Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Hydrogen cyanide (HCN)
The term “cyanide” sets off alarm bells with the general public, despite there being numerous positive uses for cyanide-containing materials. Popular culture often uses the term in movies and other forms of entertainment as a catch-all phrase for poison. Indeed, cyanide-containing compounds have been used in the 20th century in the arsenal of chemical weaponry, and it is classified as a blood agent. But as we will discuss, hydrogen cyanide is useful in several organic chemical transformations, and in the production of several chemicals that are produced on very large scales.
9.1 Method of production
Hydrogen cyanide production begins with methane and ammonia. Ammonia, for many years, has been a chemical produced in one of the two greatest amounts on Earth (the other is sulfuric acid). Methane is the major component of natural gas, and is extracted from natural gas wells throughout the world. The simplified reaction chemistry is shown in Figure 9.1.
![]()
Fig. 9.1: Hydrogen cyanide production.
Once again, the reaction does not provide all the necessary details, as this reaction requires a platinum catalyst and temperatures of approximately 1200 °C. Also, the reaction is exothermic enough that the heat generated by it is often used to help other reactions. This reaction is called the Andrussow Oxidation or Andrussow process, after its inventor, the chemical engineer Leonid Andrussow, who was working within IG Farben at the time of its discovery. Additionally, hydrogen cyanide can be made through what appears to be a more direct combination of methane and ammonia, as seen in Figure 9.2.
![]()
Fig. 9.2: Direct combination of methane and ammonia for hydrogen cyanide production.
This is referred to both as the Degussa process, because Degussa is the firm that first developed it, as well as the BMA process which is an abbreviation of the German terms “Blausaeure, Methan, Ammoniak” (in English: hydrogen cyanide, methane, and ammonia). The process is endothermic, and historically has required platinum-coated piping and temperatures of about 1400 °C. Thus, it is not used to as large an extent as the Andrussow process.
The Shawinigan process is another electrolytic method of production of hydrogen cyanide. In this process, ammonia and propane are fed as a mixture over coke particles. While this produces yields of HCN over 80%, it runs at 1350—1650 °C, and thus is expensive in terms of energy.
9.2 Volume of production annually
Because of its poisonous nature, a great deal of hydrogen cyanide production occurs at or near the site where the material will be used further reaction chemistry so it does not need to be transported. Thus, it becomes difficult to put a number on the total amount produced annually, since this captive HCN is not usually counted. Additionally, any of the material that is stored by the military of any nation is generally not published or known, for reasons of security. However, estimates are that approximately 1 billion pounds of HCN are manufactured annually. At its website, IHS Chemical states
In 2012, over fifty companies operated more than seventy hydrogen cyanide (HCN) production facilities in the world. Direct production accounts for about 68% of total capacity and the balance is derived as coproduct material from acrylonitrile production. DuPont had been the major global producer, with about 20% of this capacity, until it sold its fibers unit to Koch Industries, Inc. Koch now has about 14% of global capacity. Evonik—Degussa and Butachimie are also significant players, with about 8% and 10% of global capacity in 2012, respectively (IHS Chemical, 2014).
Clearly, HCN is manufactured and used on a large scale, and in a wide variety of uses and applications.
9.3 Uses
We have seen in Chapter 8 that HCN is used in the production of hexamethylene diamine, and thus in nylon. There are several other major uses, which are discussed in the following subsections.
9.3.1 Mining
Mining consumes a significant amount of cyanides, usually sodium and potassium cyanide, because cyanide has a high affinity for metal ions. When the metal is of high enough value, such as gold, it is economically worthwhile to use cyanide salts to capture the gold from any production and waste stream. The reaction chemistry by which sodium cyanide is produced is shown in Figure 9.3.
![]()
Fig. 9.3: Sodium cyanide production.
It is perhaps obvious that this reaction also consumes a significant amount of sodium hydroxide, another chemical produced in large quantities annually. The reaction chemistry in which sodium cyanide reacts with gold is straightforward, as shown in Figure 9.4.
![]()
Fig. 9.4: Gold complexation by sodium cyanide.
It is this high affinity for the cyanide ion to gold that makes the reaction so important in extracting gold from deposits where it is a minor component. The process is highly efficient and cost effective. The production and use of potassium cyanide mirrors that of sodium cyanide.
9.3.2 Adiponitrile
This material is the precursor to hexamethylene diamine, and that to nylon-6,6, as discussed in Chapter 8.
9.3.3 Acetone cyanohydrin
This chemical is used in one of the methods of production for methyl methacrylate, and is shown in Figure 7.2.
9.3.4 Methionine
This amino acid is defined as an essential amino acid, meaning it is not produced in the body, and thus it is essential that it be consumed. The large-scale synthesis of methionine is shown in Figure 9.5, where it can be seen that potassium cyanide is a required reagent.
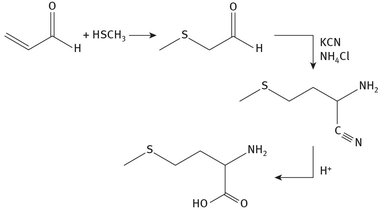
Fig. 9.5: Methionine production.
9.3.5 Cyanuric chloride and cyanogen chloride
The former of these two is prepared through the second as an intermediate compound. All of this is consumed in the production of the pesticide atrazine, an ethyl-isopropyl-derivative of CNCl3. Over 100,000 tons have been produced annually for the past several years.
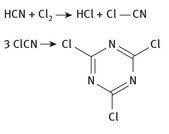
Fig. 9.6: Cyanuric chloride production.
9.3.6 Chelators
Hydrogen cyanide or sodium cyanide is also used in the production of the chelator ethylene diamine tetraacetic acid, commonly abbreviated as EDTA. The resulting EDTA or EDTA salt finds numerous uses in a wide variety of applications, such as metal ion sequestration in textile dyeing, or even in the paper and pulp industry. The synthesis is shown in Figure 9.7.
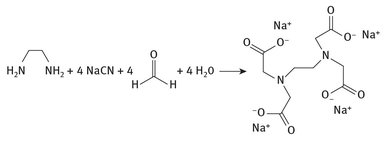
Fig. 9.7: EDTA production.
9.4 Recycling and reuse
Since hydrogen cyanide is never used as a material that is itself an end product, there is none that is recycled. However, since it is so poisonous, all industries that use the material have stringent safeguards in place to recover and reuse any HCN that is not consumed in the reaction. Releases of HCN are considered extremely dangerous, and thus every industry that uses it recovers all that does not react in some process.
The collection of different cyanide salts from mining waste streams is not a matter of recycling so much as a matter of containment. The liquid effluent and waste water from different mining operations can be very toxic, and containing it so that these materials are not released into the greater environment has become a matter of importance to governmental agencies, as well as to industries.
Bibliography
IHS Chemical. Website. (Accessed 15 May 2014, as: http://www.ihs.com/products/chemical/planning/ceh/hydrogen-cyanide.aspx)