Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Acetic anhydride
4.1 Method of production
Acetic anhydride finds a large number of uses in the production of various commodity chemicals as well as in some consumer end products, such as aspirin and acetaminophen. It is manufactured from methyl acetate which in turn is made from methanol. Going back further, methanol is routinely produced from syn-gas, the combination of carbon monoxide and hydrogen gas in the presence of a metal or metal-oxide catalyst at elevated temperature and pressure (generally, 250 °C and 60—100 atm).
Thus, the reactions whereby acetic anhydride is produced from a single carbon atom source are shown in Figure 4.1. The final reaction requires rhodium iodide and lithium iodide as catalysts.
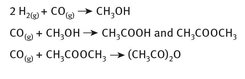
Fig. 4.1: Production of acetic anhydride from carbon monoxide.
The final reaction, shown in terms of Lewis structures, is shown in Figure 4.2.
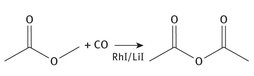
Fig. 4.2: Acetic anhydride production from methyl acetate.
4.2 Production
The following companies are major manufacturers of acetic anhydride. By the names, it is evident that this material is produced widely across the globe, in a large number of countries.
Anhua Group
At their website (Anhua Group, 2014), Anhua states that it produces 80,000 metric tons of acetic anhydride annually.
BP
BP states at its website: “Over 75% of acetic anhydride is used in making cellulose acetate. It is found in filter tow, textiles, plastics, photographic, and X-ray film, and a diverse range of other applications. Acetic anhydride is used in the synthesis of a number of pharmaceuticals such as aspirin and paracetamol. It is also used in the manufacture of a bleach activator, which is extensively used in washing powder formulations” (BP, 2013, 2014). This statement and similar ones from other manufacturers confirm that the use of acetic anhydride for production of cellulose acetate is the primary use for this material.
Eastman Chemical
Eastman Chemical also produces acetic anhydride for a wide range of uses, including a kosher grade for food use (Eastman methyl acetate, 2013). Such high-grade material can be used for several modified starches, which in turn are given the numbers E1414, E1420, and E1422 by the International Starch Institute (International Starch Institute, 2014). E1414 is an aceytlated di-starch phosphate, E1420 is simply an acetylated starch with modification at the −OH, and E1422 is an acetylated starch that can also be described as a di-starch adipate. These and other modifications are designed to adjust such properties as viscosity and cold or hot water solubility of the product.
Celanese Chemicals
Celanese Chemicals, USA, also produces cellulose acetate as the major material from its acetic anhydride (Celanese, 2014).
Hoechst
Hoechst has an extensive history of using acetic anhydride in the production of acetaminophen (paracetamol), and pioneered the current process for its production.
IHS Chemical
IHS Chemical (2014) states at its website that: “Sixty-two percent of the acetic anhydride consumed globally in 2012 went to the production of cellulose acetate flake. Cellulose acetate flake, in turn, is converted to cigarette filter tow, filament yarns and plastics” (IHS Chemical, 2014).
Wacker-Chemie
Wacker-Chemie has been producing acetic anhydride for nearly a century, and developed the ketene process for its manufacture (Wacker, 2014; UNEP OECD SIDS, 2013).
4.3 Uses
While there are a variety of uses for this material, all of them acetylations, the reaction with cellulose to form cellulose acetate is generally the largest commercial application, as mentioned above. For example, this accounts for 75% of BP’s use of acetic anhydride, as mentioned above (BP, 2013, 2014). Most of the remainder is used in the production of aspirin and acetaminophen, the latter sometimes called paracetamol (BP, 2013, 2014).
Cellulose acetate finds use in photographic film coatings, cigarette filters, some diapers, as well as in other specialty coatings (see Figure 4.3). Apparently, Lego building blocks were made from cellulose acetate for several years starting in the late 1940’s.
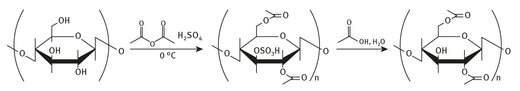
Fig. 4.3: Cellulose acetate production.
The production of aspirin is now a mature industry, but is one that requires a continuous input of acetic anhydride for the acetylation of salicylic acid (see Figure 4.4). In turn, the acid is produced from phenol, meaning that crude oil becomes the ultimate material source for aspirin.
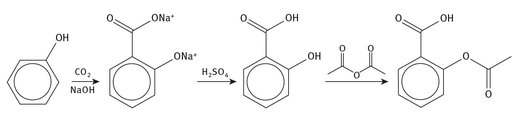
Fig. 4.4: Production of aspirin.
As can be seen from the production reactions for acetaminophen (see Figure 4.5), the starting materials are now ultimately oil once again, and hydrofluoric acid. Production and use of fluorine compounds are discussed in Chapter 22.
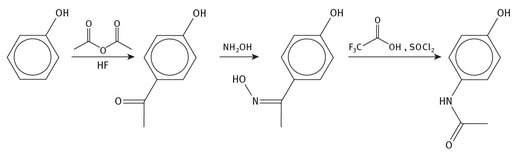
Fig. 4.5: Production of acetaminophen.
4.4 Recycling and reuse
All acetic anhydride is consumed in the production of other chemicals, and thus there are no recycling programs for it. There are also no large-scale recycling projects for the end-use products listed above.
Bibliography
Anhua Group. Website. (Accessed, 23 April 2014, as: http://www.danhua-group.com/pro10e.html).
BP. Website. (Accessed 28 December 2013, as: http://www.bp.com/sectiongenericarticle.do?categoryId=9008844&contentId=7016477)
BP, Acetic anhydride. Website. (Accessed 23 April 2014, as: http://www.bp.com/en/global/corporate/products-and-services/bp-petrochemicals/what-we-do.html).
Celanese. Website. (Accessed 12 May 2014, as: http://www.celanese.com/cellulose-derivatives/About-Us.aspx).
Eastman Kodak. Website. (Accessed 28 December 2013, as: http://www.eastman.com/Pages/Home.aspx)
Eastman methyl acetate. Website. (Accessed 28 December 2013, as: http://www.eastman.com/Pages/ProductHome.aspx?product=71001121) and acetic anhydride. Website. (Accessed 12 May 2014, as: http://www.eastman.com/Products/Pages/ProductHome.aspx?Product=71016156&list=Chemicals).
IHS Chemical. Website. (Accessed 8 May 2014,as: http://www.ihs.com/products/chemical/planning/ceh/acetic-anhydride.aspx).
International Starch Institute. Website. (Accessed 13 May 2014, as: http://www.starch.dk/isi/applic/E-numbers.htm).
Lux, S.; Winkler, T.; Siebenhofer, M. Synthesis and isolation of methyl acetate through heterogeneous catalysis with liquid/liquid extraction. Website. (Accessed 28 December 2013, as: http://www.iscre.org/iscre21/abstracts/15.pdf)
UNEP OECD SIDS. Website. (Accessed 28 December 2013, as: http://www.chem.unep.ch/ and http://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html)
Wacker. Website. (Accessed 13 May 2014, as: http://www.wacker.com/cms/en/wacker_group/wacker_facts/history/history.jsp).
Zoeller, J. R.; Agreda, V. H.; Cook, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pond, D. M. Eastman Chemical Company Acetic Anhydride Process, Catal. Today 13, 1 (1992) 73—91.