Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Glass
Broadly defined, glass is any amorphous material which can exist in a hard, usually brittle state as well as in a molten, generally soft but viscously liquid state, and that can be transformed from one to the other repeatedly. Most people think of glass in less technical terms, considering it to be in some way a transparent, hard, brittle material that is not metallic. Using this as a broad definition, glass could be a polymer, such as a polycarbonate. This chapter will focus only on traditional glass, meaning the material made from silica and other inorganic materials.
23.1 Raw materials
Most glass requires three materials: silicon dioxide (sometimes just called sand, although it must be quite free of impurities), soda ash, and calcium oxide, still often called lime. These three materials are so common that glass can be produced in almost any country. The United States Geological Survey does not track glass in its Mineral Commodity Summaries which are published each year, but does track all three of the above ingredients, sand, soda ash, and lime (USGS, 2013). Of the three, soda ash finds its largest use in glass making. Perhaps obviously, sand sees much larger volume uses in construction and cement making and lime sees its major use in steel manufacturing.
23.1.1 Compositions
Various other materials are added to glass mixtures, always to change one property or another, to arrive at a material with some desired combination of properties and abilities. Examples of several different types of glass are shown in Table 23.1. Numbers indicate percentages of components.
While this variety of glass types shows the versatility of it as a material, soda-lime-silica glass is the formulation used on the largest scale by far. Production and sales do change from one year to the next, but roughly 90% of glass manufacturing is for this type. There are also several types of glass made in smaller quantities, for niche uses. Overall though, glass is used on such a large scale that several trade organizations exist which are devoted to the manufacture, sale, and use of numerous types of glass (Glass Association of North America, 2014; National Glass Association, 2014; Glass Manufacturing Industry Council, 2014; Glass Build America, 2014; British Glass, 2014; Bundesverband Glasindustrie, 2014; Glass Alliance Europe, 2014).
Table 23.1: Common types of glass.
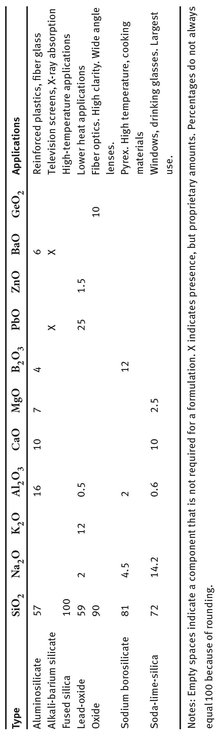
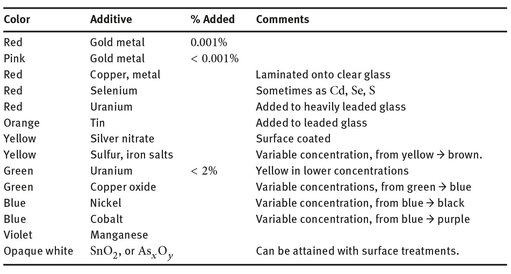
Table 23.2: Color additives for glass.
23.1.2 Colors
Various additives are incorporated into glass mixtures when they are molten or semi-molten to create specifically colored glasses. These are collectively referred to as “pot metal glass.” Others are applied to a surface, and then re-heated to fuse the colored layer to a clear base of glass. Glasses made in this fashion are called “flashed glass.”
Historically, churches have used these specialty, colored glasses in what are called stained glass windows, which are often made from large numbers of different colored, small panes of glass. This form of architectural adornment started in Europe, but has since spread throughout the world, and still occupies a niche in the glass making industry today.
Table 23.2 illustrates several additives that create various colors. Since there is no theory concerning how such colors form, the table simply lists them in the order of a rainbow using the old mnemonic for colors, Roy G. Biv, from red to violet.
The reason percentages are not listed for each color is that there is significant variation which produces a range of color (from red to yellow, for example), and the processes by which specific colors are produced are still proprietary in some cases.
23.2 Production
Although glass has been produced for millennia, there are still only two main ways to manufacture it. One, simply called glass blowing, goes all the way back to the origins of glass production, where it was used to make small bottles, cups, and other containers. While much of this is automated today, the process remains essentially the same.
