Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Methyl methacrylate
Several of the major, large-volume chemicals are produced on a large scale for later use in many different materials or consumer end products. Others, methyl methacrylate (MMA) included, tend to be produced for more limited purposes, although they are still produced on a very large scale. MMA is used predominantly for the production of the polymer, polymethyl methacrylate (PMMA), as well as for co-polymers. The reaction chemistry for the production of PMMA can be shown simply, as seen in Figure 7.1.
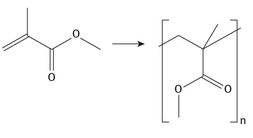
Fig. 7.1: PMMA synthesis.
7.1 Method of production
There is more than one method for the production of methyl methacrylate, but the largest has for some years been the acetone cyanohydrin method (ACH method), named after its starting materials.
7.1.1 The ACH method
The ACH production route for methyl methacrylate has been examined and its yields maximized over the years. Figure 7.2 shows the simplified reaction chemistry.
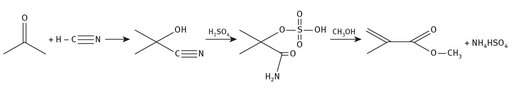
Fig. 7.2: ACH production of methyl methacrylate.
Acetone itself is produced industrially by the oxidation of cumene (isopropylbenzene), which co-produces phenol. Hydrogen cyanide is another chemical produced on a large scale annually, and is examined in Chapter 9. Sulfuric acid is the largest commodity chemical produced in the world, and in the United States it is produced in over 100 facilities within 29 states (United State Geological Survey, 2013). Methanol is yet another large commodity chemical, which is produced from syn gas. Methyl methacrylate production is only one of many uses for methanol.
Although this process is used to produce large quantities of methyl methacrylate, the ACH route also produces by-products in stoichiometric ratios. Specifically, ammonium hydrogen sulfate is a by-product that is always present in this process. Indeed, the production of 1 pound of methyl methacrylate through this process also produces approximately 2.5 pounds of the ammonium hydrogen sulfate. The disposal of this co-product becomes a serious concern.
7.1.2 Production from isobutylene
Isobutylene can be used as the starting material as well, and oxidized in air to produce methacrylic acid. The acid is then reacted with methanol to derive the product. The simplified reaction chemistry is shown in Figure 7.3.
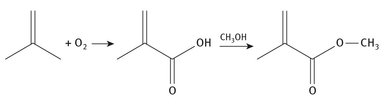
Fig. 7.3: Isobutylene production of methyl methacrylate.
Isobutylene itself can be separated from other light hydrocarbons that are present in natural gas.
7.1.3 Production from isobutyric acid
The production of isobutyric acid starts with propene (propylene), carbon monoxide, and water, and is catalyzed with hydrofluoric acid. Methacrylic acid is then produced from the isobutyric acid by dehydrogenation. Finally, the methyl group is added. The overall reaction chemistry is shown in Figure 7.4.
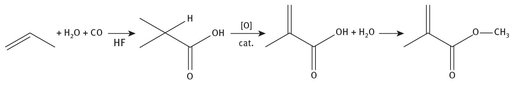
Fig. 7.4: Isobutyric acid production of methyl methacrylate.
Propene is another light hydrocarbon that ultimately has crude oil as its source material.
7.1.4 The methacrylonitrile process (MAN process)
Isobutylene is the main starting material for the MAN process, but the steps are different from those outlined in Section 7.1.2, above. Both ammonia and oxygen are required to produce methylacrylonitrile, which is then reacted with sulfuric acid to form a sulfuric acid adduct of the amide. The final step is the addition of methanol to form methyl methacrylate. The basic reaction chemistry is shown in Figure 7.5.
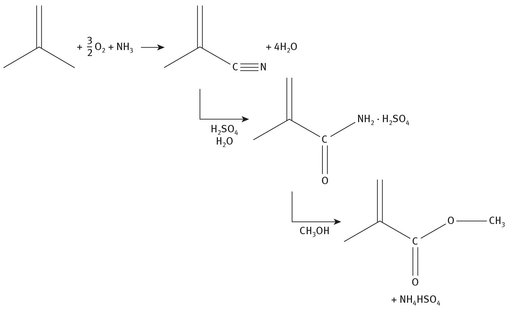
Fig. 7.5: MAN process for methyl methacrylate production.
7.1.5 Production from methyl propionate (the BASF Route)
This method begins with ethylene, and produces methyl propionate in the first step at slightly elevated pressure. The final step is the addition of formaldehyde to form the product. The simplified reaction chemistry is shown in Figure 7.6.
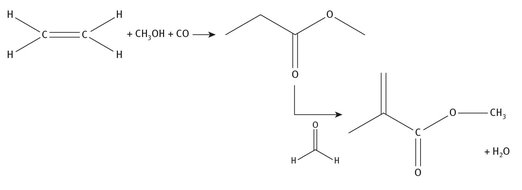
Fig. 7.6: Methyl propionate production of methyl methacrylate.
