Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Cement
As with several other large-scale chemical commodities, cement has an ancient history. People throughout the world have been using various mixtures of earth-based minerals to create substances that will act as binders, usually in the construction of buildings, walls, fortifications, or roads. Buildings from ancient times in China, south-west Asia, and parts of the Roman Empire all used some type of cement in their construction.
Only in the last few 100 years, however, has an experimental science for the formulation and production of cement been developed. The world as we know it today, with cities that have numerous high-rise buildings, roads that can be subjected to the stress of high speed, heavy traffic, and enormous hydroelectric power dams, would not be possible without the use of massive amounts of cement.
This chapter will discuss hydraulic cement, meaning cement that can set underwater, as well as nonhydraulic cement, which cannot do so. When the term “concrete” is used, it refers to mixtures of a type of cement that is further mixed with stone, sand, or other aggregate material to make some type of end product that can set in a specific, designed shape.
24.1 Sources
The source materials for cement are common throughout most of the world. The United States Geological Survey Mineral Commodity Summaries for 2013 does track cement production, but states:
“Although individual plant reserves are subject to exhaustion, cement raw materials, especially limestone, are geologically widespread and abundant, and overall shortages are unlikely in the future (USGS, 2014).
The distribution of cement production countrywise as of 2012 is shown in Figure 24.1. Countries from which the United States imports cement include: Canada, South Korea, China, and Mexico (USGS, 2014).
While it is obvious that the output of cement in China is enormous, it can also be seen that cement is produced in large quantities in many other developed countries in the world. Indeed, there are several national and international trade associations devoted to the production, manufacture, sale, and research into cement and its uses (America’s Cement Manufacturers, 2014; British Cement Association, 2014; Mineral Products Association, 2014; Verein Deutscher Zementwerke, 2014; Cembureau, 2014).
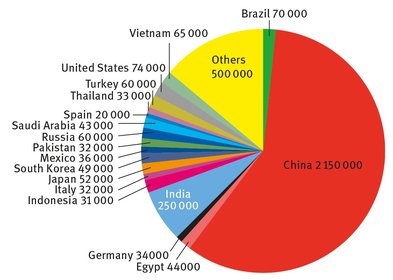
Fig. 24.1: Cement production, in thousands of metric tons.
24.2 Production and formulation chemistry
The production chemistry of various cements can be broken into two broad categories, based on whether or not the material in question can set underwater. As mentioned, any hydraulic cement is one that can set underwater, and any nonhydraulic cement does not. Hydraulic cements all require one or more materials that form sparingly soluble hydrates. Nonhydraulic cements require some carbon dioxide to set, which is usually just the carbon dioxide in the air.
24.2.1 Hydraulic cement chemistry
What is often called “Portland cement” requires one or more forms of mixed calcium—aluminum oxides. The steps in its manufacture can be broken down as follows, in what is called the “dry method”:
1. Quarrying and crushing. This is usually a mixture of limestone (CaCO3) and clay that is brought down to particle size of 6—7 cm.
2. The addition of fly ash (SiO2 and CaO) and iron ore to the mix.
3. Processing through an almost horizontal kiln, which reaches final temperatures of approximately 1500 °C. This drives off gaseous material and forms what is called clinker, pieces that are roughly 2 cm in diameter.
4. Cooling of the clinker.
5. Grinding, andtheadditionoflimestoneandgypsum(CaSO4 ⋅2H2O).
As mentioned, these are the general steps of what is called the Dry Method. The wet method is essentially the same process, but it involves the material being in a liquid slurry (America’s Cement Manufacturers, 2014).
As far as the reaction chemistry that causes cement ultimately to form as a solid, the material must first set, and then harden. Example reactions for these broad steps are shown in Figures 24.2 and 24.3.

Fig. 24.2: Hydraulic cement setting.
While the reactants and products of these three reactions may appear somewhat complex, they are all examples of the formation of single or mixed oxide hydrates. The examples of reactions which illustrate hardening in hydraulic cement, in Figure 24.3, are similar.
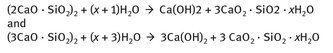
Fig. 24.3: Hydraulic cement hardening.
Both of these reactions are further examples that illustrate the formation of sparingly soluble hydrates which essentially form a solid solution. All of these reactions depend upon water, and thus can occur in or under water. While formulations change based on the specific need, the materials used by Roman engineers to build the now ruined piers at Caesarea off the coast of Israel roughly two millennia ago are similar to modern mixtures.
