Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Phosgene (carbonyl dichloride)
Throughout history, certain chemicals or materials have gained fame or notoriety for a wide variety of reasons. Gold has been valued in almost all civilizations from ancient times to the present for no reason except its visual beauty. Iron has been valued because of its ability to hold an edge, which makes it useful in forming tools and weapons. Arsenic and arsenic compounds have been known to be poisons in several cultures — and indeed, have been considered a weapon within an assassin’s arsenal for hundreds of years. More recently, both phosgene and one of its starting materials, chlorine, have been considered one of the worst of battlefield weapons, namely, poison gas.
Elemental chlorine gas was indeed the first chemical warfare agent widely used on the western front of the First World War. Although the gas is poisonous, at the concentrations that were delivered by artillery in that war, it was seldom lethal by itself. It is denser than air, thus seeping and pouring into the trenches, forcing soldiers without gas helmets to rise up for air, where enemy soldiers could then shoot at them. Phosgene, used later in the war, was far more lethal. When inhaled even in small amounts, HCl forms in the lungs, affecting what is called “dry land drowning” as lung tissue was destroyed. The limited number of survivors of phosgene attacks claim that small doses of it smell like newly mown hay, or fresh-cut wheat.
It is staggeringly ironic than that elemental chlorine is today used as an inexpensive antibacterial in water, and thus has saved countless people from a wide variety of diseases. Even more so that phosgene is used as a starting material for several very useful plastics, all of which are produced in large volumes.
2.1 Method of production
The reaction chemistry that illustrates the synthesis of phosgene is a deceptively simple addition reaction. It can be represented as
![]()
This does not however give any details about the reaction conditions, which are important for optimal yield of phosgene. The two reactants are passed through activated carbon, sometimes called activated charcoal. This serves a catalytic role. Since the reaction is exothermic and usually runs at a temperature zone of 50—150 °C, the reactor is typically cooled during the process.
The chlorine reactant is produced as one of the three products in what is called the chlor-alkali process. The other products of this process are sodium hydroxide and hydrogen gas. Carbon monoxide is usually produced by the reaction of carbon dioxide and carbon at elevated temperatures.
2.2 Volume of production annually
It is difficult to put a number on phosgene production annually, because the chemical’s toxicity dictates that it is immediately used for the production of other commodity chemicals at the location at which it is generated. Because of its ability to be used as a chemical weapon, large producers must be reported to the Organization for the Prohibition of Chemical Weapons (Organization for the Prohibition of Chemical Weapons, 2014).
It is however possible to find the production figures for the more common isocyanates, which are produced from phosgene. Totaled, several million tons of the most common are produced annually.
2.3 Sales
Almost no phosgene is sold as a commodity chemical. Rather, most is produced at the site where it will be further used in the production of other commodity chemicals, such as diamines and isocyanates.
2.4 Uses
As mentioned, phosgene had an infamous debut, but has evolved into a highly useful starting material for several bulk, organic chemicals.
Today, the major use of phosgene is in the production of isocyanates, almost all of which are further used in the production of polyurethanes. While there are many isocyanates, methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) are the two most commercially important, and thus the two that are made in the largest volumes. There are several large producers of MDI, including (in alphabetical order):
1. BASF, Germany
2. Bayer, USA
3. BorsodChem
4. Dow, USA
5. Huntsman
6. Nippon Polyurethane, Japan
7. OCI, South Korea
8. Yantai Wanhua, China, producing approximately 1.1 million tons (BASF Polyurethanes, 2014; Bayer Polyurethanes, 2014; BorsodChem, 2014; Dow, 2014; Huntsman Polyurethanes, 2014; Nippon Polyurethane Industry, 2013; OCI, 2013; Yantai Wanhua, 2013).
There are other, smaller producers as well (International Isocyanate Institute, 2013). For the past several years, roughly 5 million tons of isocyanates have been produced annually. The production of isocyanates is a matter of reacting an amine with phosgene. Generically, the reaction is as shown in Figure 2.1.
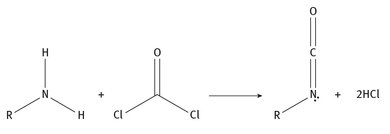
Fig. 2.1: Isocyanate production.
The more specific reaction to form MDI proceeds is shown in Figures 2.2 and 2.3.
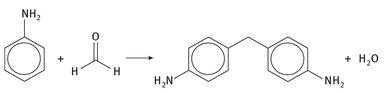
Fig. 2.2: Formation of methylene dianiline.
Aniline and formaldehyde form a precursor diamine. This is then reacted with 2 molar equivalents of phosgene to form the functional and reactive isocyanate ends, as seen in Figure 2.3.
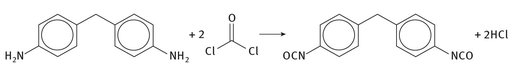
Fig. 2.3: MDI production.
In both cases, after the isocyanate has been formed, most are immediately converted to polyurethanes. The basic repeat unit for this is shown in Figure 2.4.
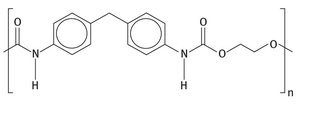
Fig. 2.4: Polyurethane repeat unit.
These diisocyanate units are reacted with a di-alcohol, generally called a diol. The resulting polymers, combinations of the isocyanate and alcohol, are polyurethanes, with the connecting −RN−CO2 -unit, called carbamates. This becomes the final functional group. Polyurethanes have a wide variety of uses, but rigid, thermosetting polyurethane foams are used as insulating materials in refrigerators, freezers, and several other consumer end use products (Allport et al., 2003).
2.5 Recycling
As mentioned earlier, phosgene is almost always used at or near the site where it is produced. Thus, none exists for any sort of recycling or reuse. The end-product polyurethanes are recycled in cases where doing so is economically feasible. The American Chemistry Council website discusses the general ways in which such materials can be recycled (American Chemistry Council, 2014).
Bibliography
Allport, D. C. ; Gilbert, D. S.; Outterside, S. M. (Eds.). MDI and TDI: Safety, Health and the Environment: A Source Book and Practical Guide, ISBN: 0-471-95812-3, 2003.
American Chemistry Council. Website. (Accessed 22 April 2014, as: http://polyurethane.americanchemistry.com/Sustainability/Recycling).
BASF Polyurethanes. Website. (Accessed 22 April 2014, as: http://www.polyurethanes.basf.us/).
Bayer Polyurethanes. Website. (Accessed 22 April 2014, as: http://www.polyurethanes.bayer.com/).
BorsodChem. Website. (Accessed 22 April 2014, as: http://www.borsodchem-group.com/About-us/The-present.aspx).
Dow.
Huntsman Polyurethanes. Website. (Accessed 22 April 2014, as: http://www.huntsman.com/polyurethanes/a/Products/Thermoplastic%20polyurethanes).
International Isocyanate Institute. Website. (Accessed 26 December 2013, as: http://www.diisocyanates.org/).
Nippon Polyurethane Industry. Website. (Accessed 26 December 2013, as: http://www.npu.co.jp/en/)
OCI. Website. (Accessed 26 December 2013, as: http://www.oci.co.kr/eng/)
Organization for the Prohibition of Chemical Weapons. Website. (Accessed 22 April 2014, as: http://www.opcw.org/chemical-weapons-convention/).
Yantai Wanhua. Website. (Accessed 26 December 2013, as: http://en.wanhuagroup.com/AboutUsInfo/AboutUs.aspx)