Ethylene is used in a large number of other applications, most notably the production of a variety of types of polyethylene, from linear low-density polyethylene to ultrahigh density polyethylene.
7.1.6 Production from propionaldehyde
In this synthesis, propanal (propionaldehyde) reacts with formaldehyde, using a secondary amine catalytically, resulting in methacrolein. This is then oxidized to form methacrylic acid. The acid is then converted to methyl methacrylate. The reaction chemistry for this is shown in Figure 7.7.
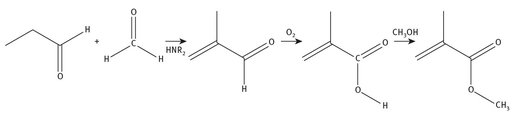
Fig. 7.7: Propionaldehyde production of methyl methacrylate.
Propanal is itself produced from ethylene in a hydroformylation. Again, the starting material is derived from a fraction of crude oil.
7.1.7 Production from propyne (aka, methyl acetylene)
In this process, propyne is reacted with both methanol and carbon monoxide using a metal catalyst and an acid. The reaction proceeds cleanly in a single step. The reaction is shown in Figure 7.8.
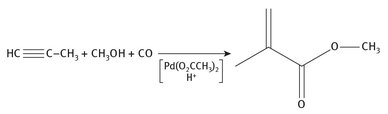
Fig. 7.8: Propyne production of methyl methacrylate.
Because this chemistry involves a metal catalyst and an acid, and proceeds at elevated pressures, it is often called Reppe chemistry, after pioneering chemist Walter Reppe, who worked extensively with various alkynes.
This series of synthetic pathways shows how wide the variety is in terms of how methyl methacrylate can be formed. Each synthesis is dependent on the availability and cost of its feedstock; but all the carbon-based molecules involved ultimately come from some fraction of petroleum.
7.2 Volume of production annually
The wide number of production routes to methyl methacrylate indicates that very large amounts are produced globally each year. One consulting firm that tracks the usage of large-scale chemical production and sales, Nexant, Inc., states
At the end of 2008, there were almost 50 methyl methacrylate manufacturing plants globally (some uncertainty exists over the precise number of plants in China where the very small localized plants are scattered over a wide geography) with plant sizes ranging from 1500 to 360,000 metric tons per year (3.3 to 794 million pounds MMA per year) ChemSystems (2014).
This level of production generally appears to be steady from year to year, with some variations occurring, usually linked to the economies of the nations where it is produced.
7.3 Uses
Roughly three-fourths of all methyl methacrylate is used for the production of PMMA, as mentioned by Dow Chemical (2014). The polymerized PMMA structure is shown in Figure 7.9.
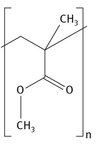
Fig. 7.9: Polymerized PMMA structure.
To a lesser extent, it is also used as a polymer material with butadiene and styrene (called MBS). This MBS ter-polymer, much like acrylonitrile-butadiene-styrene (or ABS) ter-polymer, is simply used to produce materials with desired properties, usually toughness, inertness, and proper flexibility or lack thereof for a specific application. MBS can be utilized to modify the properties of polyvinyl chloride (PVC), another large volume plastic. The MBS and ABS ter-polymer units are shown in Figure 7.10. There are other uses for methyl methacrylate as well. They include the following:
· — Other methacrylate production
· — Bone inserts
· — Cement in joint replacements
· — Acrylate polymers.
While production volumes do change each year, they have not done so drastically for the past several years.
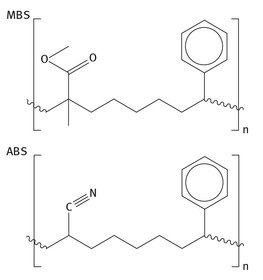
Fig. 7.10: The MBS and ABS ter-polymer repeat units.
7.4 Recycling and reuse
As with many plastics, recycling efforts for methyl methacrylate are not directed at the monomer, but rather for the polymerized material. Plastic production involves significant investments in material purity and in energy, and thus recycling is cost effective.
Since methyl methacrylate is essentially all consumed in the production of other chemicals and end-use materials, none of it is recycled.
Bibliography
ChemSystems. Website. (Accessed 25 January 2014, as: http://www.chemsystems.com/index.cfm, and as: http://www.chemsystems.com/about/cs/news/items/PERP%200809_7_MMA.cfm).
Dow Chemical. Website. (Accessed 24 January 2014, as: http://www.dow.com/products/market/packaging/product-line/methacrylates/product/methyl-methacrylate-(mma)/ ).
United State Geological Survey. Mineral Commodities Summary 2013. Website. (Accessed 20 May, 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).