Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Hexamethylene diamine (HMDA)
Many of the large commodity organic chemicals produced today find many uses, sometimes in a wide variety of end-user products, and sometimes in a variety of different intermediate roles. Hexamethylene diamine (HMDA) is quite different in that its commercial use is almost exclusively in the manufacture of nylon-6,6, one of the most common types of nylon, with only small secondary uses (IHS Chemical, 2014; Solvay, 2014; Invista, 2014).
8.1 Method of production
HMDA has a long history, and through that time has been produced in different ways, at least on a small scale. Currently, it is produced from adiponitrile, the synthesis of which is shown in Figure 8.1
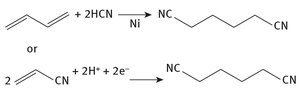
Fig. 8.1: Production of adiponitrile.
As can be seen, adiponitrile can be made in more than one way. But both methods shown in Figure 8.1 use starting materials — butadiene or acrylonitrile — that ultimately have petroleum as their source. The first method shown here has been pioneered by DuPont (DuPont, 2014) — which has been involved with the material deeply enough that two of its senior personnel have been entered in their Lavoisier Academy — and the second by Monsanto (Monsanto, 2014). The latter is an electrolytic process, which makes it energy intensive. The former requires a nickel catalyst to proceed to completion. The required hydrogen cyanide is the subject of the next chapter.
The final step that produces HMDA is the hydrogenation of the resulting adiponitrile, as shown in Figure 8.2.
![]()
Fig. 8.2: HMDA production from adiponitrile.
The reaction can be written in a very straightforward manner, but once again requires a catalyst for it to occur, usually an iron-based or cobalt-based catalyst. Also, there are side products that are isolated from the reaction, two of which are shown in Figure 8.3. The reaction is also a significant use for hydrogen.
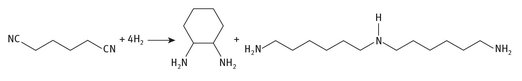
Fig. 8.3: By-product production during HMDA manufacture.
8.2 Volume of production and uses
Slightly more than 1 million tons of HMDA are produced annually. The vast majority of this is used in the production of plastics of one form or another. Perhaps the best known large scale use of it is for the production of nylon-6,6, this being a co-polymer with adipic acid. The chemistry can be represented in a straightforward manner, as is shown in Figure 8.4.
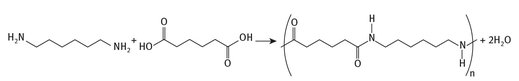
Fig. 8.4: Production of nylon-6,6.
Considerably less is used in the production of hexamethylene-diisocyanate. This latter material does find specialty applications though, usually in coatings.
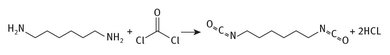
Fig. 8.5: Production of isocyanate from HMDA.
These now classic syntheses have become sources of materials for a very wide variety of uses. Nylon can be spun into fibers of various gages, and used to create numerous durable fabrics for a multitude of end uses. It can also be shaped into a wide variety of consumer products or incorporated into them. Some of the most common are shown in Table 8.1.
Table 8.1: Nylon-6,6 uses.
Nylon-6,6 form |
Use |
Pellet, flake |
Shaping or extrusion |
Bulk material |
Pipes, machine parts, auto parts |
Fiber |
Airbags, clothing, sails, rope, carpet, textile (Invista, 2014) |
Fiber |
Truck and bicycle tires, zip ties, flexible consumer products (Kolon, 2014) |
8.3 Recycling and reuse
Since hexamethylene diamine is used almost entirely in the production of nylon, with other downstream products representing the rest of its use, the raw material is never reused or recycled.
Nylon and the other plastics made from HMDA can be recycled, and sometimes are. This usually depends on recycling laws in different states and provinces. In several parts of the world, tires are down scaled, meaning some second use is found for them, usually after their treads have been worn or compromised. Such uses include berm and dam material, or matting, after they have been mechanically shredded.
Bibliography
Brendt, D. Polymer Data Handbook. Oxford University Press, Inc. 1999, ISBN: 978-0-195-10789-0.
DuPont. Website. (Accessed 15 May 2014, as: http://www.dupont.com).
IHS Chemical. Website. (Accessed 15 May 2014, as: http://www.ihs.com/products/chemical/planning/ceh/hexamethylenediamine-adiponitrile.aspx).
Invista. Website. (Accessed 14 May 2014, as: http://www.invista.com/en/hmd/index.html).
Invista. Website. (Accessed 14 May 2014, as: http://www.invista.com/en/polymers/nylon-polymer.html).
Kolon Industries. Website. (Accessed 15 May 2014, as: http://www.kolonindustries.com/Eng/Product/product02_01.asp).
Monsanto no longer displays any information related to such production on its website, and has refocused to agricultural products and chemicals.
Randall, D.; Lee, S. The Polyurethanes Book. Wiley, 2002, ISBN: 978-0-470-85041-1.
Solvay. Website. (Accessed 14 May 2014, as: http://www.solvay.com/en/binaries/ Hexamethylenediamine_GPS_rev0_sept12_RHD-139548.pdf ).