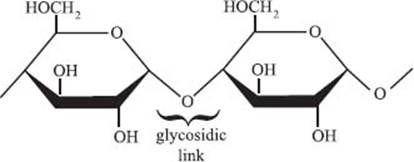
The enzymes that catalyze the breakage glycosidic linkages are called glycoside hydrolases. These are necessary to allow for glucose to be released from storage. The enzymes responsible for forming the linkages are called glycosyltransferases.
What is saponification?
Saponification is a type of reaction in which the ester functional groups of triglycerides are hydrolyzed under basic conditions. The term can also refer to the hydrolysis of any ester as well.