What is called the “float glass process” is used to produce sheets of glass. This process is newer than glassblowing and can produce large sheets of glass of uniform thickness. The process was first tried in the 1950s by Sir Alastair Pilkington. For this reason, the process is also called the Pilkington Process (Pilkington, 2014). Glassblowing steps can be divided as follows:
1. Batch house. Mixing of the materials occurs in the batch house, as the materials are fed to the furnace.
2. Furnace or hot end. The furnace is where the raw glass is made molten, and items are formed and initially shaped, usually using a variety of molds. Most furnaces produce tons of glass products per day.
3. Annealing. As the glass cools, the object is fed into an annealing oven, so that the cooling is controlled and even. Some annealing operations can take more than a day of constant slow cooling.
4. Cold end. Final treatment of bottles and containers occurs at the cold end. This always includes an inspection of the bottle for defects, and may include the application of some outer coating to the glass.
Float glass differs in that the molten glass floats on a bed of molten tin (although other metals can be used) after any mixing in the furnace. The purpose of this step is to impart a uniform thickness to the glass.
Both processes are very energy intensive, requiring temperatures as high as 1750 °C. Since the processes are of large scale (a float glass factory can be several hundred meters long), oil or natural gas is used as the fuel source to obtain the temperatures required to melt the glass batches.
23.3 Uses
The uses of the various types of glasses are myriad. Window glass, bottles, and jars are three applications that come quickly to mind in terms of consumer products that are made from glass, but there are many other uses for glass or glass-like materials as well. A partial listing, quoting the Glass Alliance Europe website, states: “Glass is used in the following nonexhaustive list of products:
· — Packaging (jars for food, bottles for drinks, flacon for cosmetics and pharmaceuticals)
· — Tableware (drinking glasses, plate, cups, bowls)
· — Housing and buildings (windows, facades, conservatory, insulation, reinforcement structures)
· — Interior design and furnitures (mirrors, partitions, balustrades, tables, shelves, lighting)
· — Appliances and Electronics (oven doors, cook top, TV, computer screens, smartphones)
· — Automotive and transport (windscreens, backlights, light weight but reinforced structural components of cars, aircrafts, ships, etc.)
· — Medical technology, biotechnology, life science engineering, optical glass
· — Radiation protection from X-rays (radiology) and gamma-rays (nuclear)
· — Fiber optic cables (phones, TV, computer: to carry information)
· — Renewable energy (solar-energy glass, wind turbines)” (Glass Alliance Europe, 2014).
23.4 Reuse and recycling
Very few materials have as much of a reuse and recycling history as glass. Glass bottles for beverages have been recycled or reused for decades. In many cases, bottles are simply deposited into large containers. The containers are then sent to glass companies that have the capability of melting the batch and remaking bottles from the material. The term “cullet” is sometimes used for this type of recycled glass. In other cases, glass bottles are returned intact to a point of origin, often a store or business, then sent back to a bottling facility. There they are sanitized and reused.
Some countries have national programs for recycling glass bottles, while other nations leave policy to regional or state governments.
Bibliography
British Glass. Website. (Accessed 1 June 2014, as: http://www.britglass.org.uk/).
Bundesverband Glasindustrie. Website. (Accessed 1 June 2014, as: http://www.bvglas.de/en/the-association/).
Glass Alliance Europe. Website. (Accessed 29 January 2014, as: http://www.glassallianceeurope.eu/en/applications).
Glass Association of North America. Website. (Accessed 1 June 2014, as: http://www.glasswebsite.com/).
Glass Build America. Website. (Accessed 1 June 2014, as: http://www.glassbuildamerica.com/).
Glass Manufacturing Industry Council. Website. (Accessed 1 June 2014, as: http://www.gmic.org/.
National Glass Association. Website. (Accessed 1 June 2014, as: https://www.glass.org/).
Pilkington. Website. (Accessed 29 January 2014, as: http://www.pilkington.com/pilkington-information/about+pilkington/education/float+process/step+by+step.htm).
US Geological Survey, Mineral Commodity Summaries 2013. Website. (Accessed 26 January 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).