Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
n-Butanol (1-butanol or n-butyl alcohol)
The term “alcohol” is used by the general public either to denote the drinkable liquid, ethyl alcohol — a mild intoxicant — or to a lesser extent isopropyl alcohol, also known as rubbing alcohol. n-Butanol does form as a fermentation product in certain natural processes, but does so only to a small degree. However, the industrial synthesis of n-butanol has become a large process in the past few decades (now more than 1 million tons annually across the globe), placing this material routinely in the top 100 chemicals produced on an annual basis.
6.1 Method of production
While an older industrial scale method for the production of butyraldehyde, which was discussed in Chapter 3, began with n-butanol as the feedstock, today the reverse is the case. Butyraldehyde is hydrogenated to form the alcohol. The reaction chemistry appears to be rather simple, as shown in Figure 6.1.
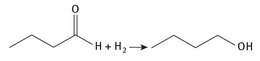
Fig. 6.1: Production of n-butanol.
This represents a significant large-scale use of hydrogen, most of which is produced from the hydrocarbon stripping of light hydrocarbons from natural gas or petroleum. However, the total amount of hydrogen used in n-butanol production is still far less than that consumed in the production of ammonia via the Haber process. Several different large chemical producers manufacture n-butanol. They include:
1. BASF (BASF (2014)) indicates at their website the broad profile of uses for which it manufactures n-butanol. It includes solvents for the coatings industry (about 50%), cellulose nitrate lacquers, as a diluent, alkyd resin paints additive to improve flow. Also
o — Solvent for dyes, e.g. in printing inks.
o — Extractant in the production of drugs and natural substances such as antibiotics, hormones, vitamins, alkaloids, and camphor.
o — Additive in polishes and cleaners, e.g. floor cleaners and stain removers.
o — Solubilizer in the textile industry, e.g. additive in spinning baths or carrier for coloring plastics.
o — Additive in de-icing fluids.
o — Additive in gasoline for spark-ignition engines (prevents carburetter icing).
o — Mobile phase in paper and thin-layer chromatography.
o — Humectant for cellulose nitrate.
o — Feedstock for the production of glycol ethers (in reaction with ethylene or propylene oxide) (BASF (2014)).
2. In Dow Chemical’s website (Dow Chemical, 2014), Dow indicates that 5.1 million metric tons of n-butanol were used worldwide in 2002.
3. Shivam Industries (2014) indicates not only its manufacture and sale of n-butanol, but of several commodity chemicals made from it.
4. Solvert (2014) indicates in its website that it focuses on “n-butanol, acetone, hydrogen, renewable energy.”
5. Ridhdhi Sidhdhi Chemicals (2014) markets n-butanol as a high purity solvent.
6. Ree Atharva Lifescience Pvt. Ltd. (Ree Atharva Lifescience Pvt Ltd, 2014) produces n-butanol in what it is called a pharma grade.
6.2 Uses
While it may seem surprising, n-butanol is referred to as a flavorant in its role as an additive to a variety of foods (OECD SIDS, 2013). The Organisation for Economic CoOperation and Development Screening Information Data Sets (OECD SIDS) states:
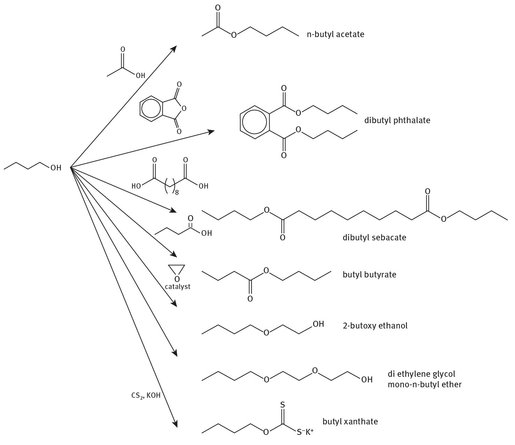
Fig. 6.2: Chemicals from n-butanol.
“In the United States, butyl alcohol is approved by the Federal Food and Drug Administration (FDA) as an indirect food additive for use only as a component of adhesives (21 CFR 175.105). It is a food additive permitted by the FDA for direction addition to food for human consumption (21 CFR 172.515)” (OECD SIDS, 2013).
n-Butanol has been approved for use in a wide variety of goods, from foods toto plastics to solvents. It is also used in the production of a wide variety of other commodity chemicals that in turn have widely differing end uses. Figure 6.2 shows the production of several other chemicals from n-butanol. Additionally, it is used in the manufacturing of materials as diverse as perfumes and vitamins, and is used in a variety of coatings and paints, as mentioned above.
6.3 Recycling and reuse
As mentioned, n-butanol is either used in end-use products or consumed in the production of other chemicals, so recycling is not possible.
Bibliography
BASF. Website. (Accessed 14 May 2014, as: http://www.basf.com/group/corporate/us/en/brand/N_BUTANOL).
Dow Chemical. Website. (Accessed 14 May 2014, as: http://www.dow.com/productsafety/finder/nbut.htm).
OECD SIDS N-Butyl Alcohol. SIDS Initial Assessment Report for SIAM 13. Website. (Accessed 28 December 2013, as: http://www.inchem.org/documents/sids/sids/71363.pdf)
Ree Atharva Lifescience Pvt Ltd. Website. (Accessed 14 May 2014, as: http://www.reeatharva.co.in/n-butanol-1055289.html).
Ridhdhi Sidhdhi Chemicals. Website. (Accessed 14 May 2014, as: http://www.rschemicals.co.in/n-butanol-429618.html).
Shivam Industries. Website. (Accessed 14 May 2014, as: http://www.shivaminds.com/n-butanol.html).
Solvert. Website. (Accessed 14 May 2014, as: http://www.solvertltd.co.uk/products.html).