Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Butyraldehyde
3.1 Method of production
There have been several methods for the production of butyraldehyde in the past (also known as butanal), including the dehydrogenation of butanol, but the dominant one today is propylene hydroformylation, often called the Oxo Process, as shown in Figure 3.1.
![]()
Fig. 3.1: Butyraldehyde production.
As mentioned in Chapter 2, carbon monoxide can be produced by the addition of carbon dioxide to carbon. Propylene is refined from the lighter fractions of crude oil. Hydrogen gas is often obtained by hydrocarbon stripping methane or other light hydrocarbons.
3.2 Volume of production annually
Each year, roughly 6 billion kilograms of butyraldehyde are produced. This figure has not changed appreciably in the past 5 years. All is consumed in further reactions, and little is sold as an end product. Because the material can be dangerous upon coming in contact with unprotected personnel, personal and environmental safety precautions are always taken, and almost all butyraldehyde is used at the site where it is generated.
3.3 Uses
Butyraldehyde has a wide profile of uses, akin to many of the large commodity chemicals produced across the world. The production of butanol and the production of 2-ethylhexanol for later use in the manufacture of phthalates are the two biggest uses, while niche uses incorporate everything from plastics components to solvent. While usage profiles from different companies will differ based on their customers, a recent profile from Dow Chemical Company appears in Figure 3.2 (Dow, 2013). Here we outline the uses of butyraldehyde in making these four products. Other uses are much smaller and are not listed here.
3.3.1 n-Butanol, production and uses
While an older method for the production of butyraldehyde began with n-butanol as a feedstock, today the reverse production is the case. Butyraldehyde is hydrogenated to form the alcohol (Dow, 2013). This will be discussed in more detail in Chapter 6.
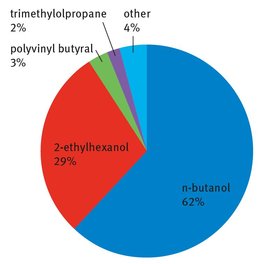
Fig. 3.2: Uses of butyraldehyde.
3.3.2 Uses of 2-ethylhexanol
2-Ethylhexanol can be produced in more than one way, but production usually involves a two-step process. What is sometimes called the Aldox process was perfected by Exxon and Royal Dutch Shell. The reactions are illustrated in Figure 3.3.
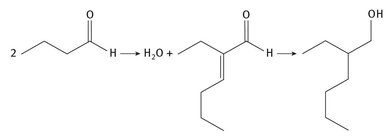
Fig. 3.3: 2-Ethylhexanol production.
The single use for virtually all 2-ethylhexanol is the production of the plasticizer: bis-(2-ethylhexyl) phthalate (usually abbreviated DEHP), which is shown in Figure 3.4. Phthalic anhydride is reacted with two moles of the alcohol resulting in the di-ester product and two moles of water as a by-product.
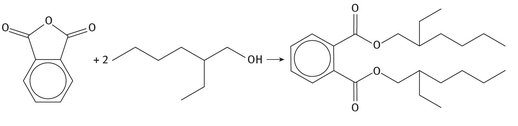
Fig. 3.4: Production of DEHP.
Since the 2-ethylhexanol has a chiral center, DEHP is produced with two chiral centers, and is sold as a mixture of the three different possible isomers.
This phthalate is used extensively as a plasticizer in several polymeric materials, most notable polyvinyl chloride (PVC), making the resultant materials more pliable and less brittle. Depending on the desired or required properties of a material, DEHP can be mixed to more than 35% by weight of the final product. It has also found use in some consumer-care products, such as sunscreens and lotions (UV Natural, 2014).
3.3.3 Uses of polyvinyl butyral
Polyvinyl butyral is a specialty plastic prepared from butyraldehyde and polyvinyl alcohol (sometimes called PVA). The reaction is shown in Figure 3.5.
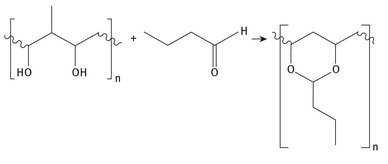
Fig. 3.5: Production of polyvinyl butyral.