24.2.2 Nonhydraulic cement chemistry
The driving force for the formation of cement in a nonhydraulic mixture is the reaction of the material with carbon dioxide. The reaction chemistry for this, in the simplified form, is shown in Figure 24.4.
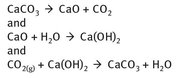
Fig. 24.4: Nonhydraulic cement formation.
While these three reactions together may seem rather circular, in that calcium carbonate is an ultimate starting material as well as a product, all the steps in Figure 24.4 are necessary. In the first reaction, calcium oxide must be made, as it is not mined. Calcium carbonate has to be heated to approximately 850 °C to form the calcium oxide. Next, the addition of water to the calcium oxide forms what is still often called the slaked lime (Ca(OH)2). Finally, the atmospheric carbon dioxide is required to provide a driving force whereby the slaked lime hardens as calcium carbonate.
24.2.3 Other types of cement
What is called “energetically modified cement” (EMC), a term coined in the 1990s, uses somewhat different starting materials than Portland cement, but still results in a cement material that can be used in applications traditionally filled by cement. Such cement is made using the following: blast furnace slag, possibly fly ash, volcanic ash, and usually some amount of Portland cement and sand. The latter two are used in smaller amounts than in established cement blends, and this becomes a use for slag and fly ash, materials that throughout history were often discarded as waste. As with other cements, formulations vary with specific applications.
At the EMC Cement website, the organization proclaims: “Compared to (Legacy) Portland Cement? Over a 90% Reduction in the Carbon Footprint and Energy Consumption! — that’s an annual saving of up to 2.4 billion tons CO2 and 2.3 trillion KWh of energy... ” (EMC Cement, 2014). Although such statements do not specify how such numbers are derived, and are obviously trying to prove a point for the industry, they are made precisely because strides are being made to produce end materials with cement in a more environmental friendly manner than what has been done historically. The use of ECM is continuing to grow, but has not yet displaced other methods of cement production.
24.3 Uses
Industrially, cement is always used as a binder in construction materials. When mixed with stone and sand, it is more properly called concrete, the common building material. The breakdown of cement uses does vary somewhat by country, but the statistics from the Mineral Products Association, from Great Britain are representative of most uses (Mineral Products Association, 2014) and are as follows:
· — Housing, 35.7%
· — Private commercial, 22.2%
· — Infrastructure, roads and bridges, 19.9%
· — Industrial buildings, 10.9%
· — Public nondomestic, 10.5%
· — Other, 0.8%
24.4 Recycling
The general population does not consider cement or concrete a recyclable material, simply because the cement and concrete applications that are present in a home are usually walls and supporting structures. But concrete can be broken into small pieces and reused, often in some further construction application. In this way, cement can be recycled. The driver to do so, as opposed to simply using old cement and concrete as a form of fill dirt, is an economic one. In other words, if it is cost-effective to reuse cement, it can be done.
Another area in which the cement industry produces a significant amount of by-product, but in which it has invested considerable time and manpower in the recent past to seek reductions, is the production of CO2, a well-known greenhouse gas. On average, carbon dioxide emissions into the atmosphere have been at or over 900 kg per ton of cement produced, at the turn of the millennium. But by the year 2009, emissions at newer plants had dropped to below 800 kg per ton (Mineral Products Association, 2014; Cembureau, 2014). While this number is still high, efforts are continuing to find ways to utilize or capture CO2 as it is generated.
Bibliography
British Cement Association. Website. (Accessed 1 June 2014, as: http://www.concretecentre.com/).
Cembureau, the European Cement Association. (Accessed 1 June 2014, as: http://www.cembureau.be/.
EMC Cement. Website. (Accessed 1 June 2014, as: http://www.emccement.com/landing4a.htm).
Mineral Products Association. Website. (Accessed 1 June 2014, as: http://cement.mineralproducts.org/).
Portland Cement Association, America’s Cement Manufacturers. Website. (Accessed 1 June 2014, as: http://www.cement.org).
US Geological Survey, Mineral Commodity Summaries 2013. Website. (Accessed 26 January 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).
Verein Deutscher Zementwerke e.V. (Accessed 1 June 2014, as: http://www.vdz-online.de/en/).
Wilson, M. L.; Kosmatka, S. H. “Design and Control of Concrete Mixtures,” 2011.