By far the major use for polyvinyl butyral (sometimes abbreviated PVB) is the laminated type safety glass required in high strength applications such as automobiles and architectural window applications. Numerous trade names for this exist. They include:
· — Butacite the DuPont trade name for PVB glass (DuPont, 2014).
· — GlasNovations safety glass used in windows, doors, and automobiles (GlasNovations, 2014).
· — Saflex Eastman trade name, safety glass used in numerous architectural applications (Eastman, 2014).
· — S-Lec trade name for Sekisui interlaminate for automotive safety glass (Sekisui, 2014).
· — Trosifol Kuraray, Ltd., the trade name for laminated glasses, mostly used in automotive applications (Kuraray, 2014).
· — WINLITE PerryChem, trade name for laminated glass used in automotive and architectural applications (PerryChem, 2014).
3.3.4 Uses of trimethylolpropane
Trimethylolpropane is produced from butyraldehyde and formaldehyde. The process is generally a two-step one, as shown in Figure 3.6.
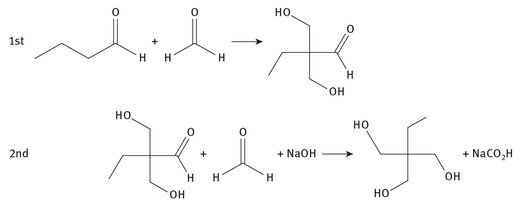
Fig. 3.6: Production of trimethylolpropane.
Sodium formate is the final by-product in the reaction. Trimethylolpropane is used in multiton quantities each year in the formation of alkyd resins — a class of polyesters — used for various applications, including multiple types of paints.
3.4 Recycling
We have seen that butyraldehyde is used completely in the production of other commodity chemicals. Thus, none is recycled. The four materials we have examined that are made with butyraldehyde are often used to create some user end-product or material. Thus, unless these materials are recycled, there is no second use for the butyraldehyde-containing material. Their use and disposal is often prescribed, discussed, or regulated through a Screening Information Dataset (SIDS), which is maintained by the Organization for Economic Cooperation and Development (OECD) (Organisation for Economic Co-operation and Development, 2013; OECD SIDS, 2013; OECD Existing Chemical Database, 2014).
Bibliography
Dow, Butyraldehyde. Website. (Accessed 27 December 2013, as: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_02b6/0901b803802b6d96.pdf?filepath=productsafety/pdfs/noreg/233-00596.pdf&fromPage=GetDoc)
DuPont, Butacite. Website. (Accessed, 23 April 2014, as: http://www2.dupont.com/SafetyGlass/en_US/products/butacite.html).
Eastman, Safelex. Website. (Accessed 23 April 2014, as: http://www.saflex.com/en/archibuildingcodes.aspx).
GlasNovations, Ltd. Website (Accessed 23 April 2014, as: http://www.glassglobal.com/profile/category66429.html).
Kuraray, Ltd. Website. (Accessed 23 April 2014, as: http://www.trosifol.com/en/).
Organisation for Economic Co-operation and Development. Website. (Accessed 28 December 2013, as: http://www.oecd.org/).
OECD SIDS N-Butyl Alcohol. SIDS Initial Assessment Report for SIAM 13. Website. (Accessed 28 December 2013, as: http://www.inchem.org/documents/sids/sids/71363.pdf)
OECD Existing Chemical Database. Website. (Accessed, 23 April 2014, as: http://www.oecd.org/chemicalsafety/risk-assessment/publishedassessments.htm #OECD_Existing_Chemicals_ Database).
PerryChem. Website. (Accessed 23 April 2014, as: http://www.perrychem.com/PVB.html).
Sekisui. Website. (Accessed 23 April 2014, as: http://www.s-lec.us/).
UV Natural. Website. (Accessed 23 April 2014, as: http://www.uvnatural.com/usa/resourcessunscreendataextractsusa.htm).